2018 Volume 66 Issue 5 Pages 535-540
2018 Volume 66 Issue 5 Pages 535-540
Twelve pseudo-ginsenosides were synthesized under a mild condition, via a simple three-step called acetylation, elimination-addition and saponification. The inhibitory effects of these twelve pseudo-ginsenosides were screened on the hemolysis of rabbit erythrocytes caused by 2,2′-azobis (2-amidinopropane hydrochloride) (AAPH). It was found that the IC50 values followed the sequence of (20Z) pseudo-protopanaxatriol (pseudo-PPT)<(20Z) pseudo-protopanaxadiol (pseudo-PPD)<(20Z) pseudo-Rh2<(20E) pseudo-PPT<(20E) pseudo-PPD<(20E) pseudo-Rh2<(20Z) pseudo-Rg2<(20E) pseudo-Rg2<Rb1<(20Z) pseudo-Rh1<Rg2<(20E) pseudo-Rh1. These compounds can be divided into three groups: accelerate the hemolysis group (7, 8), weak group (2, 11, 12) and strong group (others). Moreover, we also find that most of the Z configuration has better antioxidative activity than E configuration and the number and type of sugar moieties to the ring of triterpene dammarane influence the antioxidative activity.
Many studies have focused on the free-radical-initiated peroxidation of membrane lipid, which is associated with a variety of pathological events.1,2) Both natural and synthetic antioxidants have been used to trap peroxyl and/or initiating radicals in order to protect the membrane lipids against free radical chain reactions. Therefore, antioxidant therapy has become an attractive strategy.3,4) Red blood cell membrane is consisted of abundant polyunsaturated fatty acids that are very susceptible to free radical mediated peroxidation. Thermal decomposition at physiological temperature of a water-soluble azo compound, 2,2′-azobis(2-amidinopropane hydrochloride) (AAPH), generates initiating free radicals for attacking the erythrocyte membrane, leading to lipid peroxidation and hemolysis eventually. Since the rate of free radical generation from AAPH can be easily controlled and measured, the hemolysis induced by AAPH provides a good approach for studying the free radical induced membrane damage.5–7)
Numerous natural products reportedly possess particular health-promoting and therapeutic functions in vitro and in vivo.8,9) The knowledge of the antioxidant properties of natural and synthetic molecules is an important requirement for investigations in the fields of biochemistry, biology, food chemistry, and other disciplines. It is of particular significance in the nutritional, pharmacological and health fields, for the prevention of diseases due to unbalanced diet and cell aging.10–14) The antioxidant capacity against reactive oxidants has been assessed extensively by many groups in different systems by different methods.15–17) And many compounds have been found to be effective antioxidants in biological systems.18,19) Ginseng species such as Panax ginseng C. A. MEYER (Chinese, Korean, Asian and Oriental ginseng), Panax quinquefolius L. (popularly known as American ginseng) and Panax notoginseng BURK (Sanqi ginseng) (Araliacease family) have been used for centuries with medicinal purposes such as increasing energy, reducing stress and extending longevity.20) Panax ginseng C. A. MEYER belongs to the Araliaceae family and is widely used as a traditional medicine for thousands of years. Nowadays, it still plays a key role in natural medicine used worldwide.21,22) A number of saponins called ginsenosides have been isolated, and are regarded as the major pharmacologically active components of ginseng that target many tissues, producing an array of pharmacological responeses. Pharmacological studies on ginsenosides have reported neuroprotective, anti-human immunodeficiency virus (HIV), antioxidant and anticancer effects.23–25) The major active components ginsenosides that was used in traditional Chinese medicine has been proved to be antioxidants against AAPH-induced hemolysis of erythrocytes.26) In the previous study, many dammarane-type derivatives have been prepared and investigated for their activities.27) The pharmacological activity of Ginseng species has been attributed to its major compounds ginsenosides (i.e., Rd, Re, Rg1, Rb1, Rb2 and Rc); their clinical effects include hypoglucemic activity, cardioprotective and hepatoprotective actions, immunomodulatory activity and enhancing cognitive performance, among others. Most of these effects are thought to be related to their antioxidant activities.28–30) In current, we have described the synthesis of twelve pseudo-ginsenosides (as outlined in Fig. 1) and their comparative study of antioxidative activity in free radical induced hemolysis of rabbit erythrocytes.
