2018 Volume 66 Issue 3 Pages 327-333
2018 Volume 66 Issue 3 Pages 327-333
Palmitoyl-glycine-histidine (Pal-GH) is a new low molecular weight gelling agent. It exhibits thixotropic behavior, low viscosity, and high dissolving properties for a wide range of hydrophilic to lipophilic drugs. Orally administered ivermectin (IVM) is used to treat scabies. However, this treatment is associated with well-known side effects, thus a study is awaited to search for alternative routes of administration. Although a topical formulation of IVM could be a candidate, it requires whole body application except the head and face for several hours on a daily basis. Therefore, in this study, we prepared a gel spray formulation containing IVM as an approach for application to large skin areas with a single spray application without further contact with the applied formulation. Pal-GH gel spray formulations were prepared from its aqueous solution by a heating and cooling method. Rheological behavior and physical appearance (spraying, spreading ability, volume of spraying, and homogeneity) of the prepared formulations were evaluated. Pal-GH gel with propylene glycol demonstrated impressive rheological properties (typical thixotropic behavior) with high hysteresis area among all the tested Pal-GH gels and spreading ability. The obtained IVM concentration in the skin after topical application of 0.1% IVM-containing Pal-GH formulation onto hairless rats was much higher than the reported therapeutic concentration obtained from oral administration in humans. These results suggested that topical application of IVM using a Pal-GH gel spray formulation could be an alternative to the conventional oral forms for the scabies treatment.
Scabies (a parasitic roundworm infection) is a major worldwide public health problem in many developing countries,1) and it has become a major problem even in Japan and other developed countries due to highly aged societies. Oral administration of ivermectin (22,23-dihydroavermectin B1a; IVM) is presently used worldwide in scabies therapy. Curing the parasitic infection helps to improve QOL for patients. In people with weakened immune systems, curing the roundworm infection can reduce the risk of developing a severe or life-threatening infection. IVM belongs to a class of drugs known as antihelmintics, and it works by its paralyzing and killing the parasites. IVM is considered to be absorbed into the systemic circulation and distributed to the skin tissues through sebaceous glands after oral administration. Haas et al. reported that IVM concentration in the stratum corneum ranged from 40 to 80 ng/g in scabies patients who took 12 mg oral IVM, which is regarded as an effective dose.2) However, this treatment for scabies is potentially hazardous and associated with moderate to severe side effects for caregivers as well as patients.3,4) Furthermore, the safety and efficacy of oral IVM have not been well-established, especially in older patients with impaired liver function, in children, and in pregnant women.4,5)
Such side effects related to oral IVM prompted medical and pharmaceutical researchers to investigate alternative routes of administration and novel formulations, such topically applied cream, gel, or foam for whole body bathing as a safe and simplified therapy.6) These topical preparations probably function through IVM absorption through the skin barrier to the diseased sites with fewer systemic adverse effects than oral formulations. Thus, such topical formulations of IVM are candidate treatments. However, they still require whole-body application for several hours on a daily basis to maintain effective IVM concentrations in the skin of scabies patients.
We were surprised to observe in our preliminary experiment that topical skin application of 0.1% IVM solution provided much higher concentrations in the stratum corneum at the application site compared with the effective concentration reported by Haas et al. However, scabies spreads easily by infestation both by direct skin-to-skin contact and by contamination through clothing, bedding, and furniture.7–9) Therefore, we decided to study gel spray formulations that could cover a large skin area in a single application without further contact with the applied formulation.
Gelling agents are capable of turning liquids into “solid-like” substances by entrapping and immobilizing solvent molecules at a macroscopic level.10) They can be classified into polymeric macromolecular (>10 kDa) or low molecular weight (<10 kDa) gelling agents. Several kinds of low molecular weight building blocks can self-assemble to form one-dimensional nanofibers, and then three-dimensional entanglement of the nanofibers causes gelation of the solvents. Such hydrophilic supramolecular gelling agents can be designed at the molecular level depending on their proposed use, because of their relatively simple molecular structure.11,12) The biocompatibility and biodegradability of these gels make them ideal for drug delivery systems.13) In contrast to conventional polymeric gels, such supramolecular gels are characterized by the reversible transition between sol and gel states.
Palmitoyl-glycine-histidine (Pal-GH) is a unique molecule with hydrophilic and hydrophobic moieties consisting of glycine, histidine and palmitic acid14) (Fig. 1). In spite of its weak strength compared with conventional and marketed polymer gels, the high controllability of the molecular structure and function has led to a new gel substrate. Pal-GH can be gelled at a broad range of concentrations and even at a low level. Pal-GH has valuable properties such as thixotropic behavior, lower viscosity, and high dissolving properties for a wide range of hydrophilic to lipophilic drugs. Therefore, in this study, we planned to use Pal-GH as a gelling agent for a gel spray formulation containing IVM to be available for scabies treatment.
