2018 Volume 66 Issue 3 Pages 334-338
2018 Volume 66 Issue 3 Pages 334-338
3β-tert-Butyldimethylsiloxy-22-phenylthio-23,24-bisnorchola-5,9(11)-diene, which has a double bond between C-9 and C-11 and a phenylsulfenyl group on the terminus of the side chain, is a potential synthetic intermediate for steroids with 9,11-unsaturation or 9,11-seco skeletons. We describe here the synthesis of the title compound from 17-ethylenedioxy-3-acetoxyandrosta-3,5-dien-11-one. The introduction of an ethylene unit to 3β-tert-butyldimethylsiloxyandrosta-5,9(11)-dien-17-one by the action of ethyltriphenylphosphonium bromide under basic conditions resulted in an inseparable mixture of two stereoisomeric products (5 : 1). However, in the subsequent step, only the (Z)-isomer was susceptible to the Lewis acid-catalyzed ene reaction with formaldehyde, giving a stereochemically pure product with the desired configuration. Within three steps, the ene-product was derivatized to the title compound, with a total yield of 53% over seven steps. Reductive terminal anion formation by treatment with lithium di-tert-butylbiphenyl (LiDBB) and subsequent nucleophilic attack on a branched aliphatic aldehyde was demonstrated, with an eye toward the introduction of side chains, especially for steroids with oxygen functionality at C-23.
In both natural product syntheses and industrial production of physiologically active steroids, key compounds with proper functional groups are vital. As such, sulfenylated diene 1a may play an important role as a precursor to steroids with ergostane skeleton, e.g., 21) with unsaturation between C-9 and C-11, and marine natural 9,11-secosteroids 3,2) 43) and 54) as shown in Chart 1. Herein, we describe a synthesis of 1a from literary known 6.5)
The synthesis is shown in Chart 2. Starting material 6 is available from a commercial product, androst-4-ene-3,11,17-trione (adrenosterone). Stereoselective reduction of carbonyl groups at C-3 and C-11 with in situ formed calcium borohydride6) and the subsequent hydrolysis of the ethylene acetal furnished 7a.7) After the site-selective protection of the equatorial hydroxy group at C-3 with a tert-butyldimethylsilyl (TBS) group to form 7b, the remaining axial hydroxy group at C-11 was dehydrated by site-selective deprotonation at C-9.8) For this transformation, thionyl chloride in pyridine9) was effective to give 8 in 91% yield.
The next step was the introduction of an ethylene unit at C-17 by Wittig reaction10–12) using ethyltriphenylphosphonium bromide under basic conditions. With the use of potassium hexamethyldisilazide (KHMDS), the product 9 was obtained in almost quantitative yield. However, the ratio between the desired (Z)- and undesired (E)-isomers was only 5 : 1. The addition of hexamethylphosphoric triamide did not affect the ratio. The yield dropped to 54% when using sodium hexamethyldisilazide, with a Z/E ratio of 4 : 1. n-Butyllithium produced no alkenes in the crude product.

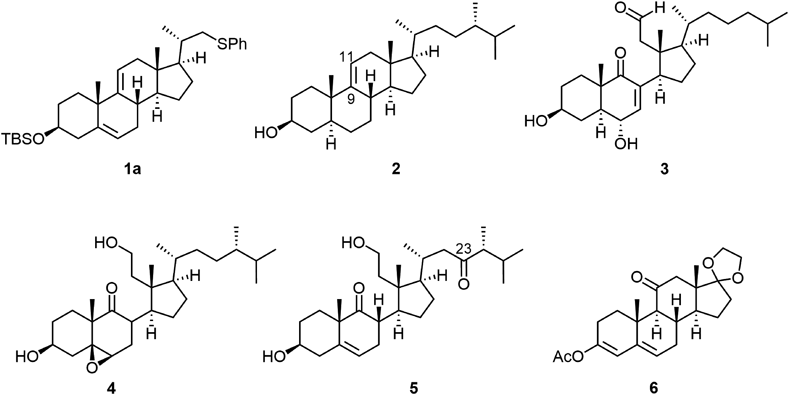
The stereoisomeric mixture of 9 was submitted to the one-carbon homologation step, i.e., the Lewis acid-catalyzed ene reaction with formaldehyde.12) Exposure of 9 to boron trifluoride etherate (BF3·OEt2) and paraformaldehyde in dichloromethane at room temperature furnished homoallylic alcohol 10 in 63% yield. To our delight, 10 was stereochemically pure with respect to the newly created chiral center at C-20, together with the recovery of unreacted (E)-9 also in stereoisomerically pure form. This observation contrasted with previous examples, such as the concomitant formation of a diastereomeric ene-product13) and the formation of an unexpected oxetane byproduct14) from (E)-isomers, in similar steroidal and related partial structures.
