Abstract
Aminopeptidase N, also known as CD13, is a transmembrance protease with many functions. CD13 is involved in inflammatory diseases and cancers. A convenient and reliable laboratory test method for detecting the suppressing effects of enzyme activity would be useful for study of CD13 inhibitors. Porcine CD13 (pCD13) was traditionally considered an enzyme source but has significant practical disadvantages. pCD13 is not a human source, and the accuracy and reliability of experimental results are greatly reduced. In this study, a modified detection method with K562-CD13 monoclonal cells, a human-derived cell line, was established to detect the suppressing effects of enzyme activity by the CD13 inhibitor. In this method, K562-CD13 monoclonal cells were used as enzyme source and L-leucine p-nitroaniline hydrochloride as substrate. Using CD13 enzyme activity analyses, we found that the ability of the catalytic substrate was weaker in K562 cells than in the other cell lines, and K562-CD13 cells expressed significantly higher levels of CD13 enzyme activity than parental K562 cells. The enzyme activity of CD13 was detected with the new method after ubenimex treatment. The enzyme activity was significantly inhibited by ubenimex in a dose-dependent manner. In summary, this study proposes a sensitive, stable, and objective laboratory method for detecting the inhibitory effect of the CD13 inhibitor.
Introduction
Aminopeptidase N (APN), also known as CD13, is a metallo-dependent integral membrane protease of the M1 family.1,2) CD13 is a Zn2+-dependent and a membrane-bound peptidase that cleaves the N-terminal peptide from small peptides.3–5) In the past few years, many studies have shown that CD13 has many functions and is involved in signal transduction and immunological responses.6) CD13 participates in tumor metastasis, angiogenesis, and virus infection.7–9) Haraguchi et al. demonstrated that CD13 is a marker of human liver cancer. In mouse xenograft models, the tumor size was significantly reduced by combining the CD13 inhibitor with reactive oxygen species (ROS)-inducing chemo/radiation therapy.10) CD13 is a potential candidate for development of targeted anticancer drugs, and synthesis of CD13 inhibitors will be of great significance. A convenient and reliable method for detecting the suppressing effect of enzyme activity by the CD13 inhibitor would be useful for the study of CD13 inhibitors. The traditional detection method uses porcine CD13 (pCD13) as the enzyme source, which is expensive and lacks accuracy and reliability. A previous work reported a new compound 16l that markedly inhibited the enzyme activity by using pCD13. However, 16l could not inhibit the enzyme activity when human and mouse tumor cells or isolated cell membranes are used as enzyme source.11) ES-2 cells are still used as the source of enzymes. However, this detection method cannot exclude the effects of other enzymes on the cell surface.12) To date, no appropriate method has been established for detecting the suppressing effect of enzyme activity by the Homo sapiens CD13 inhibitor.
This study aims to propose a new and improved method that is accurate, sensitive, stable, and cheap by replacing the enzyme source.
Investigation and Results
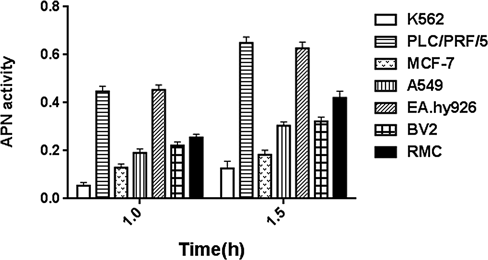
K562 Was Chosen to Overexpress CD13Considering that some enzymes can catalyze substrates and lead to nonspecific reactions, we need to screen a cell with weak ability to catalyze the substrate. L-Leucine p-nitroaniline hydrochloride was used as substrate for detecting CD13 enzyme activity. The CD13 enzyme activity in K562 cells was lower than that in the other cell lines, such as PLC/PRF/5, MCF-7, A549, EA.hy926, BV2, and RMC (Fig. 1). Therefore, K562 cells were selected for subsequent analyses.
