Abstract
We have been investigating the potential of oligoarginine-linked polymers as an adjuvant for mucosal vaccination that induces immunoglobulin G (IgG) in systemic circulation and immunoglobulin A (IgA) secreted on the mucosa. Our latest infection experiments demonstrated that mice immunized nasally with a mixture of inactivated influenza viruses and poly(N-vinylacetamide-co-acrylic acid) (PNVA-co-AA) modified with D-octaarginine were perfectly protected from homologous virus infection. On the contrary, virus infection was observed in mice immunized with the antigen alone. This difference was presumably due to insignificant induction of secreted IgA on the nasal mucosa in the latter mice. Since it was unclear whether the current induction level was sufficient for heterologous virus infection, we evaluated the effects of the chemical structures of oligoarginines conjugated to PNVA-co-AA on induction of intranasal IgA. The number and optical activity of the arginine residues and the degree of modification with oligoarginines in the polymer backbone were listed as a factor that would influence IgA induction. Mouse experiments revealed that maximization of the modification resulted in an increase in adjuvant activities of oligoarginine-linked polymers most effectively. Glycine segments inserted between oligoarginines and the polymer backbone were a prerequisite for the maximization. The highest IgA level was observed when antigens were coadministered with diglycine-D-octaarginine-linked PNVA-co-AA.
Recent advances in biotechnologies have resulted in development of diverse biomaterials. Various biomaterials have been tested in biomedical fields with the expectation that their unique properties will lead to epochal invention as drugs and drug delivery tools.1) Among them, polymeric conjugates have been widely investigated as promising candidates.2–4) For example, the conjugation of drugs and drug delivery tools to polyethylene glycol is currently used in clinic as a successful manner that increases their efficacies but decreases their toxicities.5,6)
We have been also investigating the potential of polymeric conjugates.7–9) Among such conjugates, we are currently focusing on researches and development of cell-penetrating peptides conjugated to biocompatible polymers. Cell-penetrating peptides, which are rich in basic amino acids such as arginine, has recently emerged as a promising delivery tool that enhances penetration of molecules which are poorly membrane-permeable or completely membrane-impermeable such as biologics.10,11) We designed cell-penetrating peptides-conjugated polymers with a novel strategy that they would enable poorly membrane-permeable/membrane-impermeable molecules physically mixed with them to effectively penetrate the cell membrane.9) Poly(N-vinylacetamide-co-acrylic acid) (PNVA-co-AA), which is biocompatible and non-biodegradable,12) was used as a platform to which cell-penetrating peptides were anchored. Cell studies demonstrated that membrane-impermeable plasmid DNA and proteins were taken up into cells in their active forms when incubated with PNVA-co-AA bearing D-octaarginine, which is one of typical cell-penetrating peptides. Mouse experiments revealed that the nasal absorption of antidiabetic peptide drugs such as insulin and exendin-4 was significantly enhanced when they were coadministered with D-octaarginine-linked PNVA-co-AA. We have successfully obtained valuable evidence for the potential use of our polymers as a delivery tool that enhances membrane permeation of biologics such as peptides, proteins, and oligonucleotides.9,13,14)
We have separately found that there is another potential of D-octaarginine-linked PNVA-co-AA as an adjuvant for mucosal vaccination.15–17) Mucosal vaccination is one of the most effective and adaptive immunization strategies for protecting hosts against infectious pathogens that invade epithelial cells on the mucosa such as influenza viruses.18–22) Both humoral responses: immunoglobulin G (IgG) and immunoglobulin A (IgA), are observed in the blood and on the mucosa, respectively, in hosts immunized mucosally with antigens. IgA secreted on the mucosa, which is not induced through conventional parenteral vaccines, possesses a couple of unique immunological properties. One is to block the invasion of pathogens on the mucosa with which they are primarily in contact.23,24) Perfect protection of hosts from infection itself is attributed to this property. The other is to cross-react to heterologous pathogens that differ from ones used for immunization.25,26) Since this property results in cross-protection of hosts from heterologous infection, secreted IgA is indispensable for circumvention and reduction of infection, which is caused by incorrect prediction of epidemic strains or their mutation. On the other hand, most antigens are poorly immunogenic when solely applied to the mucosa. Significant mucosal immunity are expected to be induced through coadministration of antigens with immunostimulatory adjuvants; however, such adjuvant has not been launched yet.