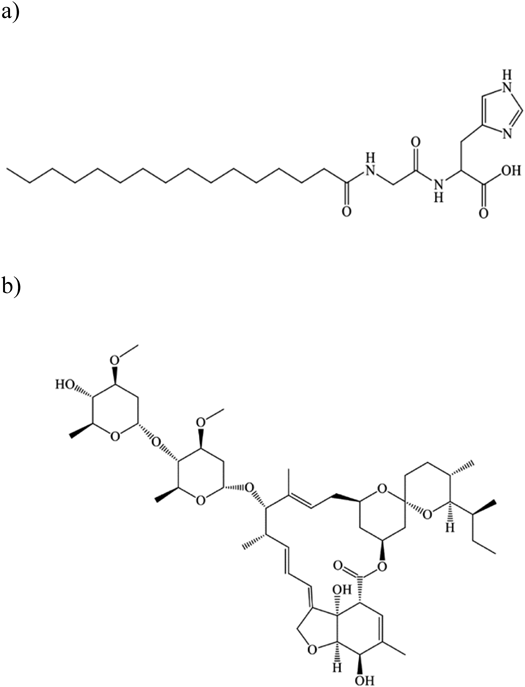
Molecular weights of ivermectin and Pal-GH are 875.1 g/mol 564 g/mol, respectively. c Log p value of ivermectin is 5.83.
Thixotropic behavior is necessary to prepare a gel spray formulation, because it exhibits an isothermal system, in which apparent viscosity decreases under shear stress, followed by a gradual recovery when the stress is removed.15) Thus, the main objective of this work was to design a topically applied Pal-GH formulation containing IVM. The formulation was prepared by a heating-and-cooling method. The rheological properties and physicochemical evaluation of the prepared Pal-GH gel spray formulations were determined. The skin concentration and IVM-release properties were also determined.
Pal-GH premix (composed of 6% Pal-GH, 30% 1,2-octanediol, 20% 1,3-butanediol, 2% polyoxyethylene lauryl ether, 1% stearic acid, and 41% purified water) and propylene glycol acetate (PGA) were obtained from Nissan Chemical Industries, Ltd. (Tokyo, Japan). IVM was purchased from Tocris Bioscience (Bristol, U.K.), and glycerin (GL) and propylene glycol (PG) were from Kanto Chemical Co., Inc. (Tokyo, Japan). Sucrose was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Other chemicals and reagents were of special grade or HPLC grade (purchased from Wako Pure Chemical Industries, Ltd.) and used without further purification.
AnimalMale hairless rats (200–250 g) were purchased either from Life Science Research Center, Josai University (Sakado, Saitama, Japan) or Ishikawa Experiment Animal Laboratories (Fukaya, Saitama, Japan). The animals were housed in temperature-controlled rooms (25±2°C) with a 12 h light-dark cycle (7 : 00–19 : 00 h), and were allowed free access to food (Oriental Yeast Co., Tokyo, Japan) and tap water. All breeding procedures and the experiments on animals were performed in accordance with the guidelines of the Animal Experiment Committee of Josai University.
Preparation of Pal-GH FormulationsThe Pal-GH formulations were prepared by a heating-and-cooling method as follows: Pal-GH premix was dissolved in purified water at 85°C. Then, IVM was completely dissolved into the Pal-GH solution at 75°C, followed by adding PG or GL at different concentrations. Furthermore, PGA aqueous solution was obtained by stirring in purified water at 85°C for 1 h. The obtained PGA solution was mixed with the Pal-GH solution containing IVM at 1000 rpm at 75°C for 1 h (until homogeny). After mixing, the formulation was kept at room temperature before use. Pal-GH gel formulations without IVM were also prepared by mixing the Pal-GH solution containing GL or PG and the PGA solution at 1000 rpm at 75–85°C for 1 h. Table 1 shows the compositions of the Pal-GH formulations.
| Pal-GH conc. | Additives | Formulation code without IVM | Formulation code containing 0.1% IVM |
|---|---|---|---|
| 2.5% | No additive (base) | F2.5 | F2.5IVM |
| Glycerin 2.5% | F2.52.5GL | F2.52.5GL-IVM | |
| Glycerin 10% | F2.510GL | F2.510GL-IVM | |
| Propylene glycol 4.0% | F2.54PG | F2.54PG-IVM | |
| 5.0% | No additive (base) | F5 | F5IVM |
| Glycerin 2.5% | F52.5GL | F52.5GL-IVM | |
| Glycerin 10% | F510GL | F510GL-IVM | |
| Propylene glycol 4.0% | F54PG | F54PG-IVM |
Abbreviations: GL, glycerin; IVM, ivermectin; PG, propylene glycol.