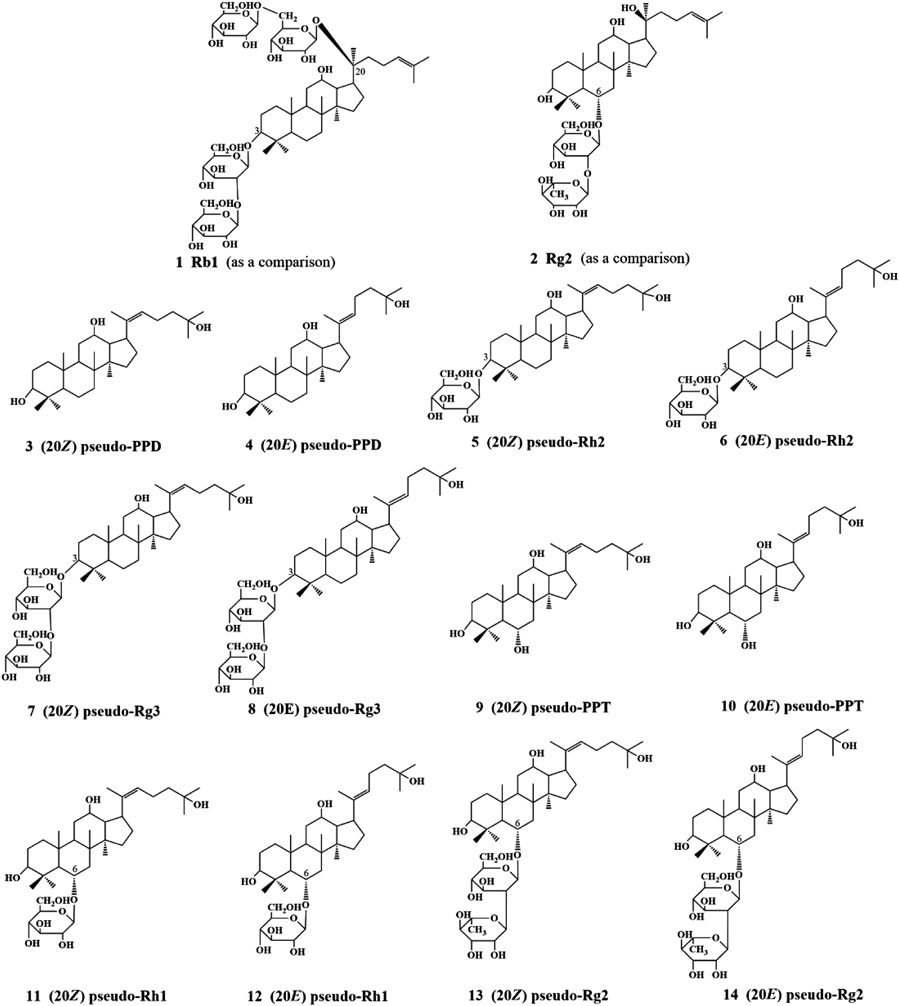
Figure 2 shows the rabbit erythrocyte hemolysis induced by AAPH, in which the final concentration of AAPH was 40 mM with the addition of the pseudo-ginsenosides whose final concentration ranges from 0 µM, as the control, to 25 µM with 5 µM interval, respectively. It is found that the hemolysis is still lagged in the absence of any pseudo-ginsenoside, indicating that the endogenous antioxidants (AH) in the erythrocyte, i.e., α-tocopherol (TOH), catalase, superoxide dismutase and glutathione, can trap the initiating and/or propagating radicals to protect the erythrocytes against free-radical-induced hemolysis.2,31) After depletion of all the endogenous antioxidants, hemolysis turns rapidly. The turning point from the inhibition period of hemolysis to the restoration period of hemolysis curve refers to the lag time of hemolysis, tlag, which can be used to estimate the activity of an antioxidant.32) In this work, the relationship between the lag time of hemolysis, where various pseudo-ginsenosides are added to the hemolysis system, respectively, and the final concentration of the corresponding ginsenoside is depicted in Fig. 3.