Our previous studies demonstrated that influenza virus antigen-specific IgG and IgA were significantly induced in sera and nasal cavities, respectively, when mice were nasally inoculated with antigens such as trivalent influenza virus hemagglutinin (HA) vaccines used clinically and inactivated viral particles in the presence of D-octaarginine-linked PNVA-co-AA. IgA secreted on the nasal mucosa in mice inoculated with a mixture of inactivated H1N1 A/Puerto Rio/8/34 (PRI) influenza viruses and D-octaarginine-linked polymers cross-reacted to recombinant HA (rHA) proteins of not only antigenically-drifted variants within a subtype of the inoculated strains but also virus strains categorized into different subtypes such as H3N2 and H5N1.15,16) A similar result was obtained when antigens were substituted with inactivated H1N1 A/New Caledonia/20/99 IVR116 influenza viruses. Our latest infection experiments using mouse-adapted PRI viruses revealed that mice immunized with inactivated PRI viruses in the presence of D-octaarginine-linked polymers were perfectly protected from homologous virus infection; however, those immunized with the antigen alone were infected with the PRI viruses.17)
Our next plan is to validate protection of heterologous virus infection through immunological performance of intranasally secreted IgA, which is distinct from serum IgG. Mucosal IgA induced in the presence of D-octaarginine-linked PNVA-co-AA possessed a broad spectrum of heterosubtype immunity against influenza virus infection.16,17) However, it is unclear if the current level of IgA induction is sufficient for the heterosubtypic cross-protection. Our previous study revealed that IgA cross-reactivity to heterologous virus strains was more clearly observed as IgA reactivity to homologous virus strains elevated.16) In the present study, we evaluated effects of chemical structures of oligoarginines conjugated to PNVA-co-AA on IgA induction.
Experimental
MaterialsPNVA-co-AA (sodium salts, N-vinylacetamide (NVA) units/acrylic acid (AA) units: 70/30, weight-average molecular weight (Mw): 350 kDa, production code: GE-160-105) was obtained from Showa Denko Co. (Tokyo, Japan). Oligoarginines with the D-configuration, concretely octaarginine, nonaarginine, dodecaarginine, tridecaarginine, diglycine-octaarginine, tetraglycine-octaarginine, and octaglycine-octaarginine, were purchased from Kokusan Chemical Co., Ltd. (Tokyo, Japan). Glycine was attached to the terminal amino groups of octaarginine. Tetraglycine-octaarginine with the L-configuration was also purchased from the same company. The terminal carboxyl groups of these oligoarginines were amidated. Other chemicals were commercial products of analytical or reagent grade and were used without further purification. Trivalent influenza virus HA vaccines (SEIKEN in the 2012–2013 season, virus strains: H1N1 A/California/7/2009, H3N2 A/Victoria/361/2011, and B/Wisconsin/1/2010) were obtained from Denka Seiken Co., Ltd. (Tokyo, Japan). HeLa cells, which is a human uterocervical carcinoma cell line, were furnished by the American Type Culture Collection (Rockville, MD, U.S.A.). Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). Opti-modified Eagle’s medium (Opti-MEM), heat-inactivated fetal bovine serum (FBS), antibiotics (penicillin: 10000 units/mL, streptomycin: 10 mg/mL), trypsin-ethylenediaminetetraacetic acid (EDTA) (0.25% trypsin and 1 mM EDTA), and fluorescein isothiocyanate-labeled bovine serum albumin (FITC-BSA) were purchased from Life technologies Japan Ltd. (Tokyo, Japan). L-Glutamine (200 mM) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
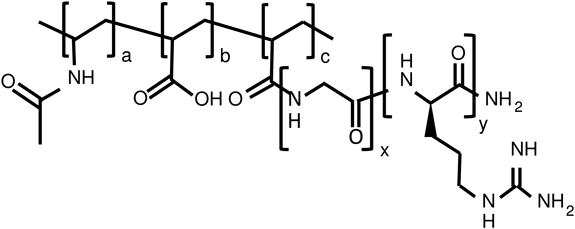
Synthesis of Oligoarginine-Linked PNVA-co-AAA series of oligoarginine-linked PNVA-co-AA were prepared in the same manner as previously described,9) irrespective of the difference of peptide structures. Briefly, N-hydroxysuccinimide ester of PNVA-co-AA was prepared through coupling of the hydroxyl groups of N-hydroxysuccinimide with the carboxyl groups of the polymer activated by N,N′-dicyclohexylcarbodiimide. Each of oligoarginines was next grafted onto the backbone of the polymeric precursor through replacement of the N-oxysuccinimide groups with the terminal amino groups of the oligopeptides. The resulting oligoarginine-linked PNVA-co-AA was purified in water and final concentration was adjusted to 10 mg/mL.