Physical characterization of the Pal-GH formulations was performed, including solid-like behavior, spraying ability, spread area, weight, and homogeneity of a single spray application. The solid-like behavior of these formulations before and after shaking was evaluated visually. Spraying ability of Pal-GH formulation was evaluated using a general spraying nebulizer (Toki Mini Spray PET vol. 20 mL, 0.35 mm i.d. nozzle, Sansho, Tokyo, Japan), and the sprayed area on a glass plate from a vertical distance of 15 cm from the nozzle to the plate was measured using imaging software (cellSens, Olympus Corp., Tokyo, Japan) and using a stereoscopic microscope (SZ61, Olympus Corp.). The weight of a single application of each sprayed formulation was measured by weighing the sample on the glass plate. Aggregation of the sprayed formulations after drying was observed to evaluate homogeneity. Moreover, a flowability test was conducted by spraying each Pal-GH formulation from a distance of 15 cm from the nozzle to a vertical glass plate. Movement of the sprayed formulation was observed using the naked eye.
Evaluation of the Rheological Properties of Pal-GH FormulationsThe rheological properties (thixotropic properties and viscosity) of Pal-GH formulations were evaluated using a rotational viscometer (Toki Sangyo Co., Ltd., Tokyo, Japan). The measurement conditions were as follows: the temperature was maintained at 25 and 32°C, corn rotor was 3°×R14, sample volume was 0.4 mL, rotation speed was increased from 0 to 100 rpm over 20 min and decreased from 100 to 0 rpm over the same period, and a flow analysis method was used. The viscosities of the Pal-GH gel formulations and sucrose solutions were evaluated. Moreover, the shear thinning of Pal-GH gel bases was evaluated by measuring their viscosity at different shear rates (0–200 s−1). The viscosity profile against time was calculated to confirm the viscosity of the formulations with good properties for maintaining the sprayed area. Hysteresis area was calculated by using VA 2000 software (Toki Sangyo Co., Ltd.).
Fourier Transform (FT)-IR Measurement of Pal-GH FormulationFT-IR spectra were measured for selected Pal-GH formulations. Samples were analyzed to confirm the interaction between IVM and Pal-GH. All spectra were recorded by FT-IR with single-reflection attenuated total reflection (ATR) using a diamond prism (IRT Tracer-100, Shimadzu Corporation, Kyoto, Japan) in the range of 400–4000 cm−1 with 4 cm−1 steps and 40 scans.
IVM Release from FormulationsIVM release from the formulations was evaluated for selected Pal-GH formulations. This experiment was performed using a side-by-side diffusion cell (effective diffusion area: 0.95 cm2) with a sheet of dialysis membrane (molecular weight cut-off; 2000–14000 Da) (Sanko Junyaku Co., Tokyo, Japan) over 24 h. The sample (0.30 g) was spread evenly in a glass cell compartment with a 0.3 mL volume and set on the membrane, and the receptor compartment was filled with 3.0 mL of soybean oil because of the high lipophilicity of IVM. The receptor cell was agitated using a magnetic stirrer throughout the experiment. The temperature in the receiver compartment was kept at 32°C using a thermostatic water bath circulating through the double glass walls of the diffusion cell. Aliquots (0.50 mL) were withdrawn from the receptor compartment at predetermined times, and the same volume of fresh medium was replaced to maintain the receiver volume. The extraction ratio of IVM from soybean oil was obtained to calculate its exact concentration.
Skin Concentration of IVMFull thickness hairless rat skin was excised from the abdomen under anesthesia by intraperitoneal (i.p.) injection of the three types of anesthesia (medetomidine, 0.375 mg/kg; butorphanol, 2.5 mg/kg; and midazolam, 2 mg/kg). Excess fat was trimmed off, and the excised skin was set in a Franz diffusion cell (effective diffusion area: 1.77 cm2) with the epidermis side facing the donor compartment. Phosphate buffered saline (PBS), pH 7.4, was added to the receiver compartments. The experiments using the skin were conducted after hydration with PBS for 60 min. The Pal-GH formulation containing IVM (1 mL) was loaded into the donor compartment (epidermal side). The receiver solution was agitated using a stirrer bar and a magnetic stirrer throughout the experiments. The diffusion cells were kept at 32°C with a water jacket connected to a water bath. In addition, at the end of the experiment (24 h) the amount of IVM that permeated through the skin was also determined, an aliquot (0.50 mL) was withdrawn from the receiver chamber. Then, the applied formulation was gently removed using a cotton swab and the skin sample was washed 20 times with 1.0 mL PBS. The permeation area of the skin was then cut into small pieces after gentle removal of excess water on the skin using Kimwipes® paper.