The rationale for the kinetic resolution between (Z)- and (E)-9 is depicted in Chart 3. Formaldehyde cannot approach (E)-9 due to steric repulsion (panel c), in contrast to the situation shown in panel a. Moreover, the six-membered transition state (panel d) would be sterically disfavored over that depicted in panel b. Although different ratios in the products between steroidal (Z)- and (E)-isomers have been reported in the diethylaluminum chloride-promoted ene reaction with methyl propionate15) and hydroboration with 9-borabicyclo[3.3.1]nonane (9-BBN),16) our present result is the first discovery of a kinetic resolution in the ene reaction with formaldehyde.
The spectral data of alcohol 1b obtained by site-selective hydrogenation at the C-16 double bond12) in triene 10 with platinum oxide (PtO2) revealed that the stereochemistry at C-17 was identical to those of the natural steroids (Chart 4). Substitution of the terminal hydroxy group in 1b with phenylsulfenyl group was performed by treatment with diphenyl disulfide and tri-n-butylphosphine17) to give 1a in 98% yield. The total yield of 1a from 6 was 53% over 7 steps.
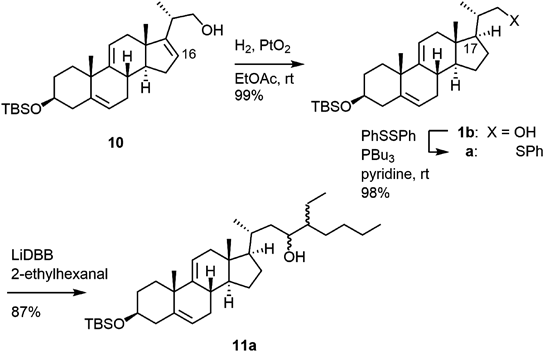
Various methods for the formation of C–C bonds to introduce side chains are expected that would capitalize on the presence of the terminal phenylsulfenyl group. In this study, we demonstrated reductive terminal anion formation by treatment with lithium di-tert-butylbiphenyl (LiDBB)18) and the subsequent nucleophilic attack19) on 2-ethylhexanal. This was the model reaction for the introduction of a branched side chain with an oxygen functionality at C-23 observed in 5 in Chart 1. Thus, treatment of 1a with LiDBB at −78°C generated the terminal primary carbanion at C-22. Subsequent addition of an excess amount of 2-ethylhexanal furnished the desired secondary alcohol 11a in 87% yield. The C–C bond formation was confirmed by the 1H-NMR signal of newly formed secondary alcohol at C-23: δ: 3.69–3.82.
In summary, a steroidal skeleton 1a with a double bond between C-9 and C-11 and a phenylsulfenyl group on C-22 was synthesized from 6 in 53% total yield over seven steps. Although the two-carbon homogation at C-17 did not proceed in a highly stereoselective manner, this problem was overcome by the subsequent Lewis acid-catalyzed ene reaction with formaldehyde. This step proceeded under kinetic resolution, and the product with the desired stereochemistry was obtained exclusively. The preliminary success in the C–C bond formation with a model branched aliphatic aldehyde suggests that 1a would be a promising precursor in the synthesis of steroids containing 9,11-unsaturation or 9,11-seco skeletons.