Characterization of Oligoarginine-Linked PNVA-co-AAProton-NMR analysis was utilized to determine the linkage level of oligoarginines in the polymer backbone. The level was expressed as the percentage of monomer units of acrylic acid grafting oligoarginines to the total number of monomer units. The weight percentages of oligoarginines excluding glycine segments grafted onto PNVA-co-AA and Mw of the polymeric conjugate were also calculated.
The z-average size diameter and ζ-potential of oligoarginine-linked PNVA-co-AA mixed with trivalent influenza virus HA vaccines in phosphate buffered saline (PBS) (pH 7.4) were determined using an electrophoretic light-scattering spectrophotometer (Zetasizer Nano ZEN5600, Malvern Instruments, Malvern, U.K.).
Immunization of Mice with AntigensAll animal experiments were approved by the Ethical Review Committee of Setsunan University. Animal experiments were performed in the same manner as previously described.15) In brief, trivalent influenza virus HA vaccines were dissolved in PBS with or without oligoarginine-linked PNVA-co-AA to prepare dosing solution. Mice (BALB/c, female, 7 weeks, ca. 20 g, not fasted, n=4) were nasally inoculated 4 times with the dosing solution at 7-d intervals. Seven days after the final inoculation, mice were sacrificed, and nasal wash fluids were collected. The samples were stored at 4°C and the antibody titration mentioned below was immediately performed.
Antibody TitrationTiters of HA vaccine-specific secreted IgA were measured in the same enzyme-linked immunosorbent assay (ELISA) as previously described.15) Endpoint titers of the antibody were determined from the x-axis intercept of the dilution curve. Each value of endpoint titers is presented as the mean±standard deviation (S.D.). The endpoint titers increase as antibody levels elevate. The titer is calculated to be 4.3 when there is no antibody induction, because sera and nasal wash fluids are minimally diluted 20-fold before antibody titration.
Cellular Uptake of FITC-BSA in the Presence of D-Octaarginine-Linked PNVA-co-AA with or without Glycine SegmentsCell studies were performed in a manner similar to that described in our previous report.14) HeLa cells were cultured in DMEM supplemented with 10% heat-inactivated FBS, 0.2% sodium bicarbonate, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine at 37°C in humidified air containing 5% CO2. The cells were incubated at 37°C for 24 h after they seeded at a density of 1.0×105 cells/0.5 mL in each well of a 24-well plate. A mixture of FITC-BSA (2.5 µg/mL) and polymers (D-octaarginine-linked PNVA-co-AA or diglycine-D-octaarginine-linked PNVA-co-AA, 12.5 µg/mL) was prepared using Opti-MEM as a solvent and then left for 15 min at room temperature. The culture medium was replaced with an equivalent volume of the mixture. After a 1 h incubation at 37°C, the medium was removed and cells were treated with 0.2 mL of trypsin-EDTA within a few minutes. The mean fluorescence intensity (MFI) of the harvested cells was measured using fluorescence-activated cell sorter (BD FACSAria™ Fusion cell sorter, Becton Dickinson Biosciences (Franklin Lakes, NJ, U.S.A.)). Excitation and emission wavelength were set to 488 and 530 nm, respectively. The same test was performed through substitution of the mixture with Opti-MEM or Opti-MEM containing FITC-BSA alone as a control.
StatisticsStatistical significance was assessed with Dunnett’s test, and p-values of 0.05 or less were considered to be statistically significant. The unpaired Student’s t-test was also performed, when necessary.
Results
Synthesis of a Series of Oligoarginine-Linked PNVA-co-AAFigure 1 and Table 1 show the chemical structure and characterization of oligoarginine-linked PNVA-co-AA, respectively. Three sets of oligoarginine-linked polymers were prepared in this study: polymers with a different number of arginine residues (Runs 1–4), ones with a different grafting degree of D-octaarginine (Runs 5–7), and ones possessing glycine segments between octaarginine and the polymer backbone (Runs 7–10). The terminal amino groups of oligoarginines were coupled to the carboxyl groups of PNVA-co-AA by replacing the N-oxysuccinimide groups of PNVA-co-AA N-hydroxysuccinimide ester. The grafting degree of oligoarginines was roughly controlled through adjustment of the molar ratio of oligoarginines as a terminal amino group equivalent to PNVA-co-AA as a carboxyl group equivalent in the coupling reaction (Table 1). D-Octaarginine was anchored chemically to half of acrylic acid units in the polymer backbone when the molar ratio was set to 0.7 (Run 1). A similar result was obtained, irrespective of the number of arginine residues (Runs 2–4). Polymers bearing D-octaarginine with a grafting degree of 10 and 20% were obtained when the molar ratio was set to 0.5 and 1.0, respectively (Runs 5 and 6). However, a further elevation of the molar ratio did not enhance the coupling reaction between D-octaarginine and the residual carboxyl groups of PNVA-co-AA. The grafting degree remained in ca. 20% even when the molar ratio was elevated to 3.0 (data not shown). The low reactivity was improved through introduction of glycine segments into the terminal amino groups of D-octaarginine. The carboxyl groups of PNVA-co-AA was completely occupied with tetraglycine-D-octaarginine when the molar ratio was set to 3.0 (Run 7). The high reactivity was not influenced by optical activities of octaarginine (Run 8) and the length of glycine segments (Runs 9 and 10).
