2018 Volume 66 Issue 7 Pages 771-772
2018 Volume 66 Issue 7 Pages 771-772
Vol./Page: Chem. Pharm. Bull. 62, 937–941 (2014)
Title: A Lanostane Triterpenoid and Three Cholestane Sterols from Tilia kiusiana
Authors: Marie Shimada, Masaaki Ozawa, Kojiro Iwamoto, Yoshiyasu Fukuyama, Akio Kishida, and Ayumi Ohsaki
Authors Comments:
Structures of compounds 3 and 4 should be revised, because the functional groups were found to be exchanged between C-3 and C-6. Thus, compound 3 was known compound, 3β-hydroxy-cholest-7-en-6-one, and trivial name kiusianin C was withdrawn. We thank Assoc. Prof. Dr. Wolfgang Robien (University of Vienna, Austria) for the private letter regarding the revision of the structures 3 and 4 using the calculated 13C-NMR data (http://nmrpredict.orc.univie.ac.at/c13robot/robot.php).
Ayumi Ohsaki
Department of Chemistry, College of Humanities and Sciences, Nihon University, Japan
April 8, 2018
| Vol. | Page | Line | Error | Correction |
|---|---|---|---|---|
| 62 | 937 | Summary | Kiusianins A–D (1–4) were isolated from the leaves of a Japanese endemic plant, Tilia kiusiana, together with 14 known compounds. The structures of a new lanostane-type triterpenoid 1 and three new cholestane-type sterols 2–4 were elucidated by spectroscopic methods, | Kiusianins A, B and D (1, 2, 4) were isolated from the leaves of a Japanese endemic plant, Tilia kiusiana, together with 15 known compounds. The structures of a new lanostane-type triterpenoid 1 and two new cholestane-type sterols 2 and 4 were elucidated by spectroscopic methods, |
| left 12 | isolation of four new compounds 1–4 named kiusianins A–D | isolation of three new compounds 1, 2, and 4 named kiusianins A, B, and D | ||
| left 15 | kiusianins A–D (1–4) | kiusianins A, B, and D (1, 2, 4) | ||
| left 24 | three new sterols 2–4, together with 3β-hydroxy- | two new sterols 2 and 4, together with 3β-hydroxy-5α-cholest-7-en-6-one (3)16), 3β-hydroxy- | ||
| 938 | Table 1 | 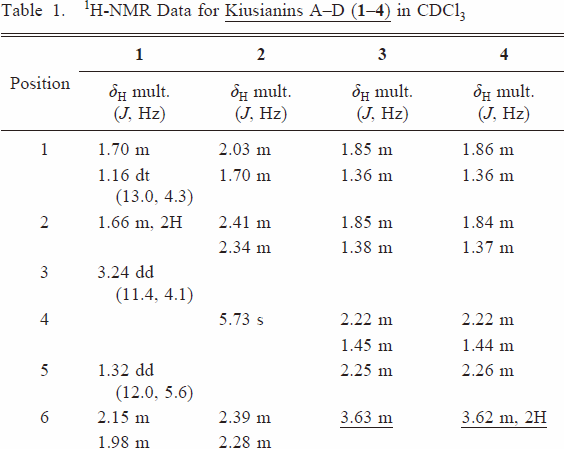 |  | |
| Table 2 |  | 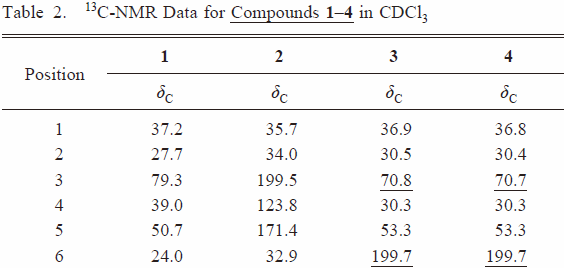 | ||
| right↑2 | cholest-7-en-3-one | cholest-7-en-6-one | ||
| right↑1 | The position of the hydroxyl group was unambiguously assigned to C-6 on the basis of HMBC correlation of H-5 (δH 2.25) to C-6 (δc 70.8) and 1H–1H COSY cross-peak of H-6/H-7. | The position of the hydroxyl group was unambiguously assigned to C-3 on the basis of HMBC correlation of H-5 (δH 2.25) to C-3 (δc 70.8). | ||
| 939 | left 4 | H-5/H-9 and H-6 | H-5/H-9 and H-3 | |
| left 8 | 6β-hydroxy-cholest-7-en-3-one and named kiusianin C. | 3β-hydroxy-cholest-7-en-6-one (3). | ||
| Fig. 1 |  |  | ||
| Fig. 2 | 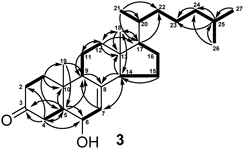 | 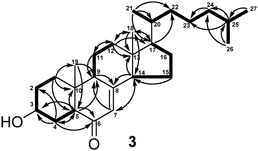 | ||
| Key 1H–1H COSY (Bold) and HMBC (Arrow) Correlations for Kiusianins A–C (1–3) | Key 1H–1H COSY (Bold) and HMBC (Arrow) Correlations for Compounds (1–3) | |||
| Fig. 3 | Key NOESY Correlations for Kiusianins A–C (1–3) | Key NOESY Correlations for Kiusianins A and B (1, 2) | ||
| Compound 3 was deleted. | ||||
| 940 | left 15 | 6β-hydroxy-cholest-7,20-diene-3-one | 3β-hydroxy-cholest-7,20-diene-6-one | |
| left 21 | New compounds 2–4 | Compounds 2–4 | ||
| 941 | left 20 | kiusianin C (3, 0.50 mg) | compound 3 (0.50 mg) | |
| left↑8 | Kiusianin C | 3β-hydroxy-cholest-7-en-6-one | ||
| References | 16) Kulig M. J., Smith L. L., J. Org. Chem., 39, 3398–3402 (1974). |