Silica gel 60 (spherical and neutral; 100–210 µm, 37560-79) from Kanto Chemical Co. (Japan) was used for column chromatography. Preparative TLC was performed with Merck Silica Gel 60 F254 plates (0.5 mm thickness, No. 5744). Melting points were measured on a METTLER TOLEDO mp 70°C and were uncorrected. 1H-NMR spectra were measured at 400 MHz on a VARIAN 400-MR spectrometer or at 500 MHz on a VARIAN 500-MR spectrometer, and 13C-NMR spectra were measured at 125 MHz on a VARIAN 500-MR spectrometer. IR spectra were measured as attenuated total reflectance (ATR) on a Jasco Fourier transform (FT)/IR-4700 FT-IR spectrometer. High resolution (HR) MS were measured on Jeol JMS-T100LP AccuTOF. Optical rotation values were recorded on a Jasco P-1010 polarimeter.
17-Ethylenedioxy-3-acetoxyandrosta-3,5-dien-11-one, 6According to the reported procedure,6) a solution of adrenosterone (2.44 g, 8.1 mmol, Tokyo Chemical Industry A1397) in acetic anhydride (20 mL) and acetyl chloride (1.4 mL) was stirred for 5 h at 100°C. After cooling, the white solid was recovered with filtration and washed with ice-cooled diethyl ether to give pure 3-acetoxyandrosta-3,5-diene-11,17-dione (2.12 g). In addition, the combined filtrate and washings were concentrated in vacuo and the residue was recrystallized from AcOEt to furnish an additional amount of the dienyl acetate (0.151 g) as a white solid. The total yield was 82%. Melting point (mp) 172.6–180.3°C (decomposed) (lit.,5) mp 197–198°C, lit.,6) mp 180–185°C). 1H-NMR (500 MHz, CDCl3) δ: 0.88 (s, 3H), 1.21 (s, 3H), 1.25 (ddd, J=5.6, 12.5, 12.5 Hz, 1H), 1.70 (dddd, J=9.5, 9.5, 12.5, 12.5 Hz, 1H), 1.91–1.97 (m, 2H), 2.00–2.06 (m, 1H), 2.09–2.22 (m, 3H), 2.14 (s, 3H), 2.28 (dd, J=9.0, 19.3 Hz, 1H), 2.34 (d, J=13.5 Hz, 1H), 2.44 (ddd, J=5.2, 5.2, 18.4 Hz, 1H), 2.48–2.60 (m, 3H), 2.67 (ddd, J=1.4, 5.6, 12.7 Hz, 1H), 5.39 (dd, J=2.7, 4.9 Hz, 1H), 5.69 (d, J=2.2 Hz, 1H). Its NMR spectrum was identical with that reported previously.5,6) This was employed for the next step without further purification.
To a solution of the above-mentioned dienyl acetate (2.62 g) in CH2Cl2 (25 mL) was added CH(OEt)3 (12.7 mL, 76.4 mmol), ethylene glycol (8.5 mL, 152 mmol) and p-toluenesulfonic acid (0.301 g, 3.7 mmol). The mixture was stirred for 2 h at room temperature. The reaction mixture was poured into saturated aqueous NaHCO3 and extracted with AcOEt. The extract was washed with brine, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography (60.0 g, hexane/AcOEt=30/1 to 7/1) to give 10 (2.63 g, 89%) as a white solid. An analytical sample was obtained by recrystallization from MeOH/acetone. Mp 133.3–134.5°C (decomposed) (lit.,5) mp 141.5–143.5°C). 1H-NMR (500 MHz, CDCl3) δ: 0.84 (s, 3H), 1.19 (s, 3H), 1.23 (ddd, J=5.6, 12.5, 12.5 Hz, 1H), 1.39 (dddd, J=6.1, 11.9, 11.9, 11.9 Hz, 1H), 1.80–1.87 (m, 1H), 1.91–2.14 (m, 8H), 2.13 (s, 3H), 2.31 (ddd, J=4.7, 18.3, 18.3 Hz, 1H), 2.47–2.54 (m, 1H), 2.63–2.68 (m, 2H), 3.83 (m, 2H), 3.93 (m, 2H), 5.36 (dd, J=2.7, 4.9 Hz, 1H), 5.67 (d, J=1.9 Hz, 1H). Its NMR spectrum was identical with that reported previously.5)