Table 1. Preparation and Characterization of Oligoarginine-Linked PNVA-
co-AA
| Run | Peptidyl branches | Molar ratioa) | Content of the respective unitsb) | Oligoarginine weight %d) | Mw (kDa)e) |
|---|
| Oligoarginine segment | Glycine segment | Oligoarginine/PNVA-co-AA | N-Vinylacetamide (a) | Acrylic acid (b) | Oligoargininec) (c) |
|---|
| Optical activity | No. of residues (y) | No. of residues (x) |
|---|
| 1 | D | 8 | 0 | 0.7 | 70 | 14 | 16 | 75 | 1210 |
| 2 | D | 9 | 0 | 0.7 | 70 | 20 | 10 | 67 | 960 |
| 3 | D | 12 | 0 | 0.7 | 70 | 16 | 14 | 79 | 1480 |
| 4 | D | 13 | 0 | 0.7 | 70 | 17 | 13 | 79 | 1490 |
| 5 | D | 8 | 0 | 0.5 | 70 | 20 | 10 | 64 | 890 |
| 6 | D | 8 | 0 | 1.0 | 70 | 10 | 20 | 80 | 1430 |
| 7 | D | 8 | 4 | 3.0 | 70 | 0 | 30 | 76 | 2260 |
| 8 | L | 8 | 4 | 3.0 | 70 | 0 | 30 | 76 | 2260 |
| 9 | D | 8 | 2 | 3.0 | 70 | 0 | 30 | 81 | 2110 |
| 10 | D | 8 | 8 | 3.0 | 70 | 0 | 30 | 67 | 2550 |
a) The molar ratio of oligoarginine as a terminal amino group equivalent to PNVA-co-AA as a carboxyl group equivalent in their chemical reaction. b) Percentage of the number of the respective monomer units to the total number of monomer units. c) The monomer units of acrylic acid grafting oligoarginines (this value is described as the grafting degree/the linkage level of oligoarginines to PNVA-co-AA). d) Weight percentage of oligoarginines grafted onto PNVA-co-AA (oligoarginines excluding glycine segments/oligoarginine-linked PNVA-co-AA). e) Weight-average molecular weight (Mw) of oligoarginine-linked PNVA-co-AA calculated on the basis of Mw of original PNVA-co-AA and oligoarginines and the grafting degree.
Based on our previous studies,15–17) 4 times nasal inoculations with a mixture of influenza virus HA vaccines and oligoarginine-linked PNVA-co-AA were performed in mice to induce IgA secretion. Trivalent influenza virus HA vaccines were used as an antigen. The vaccine dose was set to 0.05 µg/mouse/time as done previously; however, the polymer dose was reduced to 40 µg/mouse/time (100 µg/mouse/time in the previous studies) to identify the difference in adjuvant activities between polymers.
We first evaluated effects of the number of D-arginine residues on IgA induction. Polymers modified with 8–13 D-arginine residues at a grafting degree of ca. 15% were used as a mucosal adjuvant. As shown in Fig. 2, IgA was secreted on the nasal mucosa most strongly when the HA vaccines were coadministered with PNVA-co-AA bearing D-octaarginine. However, there was no significant difference in the IgA level among four polymers under statistical analysis (Dunnett’s test) with a control of D-octaarginine-linked PNVA-co-AA.
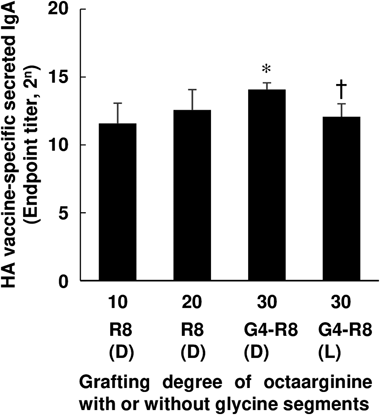
We next evaluated effects of the grafting degree of D-octaarginine on IgA induction. Tetraglycine segments were introduced between octaarginine and PNVA-co-AA as a spacer to prepare polymeric conjugates whose carboxyl groups were completely occupied with oligopeptides. As shown in Fig. 3, the IgA level increased with increasing the grafting degree. The highest level was observed for the group of mice to which PNVA-co-AA bearing tetraglycine-D-octaarginine were coadministered with the HA vaccines. The IgA level was significantly reduced when D-arginine derivatives were substituted with L-arginine ones at the same grafting degree.