IVM concentrations in full-thickness skin and viable epidermis and dermis (VED) were determined, and the amount of IVM in the stratum corneum was calculated by subtraction of its amount in the VED from that in full-thickness skin. Then, the IVM concentration in the stratum corneum was estimated under the assumption that the density of the stratum corneum would be 1.2 g/cm.3,16) The IVM concentration in VED was obtained by 20 repetitions of stripping of the stratum corneum layers from the full-thickness skin using adhesive tape (Cellotape®, Nichiban, Tokyo, Japan) just after finishing the washing process.
Determination of IVMIVM concentration in soybean oil: methanol (200 µL) was added to each soybean oil solution (200 µL), and the obtained mixture was vortexed for 10 min and centrifuged twice for 5 min to obtain the supernatant. The supernatant (100 µL) was used for HPLC assays.
Skin concentration: the obtained skin piece (0.05 g) was minced using scissors and homogenized (4°C, 5 min) with 0.45 mL of methanol for 2.5 min using a homogenizer (Polytron PT-MR 3000; Kinematica Inc., Littau, Switzerland). Methanol (0.5 mL) was added and homogenized again for 2.5 min, then agitated for 15 min. After centrifugation (5000 rpm, 4°C, 5 min), the supernatant (100 µL) was mixed with the same volume of methanol then agitated and centrifuged again using the same conditions. The obtained supernatant (100 µL) was injected into the HPLC system, and the measurement was obtained using the same conditions as described below.
The HPLC system (Shimadzu Corporation) consisted of a system controller (CBM-20 A), pump (LC-20AD), auto-sampler (SIL-20AC), column oven (CTO-20 A), UV detector (SPD-M20A), and analysis software (LC Solution). The column was an Inertsil® ODS-3 (5 µm, 4.6×250 mm) (Nihon Waters K.K., Tokyo, Japan), which was maintained at 40°C. The mobile phase was acetonitrile–methanol–water=6 : 3 : 1 (0–12 min). The flow rate was adjusted to 1.0 mL/min. IVM was detected at 245 nm.
Statistical AnalysisStatistical analysis for the skin concentration of IVM and in vitro release of IVM were performed using Student’s t-test, and p values less than 0.05 were considered to be significant.
Figure 2 shows photographs of the Pal-GH gel formulation before (Fig. 2a) and after (Fig. 2b) shaking the bottles to evaluate the ability to form a tight gel. Only the results of F2.54PG-IVM are displayed as examples.
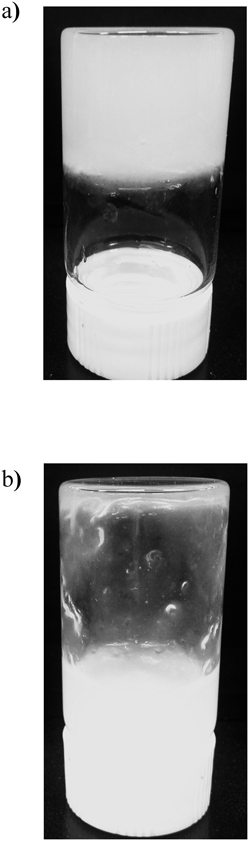
The Pal-GH gel shows a tight gel (solid like) before shaking, and then a sol just after shaking.
Table 2 summarizes the results of the physical characterizations (gel-forming property after shaking, spraying ability, sprayed area, weight of sprayed formulation, and homogeneity) of 18 Pal-GH formulations with 2.5 and 5% Pal-GH premix in different additives and with and without IVM. When spraying these formulations, almost the same weight (0.15–0.22 g) could be sprayed using a general spraying nebulizer (Toki Mini Spray PET, details in Experimental) independent of the formulations. Most of the formulations showed a sol-gel transition behavior before and after shaking (see Fig. 2). In addition, the sprayed areas were dependent on the formulation, although all formulations could be sprayed using the nebulizer. Larger sprayed areas were observed in F5 formulations, except for F5 and F5IVM. On the other hand, the sprayed areas obtained from the F2.5 formulations were smaller than those obtained from the F5 formulations. Furthermore, the homogeneity of the sprayed formulations was also evaluated. All formulations showed good homogeneity, and almost the same weight of formulation was sprayed from the nebulizer. No aggregation spots were observed in the sprayed area after drying (data not shown).