3β,11β-Dihydroxyandrost-5-en-17-one, 7aTo a suspension of NaBH4 (331 mg, 8.8 mmol) and CaCl2 (559 mg, 5.0 mmol) in EtOH (17.0 mL) was added a solution of 10 (582 mg) in CH2Cl2 (4.5 mL) dropwise at −15°C. After stirring for 12 h at −15°C, the mixture was warmed to room temperature and stirred for additional 13 h. To the mixture was added aqueous HCl (2 mol/L, 4.0 mL) and the mixture was stirred for further 2 h. To the mixture, AcOEt was added and the mixture was neutralized with saturated aqueous NaHCO3. After separation of the organic phase, the aqueous phase was extracted with AcOEt and the combined extract was washed with water and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (10.0 g, CHCl3/AcOEt=20/1 to 1/1) to give 11 (478 mg, quant.) as a white solid. An analytical sample was obtained by recrystallization from hexane/AcOEt. Mp 189.3–190.3°C (lit.,7) mp 190–192°C). 1H-NMR (500 MHz, CDCl3) δ: 1.06–1.09 (m, 2H), 1.14 (s, 3H), 1.20–1.30 (m, 2H), 1.29 (s, 3H), 1.46–1.61 (m, 3H), 1.64 (dddd, J=8.8, 8.8, 12.5, 12.5 Hz, 1H), 1.70–1.77 (m, 1H), 1.86–1.90 (m, 1H), 1.96–2.17 (m, 5H), 2.26–2.33 (m, 3H), 2.51 (dd, J=8.6, 18.8 Hz, 1H), 3.50–3.57 (m, 1H), 4.48–4.50 (m, 1H), 5.26–5.29 (m, 1H).
3β-tert-Butyldimethylsilyloxy-11β-hydroxyandrost-5-en-17-one, 7bTo a solution of 11 (742 mg, 2.4 mmol) and imidazole (376 mg, 5.5 mmol) in N,N-dimethylformamide (DMF) (10 mL) was added TBSCl (550 mg, 3.7 mmol). The mixture was stirred for 30 min at room temperature and then quenched with saturated aqueous NH4Cl, and the organic materials were extracted with AcOEt. The combined extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (20.0 g, hexane/AcOEt=30/1 to 8/1) to give 7b (976 mg, 96%) as a white solid. An analytical sample was obtained by recrystallization from hexane/AcOEt. Mp 190.6–191.6°C. 1H-NMR (500 MHz, CDCl3) δ: 0.06 (s, 6H), 0.89 (s, 9H), 1.05–1.08 (m, 2H), 1.13 (s, 3H), 1.17–1.28 (m, 2H), 1.28 (s, 3H), 1.48–1.79 (m, 5H), 1.94–2.37 (m, 8H), 2.50 (dd, J=8.8, 19.3 Hz, 1H), 3.46–3.52 (m, 1H), 4.47–4.50 (m, 1H), 5.24–5.25 (m, 1H). 13C-NMR (125 MHz, CDCl3) δ: −4.6, 15.7, 18.2, 21.7, 22.6, 25.9, 27.9, 31.1, 31.8, 35.3, 36.9, 36.9, 41.0, 41.9, 46.7, 53.7, 54.2, 68.2, 21.9, 119.5, 142.5, 219.6. IR cm−1: 3506, 2955, 2932, 2911, 2896, 2852, 1732, 1249, 1086, 1022, 889, 872, 832, 777, 514. HR-MS (electrospray ionization (ESI+)) Calcd for C25H42Na03Si [M+Na]+ 441.2801. Found 441.2777. [α]D25 −7.3 (c=0.96, CHCl3).