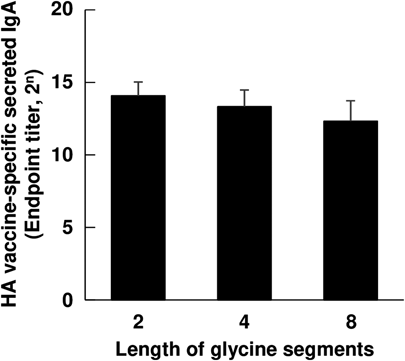
As shown in Fig. 4, effects of the length of glycine segments on IgA induction were finally evaluated. There was a tendency for the IgA level to decrease with an extension of the length of glycine segments. Secretion of IgA was enhanced most strongly when the HA vaccines were coadministered with PNVA-co-AA bearing diglycine-D-octaarginine. However, insignificant difference in the IgA level among three polymers was observed under statistical analysis (Dunnett’s test) with a control of tetraglycine-D-octaarginine-linked PNVA-co-AA which was statistically superior to D-octaarginine-linked PNVA-co-AA from a perspective of adjuvant activities (Fig. 3).
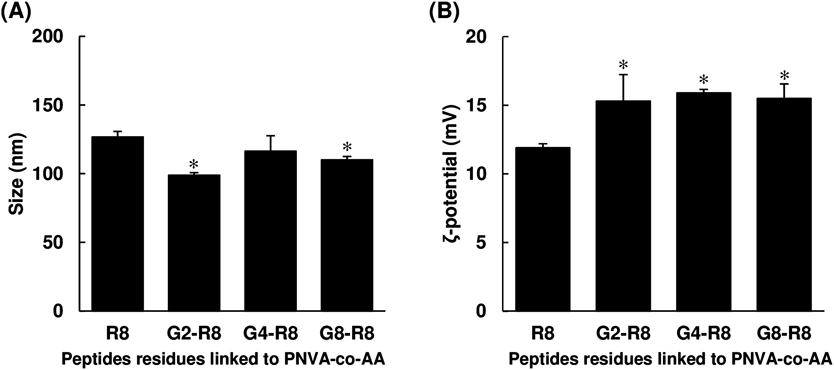
Comparison of Physicochemical Properties of Oligoarginine-Linked PNVA-co-AA with or without Glycine SegmentsZ-Average of diameters and ζ-potentials were measured to estimate effects of introduction of glycine segments into D-octaarginine-linked polymers on their physicochemical properties. Prior to the measurement of both physicochemical properties, the polymers were mixed with the HA vaccines. As shown in Fig. 5, electrophoretic light-scattering spectrophotometry revealed that there were cationic and submicron-sized particles in neutral PBS. Z-Average diameters and ζ-potentials of the particles reduced and elevated, respectively, through introduction of glycine segments; however, it seemed that the length of segment did not affect the magnitude of both parameters.
Cellular Uptake of FITC-BSA in the Presence of D-Octaarginine-Linked PNVA-co-AA with or without Glycine SegmentsIn vitro cellular uptake efficiency of PNVA-co-AA bearing diglycine-D-octaarginine was compared with that of glycine segments-free polymers. As shown in Fig. 6, FITC-BSA was rarely taken up into cells under the polymer-free conditions. The average uptake efficiency of the former polymer was higher than that of the latter one. However, the difference in the efficiency between both polymers was statistically insignificant.
Discussion
A primal objective of the present study is to evaluate effects of chemical structures of oligoarginines conjugated to PNVA-co-AA on induction of secreted IgA in nasal cavities. The study simultaneously enabled us to obtain diglycine-D-octaarginine-linked PNVA-co-AA as an adjuvant that induced humoral immunity on the mucosa most strongly. Our previous studies have indicated that a high level of intranasally-secreted IgA is advantageous to protection of heterologous virus infection.16) The level of IgA induced though nasal coadministration of trivalent influenza virus HA vaccines with PNVA-co-AA bearing diglycine-D-octaarginine was 8 times that with one bearing D-octaarginine as an average of endpoint titers. Previous infection experiments demonstrated that the latter IgA level resulted in perfect homologous virus infection and that IgA exhibited relative reactivity to heterologous virus strains in the 15 to 30% region.17) Therefore, we predict that an 8-fold elevation of the IgA level is sufficient for heterosubtypic cross-protection.