| Sample code | Gel form after shaking | Spraying ability | Sprayed area (cm2) | Weight of sprayed formulation (g) (n=3) | Homogeneity |
|---|---|---|---|---|---|
| F2.5 | ○ | ○ | 50.5 | 0.150±0.02 | ○ |
| F2.52.5GL | ○ | ○ | 153.86 | 0.186±0.01 | ○ |
| F2.510GL | ○ | ○ | 176.625 | 0.190±0.017 | ○ |
| F2.54PG | ○ | ○ | 113.04 | 0.190±0.01 | ○ |
| F2.5IVM | ○ | ○ | 38.46 | 0.215±0.05 | ○ |
| F2.52.5GL-IVM | ○ | ○ | 54.4 | 0.193±0.006 | ○ |
| F2.510GL-IVM | ○ | ○ | 63.58 | 0.197±0.006 | ○ |
| F2.54PG-IVM | ○ | ○ | 91.2 | 0.190±0.005 | ○ |
| F5 | ○ | ○ | 38.46 | 0.156±0.01 | ○ |
| F52.5GL | ○ | ○ | 145.19 | 0.186±0.01 | ○ |
| F510GL | ○ | ○ | 171.9 | 0.180±0.02 | ○ |
| F54PG | ○ | ○ | 132.6 | 0.187±0.02 | ○ |
| F5IVM | ○ | ○ | 38.46 | 0.190±0.037 | ○ |
| F52.5GL-IVM | ○ | ○ | 132.6 | 0.186±0.02 | ○ |
| F510GL-IVM | ○ | ○ | 153.86 | 0.186±0.015 | ○ |
| F54PG-IVM | ○ | ○ | 132.6 | 0.170±0.05 | ○ |
Symbols; ○: confirmed gel forming, spraying ability, or homogeneity. ×: not confirmed. The sprayed area on a glass plate was measured using imaging software after spraying at a vertical distance of 15 cm from the plate. Weight of sprayed formulation was measured to evaluate the amount of one spray.
Moreover, suitable viscosity for easy of spraying and flowability of the sprayed formulation on skin were determined qualitatively using different concentrations of sucrose, because sucrose solutions show a typical Newtonian flow. Table 3 summarizes the spraying ability, spreadability, and flowability of the Pal-GH formulations on skin as a function of viscosity. As a result, viscosity ranging between 51 and 250 mPa·s was considered to be suitable for topical application using spray formulations. In addition, the highest viscosity that was easily sprayed using a general spraying nebulizer was determined to be 250 mPa·s, and the lowest viscosity in which the vehicle maintained the sprayed area on the skin was 51 mPa·s.
| Viscosity range (mPa·s) | Spraying ability | Spreadability | Flowability on skin |
|---|---|---|---|
| 1–10 | Spraying possible | >400 cm2 | Spread over the sprayed area by flowing down of the formulation |
| 11–50 | Spraying possible | 100–400 cm2 | Flowing down of the formulation |
| 51–250 | Spraying possible | <100 cm2 | Maintained across the sprayed area over the time |
| 251–400 | Leakage only | No spreadability | Maintained across the sprayed area over the time |
| >400 | Impossible to spray | No spreadability | Maintained across the sprayed area over the time |
All observations were done using sucrose viscous solutions.
The thixotropic properties of the Pal-GH gel formulations were further investigated. Figure 3 shows rheograms of several Pal-GH gel formulations at Pal-GH concentrations of 2.5% (Fig. 3a) and 5% (Fig. 3b). Closed and open symbols indicate increasing shear rate and decreasing shear rate, respectively. Thixotropic behavior was clearly observed in all formulations containing IVM (Fig. 3). The hysteresis area of these F2.5 and F5 changed with the Pal-GH premix concentration as well as with the addition of IVM and PG or GL to the formulation. Among the formulations, higher hysteresis areas were observed in F2.54PG-IVM and F54PG-IVM, and the hysteresis areas obtained from F2.54PG-IVM and F54PG-IVM were 725 and 2611 Pa⋅s−1, respectively. On the other hand, F510GL-IVM and F52.5GL-IVM also showed higher sprayed areas (Table 2), but the hysteresis area was lower than those in F2.54PG-IVM and F54PG-IVM. The yield value obtained in the rheograms for F2.54PG-IVM and F54PG-IVM were 3.29 and 13.8 Pa, respectively. Thus, further experiments were conducted with F2.54PG-IVM and F54PG-IVM.