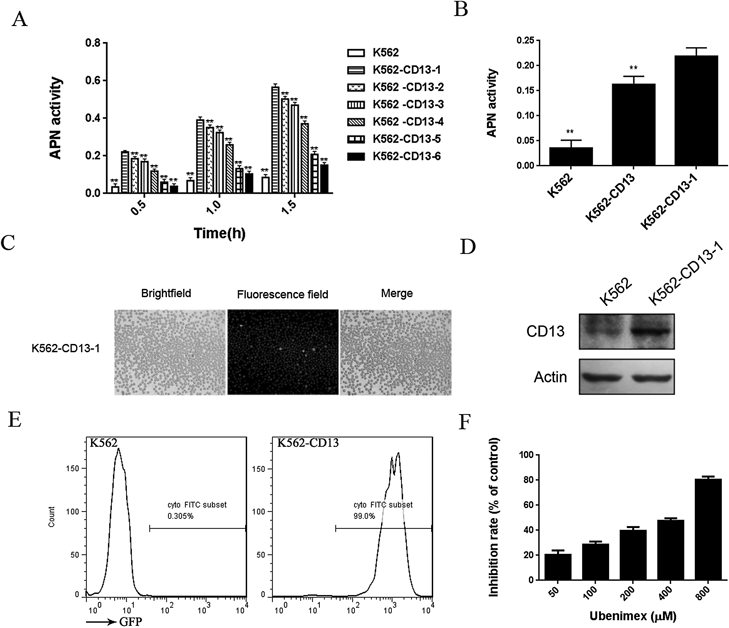
K562/CD13 Cells Could Be Used to Detect the Suppressing Effect of Enzyme Activity by the Aminopeptidase N InhibitorMyeloid lymphoblastoma cells, K562, were infected with the recombinant lentivirus vector to overexpress CD13. The cells were selected to separate monoclonal cells. Six monoclonal cells were obtained, and enzyme activity was measured. Parental K562 cells were used as control. The CD13 enzyme activity in K562-CD13-1 cells was higher than that in the other monoclonal cells and parental cells. K562-CD13-1 cells were selected for the next experiment (Fig. 2A). The CD13 enzyme activity in monoclonal K562-CD13 cells was higher than that in parental and polyclonal cells (Fig. 2B). Green fluorescence, which represents the stable expression of CD13, was observed (Fig. 2C). The expression of CD13 on these cells was analyzed by Western blot and FACS. The expression of CD13 in monoclonal K562-CD13 cells was significantly higher than that in parental K562 cells (Figs. 2D, E). As shown in Fig. 2F, after 30 min of ubenimex treatment (a CD13 inhibitor commonly used in clinic), the CD13 enzyme activity was inhibited by ubenimex in a dose-dependent manner.
Discussion
CD13 is a widely expressed plasma membrane extrapeptidase involved in pleiotropic functions. CD13 plays an important role in tumor cell invasion, neovascularization, and immune regulation. Therefore, CD13 is a potential candidate for development of targeted anticancer drugs, and CD13 inhibitors have been increasingly studied. A method for detecting enzyme activity was used to evaluate the suppressing effect of CD13 inhibitors. One of the two methods for detecting enzyme activity uses pCD13 as the enzyme source. However, the amino acid sequences of the enzyme differ between porcine and Homo sapiens (Supplementary Fig. 1). Therefore, this method cannot accurately reflect the suppressing effect of CD13 inhibitors. The other method uses the enzyme on the surface of ES-2 cells as the source. This method cannot exclude the effects of other cell surface enzymes to catalyze the substrate. That is, both methods may lead to a false positive test result, which incorrectly indicates the suppressing effects of enzyme activity by CD13 inhibitors.
In this study, the CD13 enzyme activity in K562 cells was lower than that in the other cells. Hence, K562 cells can weakly catalyze the substrate. The weak reaction between the substrate and K562 cells can be used to avoid the the actions of other enzymes on the substrate. In addition, K562 cells, which are derived from humans, are suspension and not adherent cells and need no digestion and centrifugation. Given the low interference of other enzymes and the convenience of handling, we selected K562 cells as source for detecting the suppressing effect of enzyme activity by CD13 inhibitors.
In this method, K562-CD13 cells, which were infected with CD13 vectors, were selected as enzyme source and L-leucine p-nitroaniline hydrochloride as substrate. K562-CD13 cells can integrate green fluorescent protein (GFP) and carry puromycin resistance. Infection efficiency can be evaluated by the microscopic observation of green fluorescence, and the overexpressed cells can be screened and purified based on puromycin resistance. K562-CD13 monoclonal cells can stably overexpress CD13, thereby constantly providing human aminopeptidase N. Human APN is located on the surface of the cell membrane and can directly catalyze the substrate. Furthermore, APN is suitable for evaluating the inhibitory activity of the compound. In this study, the CD13 inhibitor, ubenimex, was used as positive control to evaluate the suppressing effect of CD13 enzyme activity. We confirmed that the CD13 enzyme activity could be inhibited by ubenimex in a dose-dependent manner.
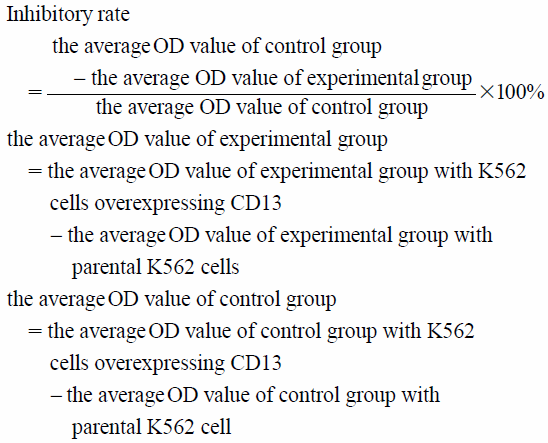
A special calculation method was employed, as described in Experimental, to exclude the influence of other enzymes and ensure the specificity of CD13 enzyme activity detection. In the calculation, we subtracted the optical density (OD) value of K562 cells from the OD value of CD13 overexpressing K562 cells. Therefore, the value of the other enzymes catalyzing the substrate was deduced, and the value obtained reflects the suppressing effect of CD13 enzyme activity by the CD13 inhibitor. This calculation can compensate for the shortcomings of the detection method using ES-2 cells as enzyme source.