The number and optical activity of arginine residues and the grafting degree of oligoarginines were listed as factors that would influence IgA induction. PNVA-co-AA whose NVA units/AA units and Mw were 70/30 and 350 kDa, respectively, was used because we had confirmed that this polymeric platform contributed to stable acquisition of cross-reactivity when compared with one with a higher molecular weight.16) The number of monomer units of acrylic acid to which oligoarginines were anchored was constantly set to 30% in the present study.
Futaki et al. have reported that a trend of cellular uptake of oligoarginines with stearate-modified terminal amino groups was 8 arginine residues ≥12≥4>16 and that oligoarginines with the D-configuration was taken up into cells more efficiently when compared with those with the L-configuration.27) A similar trend has been observed in our animal experiments using mice to which insulin was nasally administered with PNVA-co-AA modified with either D-octaarginine, L-octaarginine, or D-dodecaarginine.9) Such past studies indicated that adjuvant activities of our polymer would be also influenced by the chemical structures of oligoarginines. However, since a correlation between chemical structures of oligoarginines conjugated to PNVA-co-AA and their adjuvant activities have not been examined yet, we first evaluated effects of the number of arginine residues on IgA induction. D-Nonaarginine and D-tridecaarginine were used as oligoarginines possessing free 8 and 12 arginine residues, respectively, that did not participant in chemical reactions with polymers. Among PNVA-co-AA bearing 8–13 D-arginine residues at a grafting degree of roughly 15% (Runs 1–4, Table 1), the highest level of IgA induction was observed when D-octaarginine-linked PNVA-co-AA was used as a mucosal adjuvant (Fig. 2). There may be a consistent trend in abilities of oligoarginines which depended on the number of arginine residues.
Researches on cell-penetrating peptide-linked polymers have been initiated with our hypothesis that recognition of peptidyl branches conjugated to polymers triggers off macropinocytosis on cell membranes and that poorly membrane-permeable/membrane-impermeable molecules located in the periphery of the peptidyl branches are subsequently taken up into cells through macropinocytosis caused extensively in the multiple peptide access points at the polymeric platform.9) This hypothesis indicates that abilities of cell-penetrating peptide-linked polymers are enhanced as the linkage level elevates. The appropriateness of this indication may be supported by our past mouse experiments. Nasal absorption of insulin coadministered with D-octaarginine-linked PNVA-co-AA with a grafting degree of 17.4% was higher than that with one with a grafting degree of 1.9%.9) However, since the 3-dimentionally-complicated structure of D-octaarginine resulted in a steric hindrance to chemical reactions with PNVA-co-AA, one-third of acrylic acid units of the polymers were not occupied with D-octaarginine (the grafting degree: ca. 20%) even when an excess amount of oligopeptides was applied. Glycine, which is an amino acid with the smallest molecular weight, was introduced into the terminal amino groups of D-octaarginine with the aim of improving the reactivity through a reduction of the steric hindrance.28) This idea worked well so that PNVA-co-AA of which acrylic acid units were completely occupied with D-octaarginine possessing tetraglycine segments was successfully obtained (Run 7, Table 1). As indicated, the level of IgA induction increased with an increase in the grafting degree (Fig. 3). We successfully demonstrated that maximization of D-octaarginine modification in the polymer backbone was an effective approach to increase activities of our polymeric adjuvants. On the other hand, adjuvant activities of octaarginine conjugated to PNVA-co-AA were reduced through substitution from the D-configuration to the L-configuration. This finding was consistent with the results observed previously for absorption enhancement of peptide drugs by octaarginine-linked polymers in mice.9,27)
As shown in Fig. 4, the highest level of IgA induction was observed when HA vaccines were coadministered with PNVA-co-AA bearing D-octaarginine with diglycine segments; however, it seemed that the length of segments less contributed to adjuvant activities, when compared with other factors that influenced IgA induction. These results also indicated that adjuvant activities were not improved through introduction of glycine segments but an elevation of grafting degrees of D-octaarginine. Glycine segments enabled us to stably synthesize PNVA-co-AA highly modified with D-octaarginine. It seems that the length of glycine segments should be primarily determined from a synthesis aspect including cost performance, although adjuvant activities tended to increase with a decrease in the length.