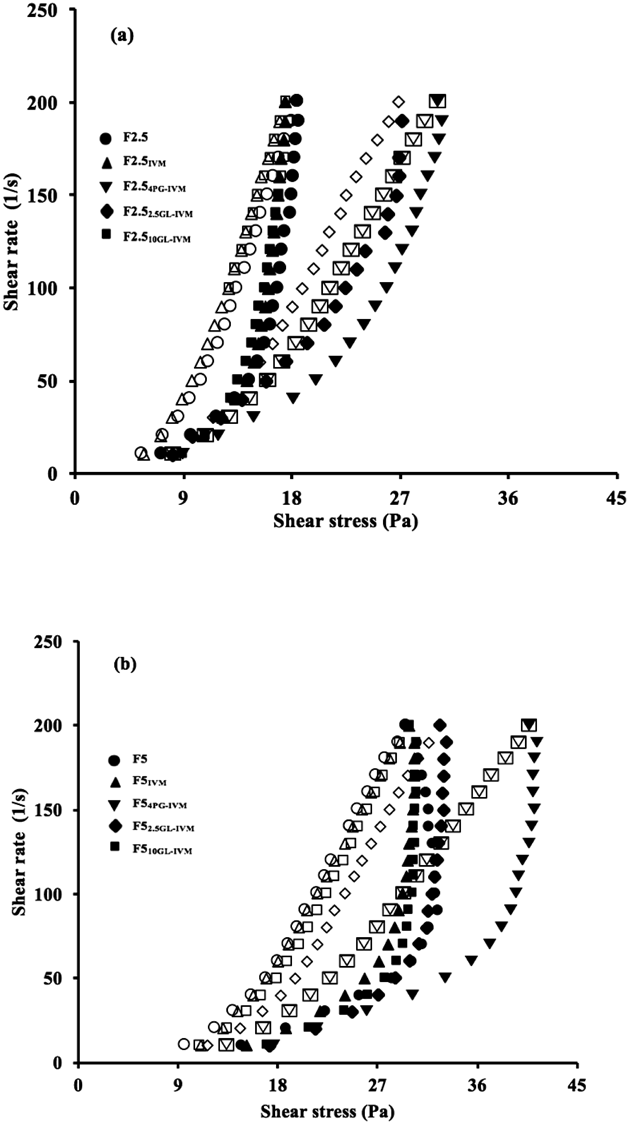
Closed and open symbols indicate increasing and decreasing shear rate, respectively. Thixotropic behavior was clearly observed in all formulations. Symbols; ○ and ●: F2.5 (in Fig. 3a) or F5.0 (in Fig. 3b), △ and ▲: F2.5IVM (in Fig. 3a) or F5.0 IVM (in Fig. 3b), ▽ and ▼: F2.54PG-IVM (in Fig. 3a) or F54PG-IVM (in Fig. 3b), ◇ and ◆: F2.52.5GL-IVM (in Fig. 3a) or F52.5GL-IVM (in Fig. 3b), □ and ■: F2.510GL-IVM (in Fig. 3a) or F510GL-IVM (in Fig. 3b).
Because IVM is a highly lipophilic compound, soybean oil was used as a receiver medium in the in vitro release studies of IVM from formulations. The cumulative percentage of IVM released from F2.54PG-IVM and F54PG-IVM was about 4.9 and 5.7% over 24 h, respectively (no significant differences were observed among all the tested Pal-GH formulations), whereas a released value of 13.6% was observed from the PG solution (Fig. 4).

Significant differences were not observed between Pal-GH formulations but were observed with between control and Pal-GH formulations (*: p<0.05). Symbols; ○: 0.1% IVM in PG (control), △: F2.54PG-IVM, □: F54PG-IVM.
Figure 5 shows the skin concentration of IVM 24 h after topical application of F2.54PG-IVM and F54PG-IVM onto hairless rat skin. PG solution containing 0.1% IVM was used for comparison. The IVM concentration in the VED and stratum corneum was also investigated. A markedly higher IVM concentration in the stratum corneum was observed in all topically applied formulations compared with the effective concentration reported by Haas et al. (40–80 ng/g). The IVM concentration in the stratum corneum that obtained from 0.1% IVM in PG was much higher than those with F2.54PG-IVM and F54PG-IVM. However, the IVM concentrations in VED after topical application of F2.54PG-IVM and F54PG-IVM were significantly increased compared with that after topical application of 0.1% IVM in PG. Despite the IVM skin concentration observed with all formulations, no skin permeation of IVM was observed.