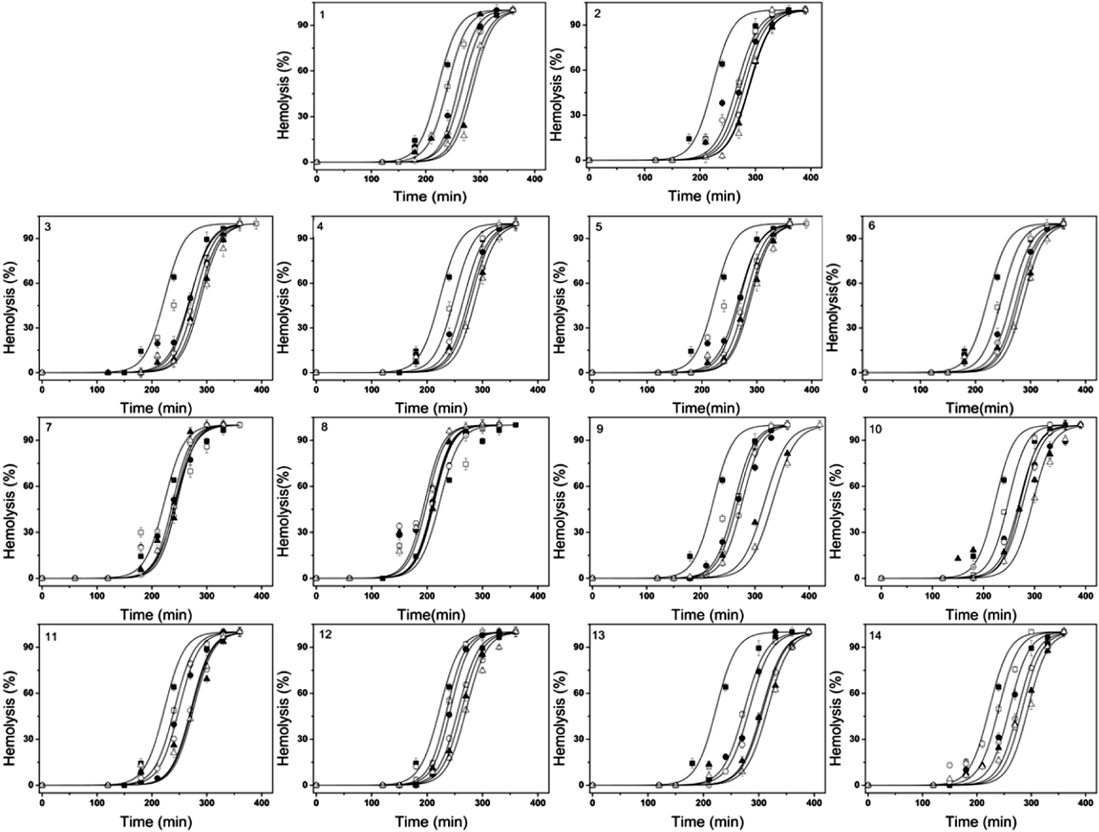
The final concentrations of various individual pseudo-ginsenosides, are 0 µM (■), 5 µM (□), 10 µM (●), 15 µM (○), 20 µM (▲) and 25 µM (△).

As can be seen from Fig. 3(A), 1, 3, 4, 5, and 6 as members in the group of protopanaxadiol (PPD)-type are able to increase the lag time of hemolysis when the final concentration exceeds 15 µM, indicating that these three ginsenosides play antioxidative roles in AAPH-induced hemolysis. On the other hand, 7 and 8, decrease the lag time of hemolysis with the final concentration being increased. These results indicate that 7 and 8 play prooxidative roles in AAPH-induced hemolysis. As depicted in Fig. 3(B), all these ginsenosides except 11 can be regarded as antioxidants since the lag time of hemolysis is increased while the final concentration is larger than 15 µM. 11, however, functions as a prooxidant since the lag time of hemolysis is decreased at relative high final concentration.
In order to compare the antioxidative activity of various pseudo-ginsenosides quantitatively, it is essential to establish the relationship between the concentration of pseudo-ginsenosides and inhibition percent, in which 4 h is appropriated to be the incubation period in this case. Figure 4 shows the relationship between the inhibitory percent, and the corresponding concentration of various pseudo-ginsenosides. The cross points between all the experimental lines and the dash line indicate the IC50 of various ingredients of pseudo-ginsenosides, respectively. All of the IC50 values are collected in Fig. 5, and a low value means that the ginsenoside possesses high antioxidant effect.