A degree of D-octaarginine modification affected the physicochemical properties of polymeric conjugates in water. Introduction of glycine segments improved reactivity between the amino groups of oligopeptides and the carboxyl groups in the backbone of PNVA-co-AA. Disappearance of anionic carboxyl groups which was accompanied by substitution of cationic arginine residues (Table 1) resulted in an elevation of ζ-potentials of D-octaarginine-linked polymers with the different length of glycine segments (Fig. 5B). Not only was an absolute amount of D-octaarginine on the surface of nanoparticles composed of glycine segments-free polymeric conjugates reduced, but biological activities of arginine residues might be also disturbed through inappropriate conformation caused by electrostatic interactions with free carboxyl groups. Less interactions possibly resulted in a size reduction that indicated compact nanoparticle formations between the HA vaccines and the polymers (Fig. 5A).
There are several issues that should be solved prior to the clinical use of cell-penetrating peptides-linked polymers as mucosal adjuvants, such as protection from heterologous virus infection, safety of the polymers, mechanism on antibody induction, etc. Our previous safety studies in mice indicated that D-octaarginine-linked PNVA-co-AA was non-toxic even when the polymer was given nasally at a dose of 100 µg/mouse which was 2.5 times the dose in the present study.15) We expect that D-octaarginine-linked PNVA-co-AA with glycine spacers possesses a similar safety profile because there is minor difference in chemical structures between both polymers. The mechanism of antibody induction is currently under investigation. There are several requirements for mucosal vaccines such as stability in administration sites, delivery to immune inductive sites, retention in mucous membranes, transport through epithelial barriers, and capture by appropriate antigen presenting cells.29) We have confirmed that D-octaarginine-linked PNVA-co-AA without glycine segments extends the retention of antigens at the nasal mucosa in mice and that the polymer enhanced in vitro uptake of antigens by Calu-3 cells, which are a human epithelial bronchial adenocarcinoma cell line. On the other hand, current uptake experiments using HeLa cells did not clearly support the improvement of adjuvant activities of D-octaarginine-linked polymers through introduction of glycine segments, although the average efficiency of polymers with glycine segments was superior to that of glycine segments-free ones (Fig. 6). It appeared that an elevation of ζ-potentials of D-octaarginine-linked polymers through introduction of glycine segments resulted in more extended retention of antigens and that a couple of effects complementarily enhanced adjuvant activities of diglycine-D-octaarginine-linked polymers. Otherwise, since our other uptake experiments using several types of proteins revealed that FITC-BSA was taken up into Hela cells with relatively high efficiency (data not shown), phenomena observed in the present study might be attributed to saturation of the uptake. The successive study will be discussed in future reports.
Conclusion
The effects of chemical structures of oligoarginine-linked PNVA-co-AA on induction of humoral immunity on the nasal mucosa were evaluated. Among PNVA-co-AA bearing 8–13 D-arginine residues at a grafting degree of roughly 15%, the highest level of IgA induction was observed when D-octaarginine-linked PNVA-co-AA was used as a mucosal adjuvant. Adjuvant activities of the D-octaarginine-linked polymers were enhanced through an elevation of the grafting degree for which glycine segments between the terminal amino groups of D-octaarginine and the carboxyl groups in the polymer backbone were prerequisite. The activities were reduced through substitution from the D-configuration to the L-configuration. Through the study, we successively obtained diglycine-D-octaarginine-linked PNVA-co-AA as an adjuvant that induced mucosal IgA most strongly.
Acknowledgment
This work was financially supported in part by Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) Seeds Validation (Research No.: AS2531303Q) from Japan Science and Technology Agency (JST).
Conflict of Interest
Shinji Sakuma received a research grant from Japan Science and Technology Agency (JST). Kohei Miyata, Kyohei Ochiai, Ken-ichiro Hiwatari, Kazufumi Tsubaki, Koichi Shigeno, and Etsuo Tobita are employees of ADEKA Co. Other authors have no conflict of interest.
References
- 1) Zhang Y., Chan H. F., Leong K. W., Adv. Drug Deliv. Rev., 65, 104–120 (2013).
- 2) Kibria G., Hatakeyama H., Ohga N., Hida K., Harashima H., Biomaterials, 34, 5617–5627 (2013).
- 3) Yamamoto H., Kuno Y., Sugimoto S., Takeuchi H., Kawashima Y., J. Control. Release, 102, 373–381 (2005).
- 4) Miura Y., Takenaka T., Toh K., Wu S., Nishihara H., Kano M. R., Ino Y., Nomoto T., Matsumoto Y., Koyama H., Cabral H., Nishiyama N., Kataoka K., ACS Nano, 7, 8583–8592 (2013).
- 5) Allen T. M., Cullis P. R., Science, 303, 1818–1822 (2004).