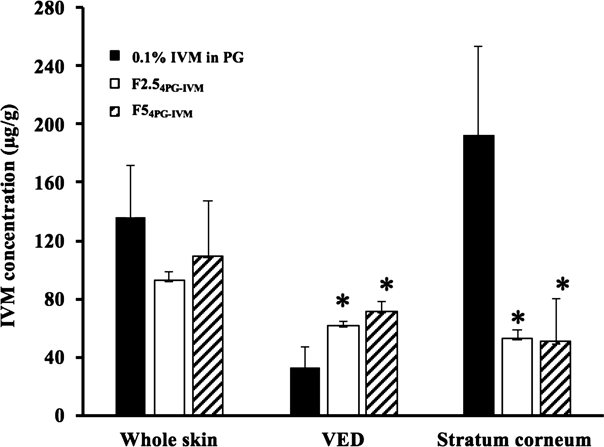
The IVM concentration of the stratum corneum was estimated by dividing the IVM amount difference between the IVM amounts in the VED and whole skin by the weight of the stratum corneum. Significant differences were observed between Pal-GH formulations and 0.1% PG solution in the stratum corneum and VED (*: p<0.05). Closed columns: 0.1% IVM in PG, Open columns: F2.54GL-IVM, hatched columns: F54PG-IVM.
FT-IR studies were carried out to investigate the interaction between IVM and Pal-GH (Fig. 6). The FT-IR spectra of IVM showed sharp peaks at: 3458.4 cm−1 due to axial deformation of free O–H; 2964.6 cm−1 characteristic of methyl groups; 2937.6 indicating axial deformation of C–H; 1730.1 cm−1 characteristic of saturated aliphatic ketone C=O stretching; 1676.1 cm−1 relating to unsaturated lactones with a double bond adjacent to the –O– group, owing to the C=C group; 1599.0 and 1454.3 cm−1 relating to moderate absorption of ketones; and 1200–1000 cm−1 showing the aliphatic ethers due to the asymmetric axial deformation of C–O–C. On the other hand, spectral peaks derived from IVM were not detected in F2.5 and F2.54PG, only a peak derived from the OH group and vibration of the C=O moiety in the carboxylates (1600 cm−1) were observed.
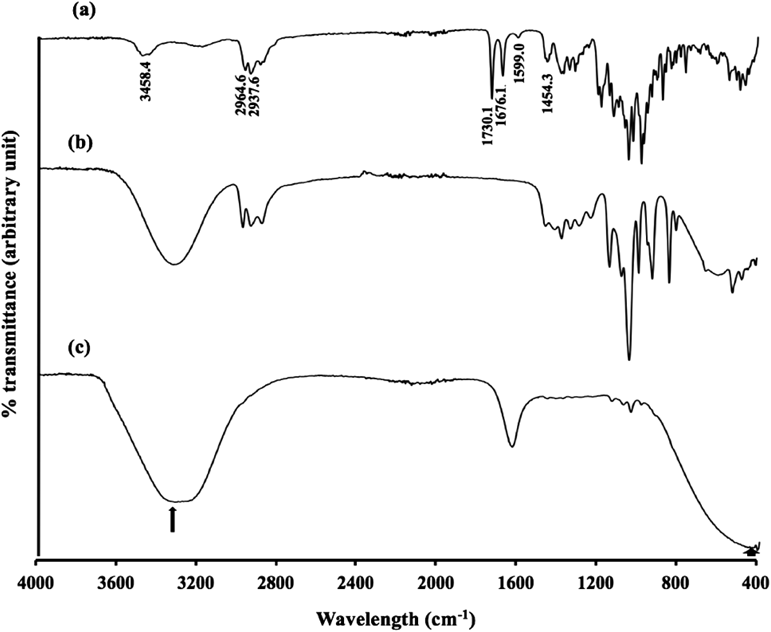
Peaks; 3458.4 cm−1 indicates free O–H; 2964.6 cm−1 is characteristic of methyl groups; 2937.6 cm−1 indicates axial deformation of C–H; 1730.1 cm−1 is characteristic of saturated aliphatic ketone C=O stretching; 1599.0 and 1454.3 cm−1 are related to moderate absorption of ketones; and 1200–1000 cm−1 indicate C–O–C. Only a peak derived from an OH group (shown in arrows) and vibration of the C=O moiety in the carboxylates (1600 cm−1) were observed in F2.5 and F2.54PG.