3β-tert-Butyldimethylsilyloxyandrosta-5,9(11)-dien-17-one, 8To a solution of 7b (471 mg, 1.1 mmol) in pyridine (11 mL) was added SOCl2 (130 µL, 1.8 mmol) at 0°C. The mixture was stirred for 20 min at the same temperature and then quenched with saturated aqueous NH4Cl. Organic materials were extracted several times with AcOEt. The combined extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (10.0 g, hexane/AcOEt=100/1 to 50/1) to give 8 (413 mg, 92%) as a white solid. An analytical sample was obtained by recrystallization from MeOH/acetone. Mp 154.9–156.3°C. 1H-NMR (500 MHz, CDCl3) δ: 0.06 (s, 6H), 0.87 (s, 3H), 0.89 (s, 9H), 1.20 (s, 3H), 1.36 (ddd, J=3.9, 13.1, 13.1 Hz, 1H), 1.49–1.86 (m, 5H), 1.95 (ddd, J=3.7, 13.0, 13.0 Hz, 1H), 2.02–2.40 (m, 8H), 2.49 (dd, J=9.0, 18.0 Hz, 1H), 3.45–3.52 (m, 1H), 5.43–5.44 (m, 1H), 5.53 (d, J=5.9 Hz, 1H). 13C-NMR (125 MHz, CDCl3) δ: −4.6, 13.7, 18.3, 22.8, 25.9, 27.7, 31.5, 32.2, 33.5, 34.0, 35.0, 36.5, 38.7, 42.7, 46.3, 49.0, 72.6, 116.0, 120.1, 140.1, 146.7, 221.8. IR cm−1: 2927, 2905, 2897, 2856, 2842, 1741, 1470, 1445, 1436, 1381, 1370, 1362, 1251, 1221, 1079, 1061, 1032, 1007, 992, 973, 964, 937, 884, 870, 852, 835, 820, 806, 772, 732, 668, 638. HR-MS (ESI+) Calcd for C25H40NaO2Si [M+Na]+ 423.2695. Found 423.2668. [α]D24 +239.4 (c=1.02, CHCl3).
3β-tert-Butyldimethylsilyloxypregna-5,9(11),17(20)-triene, 9To a solution of ethyltriphenylphosphonium bromide (432 mg, 1.2 mmol) in tetrahydrofuran (THF) (1 mL) was added a solution of KHMDS in toluene (0.5 mol/L, 2.3 mL, 1.2 mmol) at 0°C. The mixture was stirred for 2 h at 0°C, and then a solution of 8 (103 mg, 0.26 mmol) in THF (2 mL) was added to that. The mixture was stirred under reflux for 18 h. After cooling to room temperature, the reaction was quenched with water and the organic materials were extracted with AcOEt. The extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (7.5 g, hexane/AcOEt=200/1 to 50/1) to give 9 (106 mg, quant.) as a white solid. 1H-NMR (500 MHz, CDCl3) δ: 0.07 (s, 6H), 0.75 [s, 3H, C18 of (E)-9], 0.86 [s, 3H, C18 of (Z)-9], 0.89 (s, 9H), 1.19 (s, 3H), 1.23–1.40 (m, 3H), 1.56 [ddd, J=1.5, 1.5, 6.6 Hz, 3H, 21-C of (E)-9], 1.61–1.85 (m, 4 H), 1.66 [ddd, J=2.2, 2.2, 7.3 Hz, 3H, C21 of (Z)-9], 1.95 (ddd, J=3.4, 3.4, 13.2 Hz, 1H), 2.04–2.49 (m, 8H), 3.44–3.52 (m, 1H), 5.09 [qdd, J=6.6, 3.5, 3.5 Hz, 1H, C20 of (E)-7], 5.21 [qdd, J=7.1, 2.0, 2.0 Hz, 1H, C20 of (Z)-9], 5.42–5.43 (m, 1H), 5.48–5.50 [m, 1H, C11 of (Z)-9], 5.53–5.54 [m, 1H, C11 of (E)-9]. Judged from the above-mentioned 1H-NMR spectrum, the ratio between (Z)- and (E)-9 was revealed to be 5 : 1.