- 6) Peer D., Karp J. M., Hong S., Farokhzad O. C., Margalit R., Langer R., Nat. Nanotechnol., 2, 751–760 (2007).
- 7) Sakuma S., Matsumoto T., Yamashita S., Wang Y., Lu Z. R., J. Control. Release, 123, 195–202 (2007).
- 8) Ikumi Y., Kida T., Sakuma S., Yamashita S., Akashi M., J. Control. Release, 125, 42–49 (2008).
- 9) Sakuma S., Suita M., Masaoka Y., Kataoka M., Nakajima N., Shinkai N., Yamauchi H., Hiwatari K., Tachikawa H., Kimura R., Yamashita S., J. Control. Release, 148, 187–196 (2010).
- 10) Khafagy E.-S., Morishita M., Adv. Drug Deliv. Rev., 64, 531–539 (2012).
- 11) Nakase I., Takeuchi T., Tanaka G., Futaki S., Adv. Drug Deliv. Rev., 60, 598–607 (2008).
- 12) Chow K. T., Chan L. W., Heng P. W. S., Pharm. Res., 25, 207–217 (2008).
- 13) Sakuma S., Suita M., Yamamoto T., Masaoka Y., Kataoka M., Yamashita S., Nakajima N., Shinkai N., Yamauchi H., Hiwatari K., Hashizume A., Tachikawa H., Kimura R., Ishimaru Y., Kasai A., Maeda S., Eur. J. Pharm. Biopharm., 81, 64–73 (2012).
- 14) Mohri K., Morimoto N., Maruyama M., Nakamoto N., Hayashi E., Nagata K., Miyata K., Ochiai K., Hiwatari K., Tsubaki K., Tobita E., Ishimaru Y., Maeda S., Sakuma S., Bioconjug. Chem., 26, 1782–1790 (2015).
- 15) Sakuma S., Suita M., Inoue S., Marui Y., Nishida K., Masaoka Y., Kataoka M., Yamashita S., Nakajima N., Shinkai N., Yamauchi H., Hiwatari K., Tachikawa H., Kimura R., Uto T., Baba M., Mol. Pharm., 9, 2933–2941 (2012).
- 16) Sakuma S., Morimoto N., Nishida K., Murakami T., Egawa T., Endo R., Kataoka M., Yamashita S., Miyata K., Mohri K., Ochiai K., Hiwatari K., Koike S., Tobita E., Uto T., Baba M., Eur. J. Pharm. Biopharm., 92, 56–64 (2015).
- 17) Miyata K., Mohri K., Egawa T., Endo R., Morimoto N., Ochiai K., Hiwatari K., Tsubaki K., Tobita E., Uto T., Baba M., Sakuma S., Bioconjug. Chem., 27, 1865–1871 (2016).
- 18) Yuki Y., Kiyono H., Rev. Med. Virol., 13, 293–310 (2003).
- 19) Okamoto S., Matsuura M., Akagi T., Akashi M., Tanimoto T., Ishikawa T., Takahashi M., Yamanishi K., Mori Y., Vaccine, 27, 5896–5905 (2009).
- 20) Mizuno D., Ide-Kurihara M., Ichinomiya T., Kubo I., Kido H., J. Immunol., 176, 1122–1130 (2006).
- 21) Greenbaum E., Furst A., Kiderman A., Stewart B., Levy R., Schlesinger M., Morag A., Zakay-Rones Z., J. Med. Virol., 65, 178–184 (2001).
- 22) Tamura S. I., Asanuma H., Ito Y., Hirabayashi Y., Suzuki Y., Nagamine T., Aizawa C., Kurata T., Oya A., Eur. J. Immunol., 22, 477–481 (1992).
- 23) Mestecky J., McGhee J. R., Adv. Immunol., 40, 153–245 (1987).
- 24) Underdown B. J., Schiff J. M., Annu. Rev. Immunol., 4, 389–417 (1986).
- 25) Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J., Eur. J. Immunol., 14, 350–356 (1984).
- 26) Takada A., Matsushita S., Ninomiya A., Kawaoka Y., Kida H., Vaccine, 21, 3212–3218 (2003).
- 27) Futaki S., Ohashi W., Suzuki T., Niwa M., Tanaka S., Ueda K., Harashima H., Sugiura Y., Bioconjug. Chem., 12, 1005–1011 (2001).
- 28) Everette J. D., Bryant Q. M., Green A. M., Abbey Y. A., Wangila G. W., Walker R. B., J. Agric. Food Chem., 58, 8139–8144 (2010).
- 29) Kim S. H., Jang Y. S., Clin. Exp. Vaccine Res., 6, 15–21 (2017).