From the results, we can divide all these pseudo-ginsenosides into three groups, accelerate the hemolysis group (7, 8), weak group (2, 11, 12) and strong group (others). It can be concluded that all these pseudo-ginsenosides have the antioxidative potential against AAPH-induced hemolysis except 7 and 8 which accelerate the hemolysis.
We have found that 1 and 2 respectively are the most important antioxidative ingredients in the group of PPD-type and protopanaxatriol (PPT)-type in a previous work.32) In the view of IC50 of these pseudo-ginsenosides, it is found that the IC50 of 2 (14.12), 11 (14.07) and 12 (14.49) have a larger value than that of 1 (9.67), revealing that the antioxidative activity of 2, 11 and 12 is lower than that of 1. So they are classified into weak group. On the other hand, comparing 3, 4, 5, 6, 9, 10, 13 and 14 with 1, it is showing that the IC50 of 3 (7.51), 4 (7.91), 5 (7.57), 6 (8.00), 9 (6.97), 10 (7.73), 13 (9.41) and 14 (9.64) have a smaller value than 1 (9.67), revealing that the antioxidative activities of 3, 4, 5, 6, 9, 10, 13 and 14 are better than that of 1. So they are assigned to the strong group. At the same time, we compared the antioxidation of Z configuration and E configuration of each pseudo-ginsenoside. It is found that the antioxidation of 3, 5, 7, 9, 11 and 13 is greater than that of corresponding E configuration. These above facts preliminarily reveal that the antioxidative activity of most pseudo-ginsenosides is more effective than ginsenosides and the antioxidation of Z configuration is better than the E configuration.
In order to respectively compare the antioxidative activity of (20Z) pseudo-ginsenosides and (20E) pseudo-ginsenosides, it is necessary to analyze the IC50 of these two sets as shown in Fig. 6. Analyzing the IC50 of 9 (6.97), 3 (7.51), 10 (7.73) and 4 (7.91) to which no sugar moieties attaching, one can find that their antioxidation are more efficient than others, respectively, which are listed in Figs. 6(A) and 6(B). Comparing 13 (9.41) with 9 (6.97), it is found that one glucose moiety is connected with a rhamnose moiety by 2,1-ether bond at 6-position in 13 and no glucose moieties is connected in 9. Similarly, comparing 11 (14.07) with 9 (6.97), it is found that one glucose moiety is connected at 6-position. The same phenomenon can be seen in PPD-type. Comparing 5 (7.57) with 3 (7.51), it is found that one glucose moiety is connected at 3-position and no glucose moieties is connected in 3. To 5 (7.57) and 7, the structural difference between 5 (one glucose connected at 3-position) and 7 (two glucoses at 3-position) results in the IC50 of 5 (7.57) much lower than that of 7 (function as a prooxidant). These facts preliminarily indicate that the less sugar attaching to triterpene dammarane the more effective antioxidative activity. However, for 13 (one glucose connected with a rhamnose moiety by 2,1-ether bond at 6-position and 11 (one glucose connected at same position), indicating that rhamnose moiety benefits for the antioxidative activity of 13. Moreover, the same rule applies to (20E) pseudo-ginsenosides in (20Z) pseudo-ginsenosides as shown in Fig. 6(B). These results reveal that the number and type of sugar moieties to the ring of triterpene dammarane influence the antioxidative activity also it is found that the lower the number of sugar the stronger the antioxidant.

The antioxidative or prooxidative activities in AAPH-induced hemolysis is dependent upon the Z, E configuration and the difference of connective position and type of sugar moieties to the ring of triterpene dammarane. The reason why pseudo-ginsenosides with similar structure play different role in AAPH-induced hemolysis is due to a synergism antioxidant effect among the different part in a ginsenoside molecule, which remains unclear in detail now. Moreover, interaction between the endogenous antioxidants in the erythrocytes and pseudo-ginsenosides can also prolong or decrease the lag time of hemolysis, which should be discussed in detail in the further studies.