3β-tert-Butyldimethylsilyloxy-23,24-bisnorchola-5,9(11),16-trien-22-ol, 10To a mixture of 9 [71 mg, 0.18 mmol as a mixture of (Z) : (E)=5 : 1] and paraformaldehyde (35 mg, 1.2 mmol) in CH2Cl2 (2.5 mL) was added a solution of BF3·Et2O in CH2Cl2 (0.8 mol/L, 0.03 mmol) at room temperature. The mixture was stirred for 30 min at the same temperature. Then, the reaction was quenched with saturated aqueous NaHCO3 and the organic materials were extracted several times with AcOEt. The combined extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (1.6 g, hexane/AcOEt=50/1 to 2/1) to give 10 as a white solid (48 mg, 63%) also unreacted 9 was recovered (15%). An analytical sample of 10 was obtained by recrystallization from hexane. Mp 132.3–133.5°C. 1H-NMR (500 MHz, CDCl3) δ: 0.06 (s, 3H), 0.78 (s, 3H), 0.89 (s, 9H) 1.07 (d, J=7.1 Hz, 3H), 1.20 (s, 3H), 1.36 (ddd, J=3.7, 13.7, 13.7 Hz, 1H), 1.44 (dd, J=4.9, 7.3 Hz, 1H), 1.61–1.71 (m, 2H), 1.82–1.84 (m, 1H), 1.91–2.05 (m, 3H), 2.19–2.44 (m, 7H), 3.46–3.52 (m, 1H), 3.54–3.64 (m, 2H), 5.42–5.43 (m, 1H), 5.47–5.51 (m, 2H). 13C-NMR (125 MHz, CDCl3) δ: −4.6, 15.6, 18.3, 18.3, 25.9, 27.6, 31.9, 32.0, 32.3, 32.4, 35.0, 35.4, 37.1, 38.6, 42.7, 46.1, 54.2, 66.8, 72.7, 116.4, 120.5, 123.3, 139.9, 147.6, 155.8. IR cm−1: 3274, 3033, 3018, 2956, 2929, 1891, 2857, 1471, 1457, 1446, 1437, 1364, 1252, 1219, 1092, 1081, 1068, 1034, 1017, 1005, 964, 889, 871, 835, 818, 809, 769, 685, 668, 647, 627. HR-MS (ESI+) Calcd for C28H46NaO2Si [M+Na]+ 465.3165. Found 465.3160. [α]D26 +4.5 (c=1.15, CHCl3). The recovered 9 showed exclusive (E)-configuration. Two dimensional (2D)- nuclear Overhauser effect spectroscopy (NOESY) showed the correlation between the protons at C12 and C20. The purity was confirmed by 1H-NMR mentioned in the previous section.
3β-tert-Butyldimethylsilyloxy-23,24-bisnorchola-5,9(11)-dien-22-ol, 1bA mixture of 10 (48 mg, 0.11 mmol) and PtO2 (8 mg, 0.04 mmol) in AcOEt (7 mL) was stirred for 8 h under H2 atmosphere at room temperature. After the removal of insoluble materials, the combined filtrate and washings were concentrated in vacuo. The residue was purified by preparative TLC to give 1b (48 mg, 99%) as a white solid. An analytical sample was obtained by recrystallization from hexane. Mp 134.3–135.3°C. 1H-NMR (500 MHz, CDCl3) δ: 0.06 (s, 6H), 0.66 (s, 3H), 0.89 (s, 9H), 1.05 (d, J=6.6 Hz, 3H), 1.16–1.42 (m, 6H), 1.18 (s, 3H), 1.56–1.69 (m, 3H), 1.74–1.90 (m, 3H), 1.93 (ddd, J=3.4, 13.4, 13.4 Hz, 1H), 2.02–2.29 (m, 6H), 3.37–3.42 (m, 1H), 3.45–3.51 (m, 1H), 3.64–3.68 (m, 1H), 5.40–5.44 (m, 2H). 13C-NMR (125 MHz, CDCl3) δ: −4.6, 11.4, 16.4, 18.3, 25.4, 25.9, 27.6, 28.0, 32.3, 32.4, 34.3, 35.0, 38.3, 38.6, 41.3, 41.8, 42.7, 52.4, 53.4, 68.0, 72.7, 116.9, 120.7, 139.9, 146.1. IR cm−1: 3307, 2955, 2928, 2898, 2881, 2855, 1470, 1458, 1437, 1382, 1254, 1220, 1080, 1038, 1027, 1005, 987, 885, 871, 838, 819, 806, 775, 769, 685, 673. HR-MS (ESI+) Calcd for C28H48NaO2Si [M+Na]+ 467.3321. Found 467.3321. [α]D26 −14.7 (c=0.98, CHCl3).