Pal-GH is a recently developed low molecular weight gelling agent. Matsumoto et al.17) reported its rheological properties, molecular assembled states, and the morphology of its network structure.17) In the present study, we tried to develop a topically applicable gel spray formulation prototype using Pal-GH as a promising approach to increase IVM concentration in the skin of patients with scabies.
Topical drug application has many advantages, such as ease of administration and the ability to obtain high concentrations in the skin underneath the site of application, and reduced systemic side effects compared with oral administration or injection. Several reports have already revealed that topical application of IVM as a solution, lotion, or cream provided marketed improvement of scabies without serious side effects, although IVM concentration in the skin was not evaluated in these studies.18,19) On the other hand, an IVM concentration of more than 400 ng/g skin tissue was observed after application of a bath formulation to rat skin. Haas et al.2) reported that the IVM concentration in the stratum corneum ranged from 40 to 80 ng/g in patients with scabies, who responded to oral administration of IVM at a dose of 12 mg, and this is regarded as an effective dose.
In the present study, IVM application with a gel spray formulation (F2.54PG-IVM and F54PG-IVM) showed much higher skin tissue concentration, more than 40 µg/g in the stratum corneum and VED, respectively (Fig. 5), although no skin permeation was observed due to its high lipophilicity. Thus, a sufficient concentration was obtained for scabies treatment by application of Pal-GH formulations containing IVM. Because the skin concentration of drugs is generally related to their applied concentration (dose), adequate therapeutic skin concentration of IVM could be obtained by decreasing the applied concentration or dose in the formulation. The IVM concentration in VED obtained from the topical application of Pal-GH formulations was significantly (p<0.05) higher than that obtained from the topical application of 0.1% IVM in PG, although 0.1% IVM in PG showed a significantly (p<0.05) higher concentration in the stratum corneum compared with Pal-GH formulations. Because the complete removal of topically applied formulations from the skin surface is difficult, the IVM concentration in the stratum corneum might be overestimated, especially for the PG alone formulation, even after performing 20 washes to remove the formulation.
In addition, a gel spray formulation is useful to prevent scabies infection by direct skin contact, and it can be maintained at the site of application on skin by its thixotropic properties derived from the molecular interactions of Pal-GH via non-covalent bonding.20) The obtained hysteresis loop showed that structural change was obtained using externally applied force on the formulations. The drawn rheograms differed according to the additives in the formulation (Fig. 3), because the thixotropic properties were influenced by several factors, and one of them was by mixing with additives.21) Furthermore, the flowability of sprayed F2.54PG-IVM and F54PG-IVM was investigated to confirm the usefulness of the presently prepared gel spray formulation. No flowing-down of sprayed F2.54PG-IVM and F54PG-IVM was observed on the vertical glass plate (data not shown). This finding showed that our formulations were suitable to maintain the sprayed area with easy spraying.
A marked interaction between IVM and Pal-GH could not be found using the FT-IR determination (Fig. 6) because of the low concentration of IVM in this formulation. In addition, IVM release from the prepared Pal-GH formulation was not changed by an increase in Pal-GH concentration. However, thixotropic behavior was changed by increasing Pal-GH concentration as well as the addition of additives in the formulation. This result suggested that microstructural changes in the Pal-GH formulation might be obtained by the additives in the formulation.
The mechanism of the enhancement effect on the skin concentration of IVM from Pal-GH formulations is not fully understood. The drug release rate and its subsequent skin permeation are linearly related to the thermodynamic activity in the formulation.22–24) Thus, differences in the thermodynamic activity of the formulation might be a reason for the higher IVM concentration after application of the Pal-GH formulations. Moreover, freely existing Pal-GH molecules might work as a penetration enhancer for IVM penetration into the skin.
Further efforts are needed to develop Pal-GH formulations and assess the therapeutic efficiency of our formulations. The present findings strongly suggested that the Pal-GH gel spray formulation (i.e., F2.54PG-IVM, F54PG-IVM) developed here can be utilized as a promising new topical formulation for the treatment of scabies.
The present data clearly indicated that application of Pal-GH gel formulations (F2.54PG-IVM and F54PG-IVM) is a promising topical spray formulation as an alternative to conventional oral formulations for scabies treatment. This approach may contribute to enhance the skin concentration of mal-absorptive drugs from topical formulations. Further studies should be performed to clarify the mechanism of skin-penetration enhancement of drugs using Pal-GH formulations.
The authors wish to thank to Nissan Chemical Industries, Ltd. (Tokyo, Japan) for the supporting materials.
Kenji Sugibayashi and Hiroaki Todo received a research grant from Nissan Chemical Industries, Ltd.