AAPH was purchased from J&K Chemicals and used as reserved. The whole extraction of ginseng was purchased from Suzhou Star Ocean Ginseng Biopharmaceutical Co., Ltd., China, and identified by Prof. Yanping Chen. The fourteen ginsenosides were prepared and isolated from the crude product of ginseng in our laboratory. The rabbit erythrocytes were collected from a healthy adult rabbit by venipunture into heparin. Before experiment, erythrocytes were washed three times with phosphate-buffered saline (PBS: 138 mM NaCI, 5 mM KCI, 6.1 mM Na2HPO4, 1.4 mM NaH2PO4, 1 mM MgSO4 and 5 mM glucose, pH 7.4).33) During the last washing the erythrocytes were centrifuged at exactly 3000 rpm for 10 min to obtain a constantly packed cell volume, and then erythrocytes were added to PBS to obtain a 20% (v/v) suspension.
Synthesis of Twelve Pseudo-GinsenosidesGinsenoside Rh2 (3 g) and acetic anhydride (23 mL) were dissolyed and stirred in pyridine (20 mL) for 24 h at room temperature. The residue was taken up in ethyl acetate (25 mL) and water (180 mL). The organic phase was washed with brine, water, dried with anhydrous Na2SO4 and concentrated under reduced pressure. The dried (3.8 g) was rechromatographed on normal silica gel CC eluted with Et2O/cyclohexane (4 : 1→3 : 1→2 : 1) to give the acetylated product (3.64 g). To a solution of sulfuric acid/acetic acid solution (2%, 0.72 mL, 36 mL) in methanol (36 mL), the acetylated product (3.5 g) was added in one portion which was stirred for 3 h at −30°C. After being diluted with water (200 mL), the reaction mixture was extracted with ethyl acetate for three times. The organic layer was washed with a saturated aqueous solution of sodium carbonate and water, and then evaporated in vacuo to yield a white solid (3.2 g). The ethyl acetate extraction was submitted to silica gel CC eluted with Et2O/cyclohexane (5 : 1→4 : 1) to yield a fraction. The fraction was futher purified by preparative RP C18 HPLC [MeOH/H2O (9 : 1), 5 mL/min, monitored at 203 nm] to obtain compound 1 (2.47 g) and compound 2 (0.92 g), respectively. Sodium hydroxide (2.4 g) was added to the solution compound 1 (2.3 g) in methanol (15 mL) and dioxane (15 mL). The resulting mixture was stirred for 4 h under reflux at 90°C. Then the solution was diluted with water (100 mL) and extracted with an equal volume of n-butanol for three times. The organic layer was washed with water until neutral and dried over anhydrous sodium sulfate. After evaporation of the solvent under reduced press and recrystallization from methanol, the desired solid compound 6 (1.21 g) was obtained. At the same time, 5 (0.44 g) was prepared using the same procedure as described for the preparation of 6 with the above reactants and solvents.31)
The synthesis of other ten compounds is similar to 5 and 6, for these information, see Supplementary materials.
The experimental process of erythrocytes hemolysis is similar to that described in the literature.6,34) Various individual pseudo-ginsenosides were dissolved in propylene glycol directly. Then, the above solution was added to a 20% suspension of rabbit erythrocytes in PBS and incubation at 37°C (±0.1°C) for 5 min, which an AAPH/PBS was injected to initiate hemolysis. In every experiment, the concentration of AAPH was kept at 40 mM. The percentage of hemolysis at different incubation intervals was measured and compared with the absorbance of the supernatant of the erythrocytes at 540 nm of visible spectrum (complete hemolysis acted as the control value). Every experiment was repeated three times and the results were reproducible within 10% experimental error. In addition, the concentration of pseudo-ginsenosides for 50% inhibition of AAPH-induced hemolysis (IC50) relative to a control was determined graphically35) with the final concentrations ranging from 0 µM as a control to 25 µM of various pseudo-ginsenosides in the reaction mixture while 4 h was appropriated to be the incubation period in this case.
Prof. Zaiqun Liu (Jilin University) is thanked for providing important suggestion on experimental method. College of Basic Medical Sciences (Jilin University) is gratefully acknowledged for providing the healthy rabbit erythrocytes.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials (the synthesis of the other eight compounds).