3β-tert-Butyldimethylsilyloxy-22-phenylthio-23,24-bisnorchola-5,9(11)-diene, 1aTo a solution of 1b (758 mg, 1.7 mmol) and diphenyl disulfide (1.125 g, 5.2 mmol) in pyridene (34 mL) was added tri-n-butylphosphine (2.7 mL, 10.9 mmol) under Ar atmosphere. The mixture was stirred for 19 h at room temperature and then quenched with saturated aqueous NH4Cl. The organic materials were extracted several times with AcOEt. The combined extract was washed with aqueous NaOH (2 mol/L) twice and brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (40.0 g, hexane/AcOEt=100/1 to 70/1) to give 1a (898 mg, 98%) as a white solid. An analytical sample was obtained by recrystallization from MeOH/acetone. Mp 134.3–135.3°C. 1H-NMR (500 MHz, CDCl3) δ: 0.06 (s, 6H), 0.63 (s, 3H), 0.89 (s, 9H), 1.12 (d, J=6.6 Hz, 3H), 1.15–1.40 (m, 5H), 1.17 (s, 3H), 1.60–1.69 (m, 2H), 1.74–1.83 (m, 3H), 1.90–2.29 (m, 8H), 2.66 (dd, J=8.8, 12.2 Hz, 1H), 3.16 (dd, J=3.0, 12.2 Hz, 1H), 3.44–3.51 (m, 1H), 5.40–5.43 (m, 2H), 7.05–7.33 (m, 5H). 13C-NMR (125 MHz, CDCl3) δ: −4.6, 11.3, 18.3, 18.6, 25.3, 25.9, 27.6, 28.4, 32.3, 32.4, 34.3, 35.0, 36.3, 38.3, 41.1, 41.5, 41.7, 42.7, 53.5, 55.5, 72.7, 103.3, 116.8, 120.7, 125.5, 128.8, 137.8, 139.9, 146.1. IR cm−1: 3734, 3648, 2955, 2935, 2925, 2901, 2892, 2879, 2854, 1541, 1458, 1437, 1220, 1093, 1083, 774, 768, 743, 688, 673. Elemental analysis: Calcd for C34H52OSSi: C, 76.07; H, 9.77; S, 5.96. Found: C. 76.22; H, 10.04; S, 6.21. [α]D23 +20.3 (c=1.05, CHCl3).
24-Butyl-3β-tert-butyldimethylsilyloxybishomochola-5,9(11)-dien-23-ol, 11aA solution of LiDBB in THF (0.4 mol/L) was prepared, according to the reported procedure.20) To a solution of 1a (211 mg, 0.39 mmol) in THF (5.5 mL) was add a solution of LiDBB in THF (0.4 mol/L, 1.3 mmol) at −78°C dropwise over 20 min. The low reaction temperature was carefully controlled by elaborating the way of addition. The solution of pre-cooled (0°C) of LiDBB in THF was slowly introduced via the side wall of two-necked flask, which was deeply dipped in the cold bath. The mixture was stirred for 10 min and 2-ethylhexanal (0.6 mL, 3.8 mmol) was added to that at the same temperature dropwise over 10 min. After stirring 24 h, the reaction temperature was raised to room temperature and the mixture was stirred for additional 1 h. The reaction was quenched with saturated aqueous NH4Cl, and the organic materials were extracted several times with AcOEt. The combined extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (20.0 g, hexane/AcOEt=1/0 to 50/1) to give 11a (191 mg, 87%). 1H-NMR (CDCl3, 500 MHz) δ: 0.06 (s, 6H), 0.65 (s, 3H), 0.89–2.29 (m, 45H), 3.44–3.51 (m, 1H), 3.69–3.82 (m, 1H), 5.40–5.43 (m, 2H). The structure was further confirmed by acetylation to acetate 11b. Rf value of 11a (0.37, developed with a mixture of hexane/AcOEt=10/1) was changed to 0.57 for 11b. The signal δ: 3.69–3.82 in 11a was downfield-shifted to 5.04–5.09 in 11b. HR-MS (ESI+) Calcd for C38H66NaO3Si [M+Na]+ 621.4679. Found 621.4678.
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 17K01952) and acknowledged with thanks.
The authors declare no conflict of interest.