In conclusion, K562 cells with CD13 overexpression were used as enzyme source to detect enzyme activity. The proposed method addressed the shortcomings of traditional methods. This study offers a simple, sensitive, highly specific, and repeatable method for detecting the suppressing effect of enzyme activity by the CD13 inhibitor. The method can be used to evaluate the activity of antitumor compounds.
Experimental
Cell CultureHuman K562 myeloid lymphoblastoma cell line was cultured in RPMI 1640 supplemented with 10% fetal calf serum, MCF7 in MEM supplemented with 15% fetal calf serum (FCS) and 0.01 mg/mL insulin, respectively, PLC/PRF/5 in MEM supplemented with 10% FCS, A549 in RPMI 1640 supplemented with 10% FCS. EA.hy926, BV2 and RMC in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS. These cells were incubated at 37°C in a humidified atmosphere of 5% CO2.
Western BlotCells were collected, and lysed with RIPA lysis buffer containing protease inhibitor. The protein concentration was quantified using bicinchoninic acid (BCA) protein assay kit (Beijing Solarbio Science and Technology, China). Protein (30 µg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene fluoride (PVDF) membrane (Cat. IPVH00010, Millipore, MA, U.S.A.). The membrance was blocked with 5% skim milk in TBST and then incubated with goat anti-human CD13 antibody (Santa, U.S.A.) at 4°C overnight. After washed with TBST, the membrane was incubated with the horseradish peroxidase (HRP)-conjugated secondary antibodies (1 : 5000; ZsBio, Beijing, China) for 1 h at room temperature. Then the membrane was visualized by enhanced chemiluminescence ECL (Cat. WBKLS0050, Millipore).
APN Activity AssayK562 was infected with CD13 lentivirus and K562 cells were treated with different doses of ubenimex. After 30 min of treatment with ubenimex, the absorbance at 405 nm was measured with L-leucine p-nitroaniline hydrochloride as a substrate. The inhibitory rate of ubenimex on APN enzyme activity was calculated by the following formula:
Flow Cytometry AssayCells were incubated with anti-CD13 antibody for 30 min at 4°C. Afer washed twice with phosphate buffered saline (PBS), the fluorescence intensity of the cells were measured using a FACS Calibur Cytometer. Flow cytometry data were analyzed using FlowJo 7.6 software.
Lentivirus TransfectionMyeloid lymphoblastoma cells K562 were plated in a 6-well plate overnight, and then infected with recombinant lentivirus vector to overexpress CD13. Lentivirus were synthesized by Genechem (Shanghai, China). The lentivirus to overexpress CD13 was transfected into the K562 cells according to the manufacturer’s instructions. The transfection efficiency was measured with fluorescence microscope. Cells were then collected and cultured to obtain stable cell lines.
Monoclonal Cells CultureCD13 overexpressing K562 cells were diluted to 10 cells/mL with medium, and then inoculated into 96-well plates with 100 µL/well. Observed and recorded the well with only one cell under a microscope. After one week, the cells were sucked into a 6-well plate and continue to expand and cultivate.
Statistical AnalysesData were expressed as mean ± standard deviation (S.D.). Statistical analyses were evaluated by the Dunnett’s test using SPSS 17.0 statistical software. In all of the experiments, p < 0.05 was considered statistically significant difference.
Acknowledgments
This work was supported by the government of National Natural Science Foundation of China (81503108), the Project of Shandong Province Higher Educational Science and Technology Program (J15LM58, J17KA255), Weifang science and technology development plan project (2017YX064), Foundation of Weifang Scientific Committee (2017BSQD12) and college students’ science and technology innovation project (KX2017035)
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
References
- 1) Olsen J., Cowell G. M., Kønigshøfer E., Danielsen E. M., Møller J., Laustsen L., Hansen O. C., Welinder K. G., Engberg J., Hunziker W., Spiesst M., Sjöström H., Norén O., FEBS Lett., 238, 307–314 (1988).
- 2) Rawlings N. D., Morton F. R., Kok C. Y., Kong J., Barret A. J., Nucleic Acids Res., 36 (Database), D320–D325 (2000).
- 3) Bauvois B., Dauzonne D., Med. Res. Rev., 26, 88–130 (2006).
- 4) Pasqualini R., Koivunen E., Kain R., Lahdenranta J., Sakamoto M., Stryhn A., Ashmun R. A., Shapiro L. H., Arap W., Ruoslahti E., Cancer Res., 60, 722–727 (2000).
- 5) Look A. T., Ashmun R. A., Shapiro L. H., Peiper S. C., J. Clin. Invest., 83, 1299–1307 (1989).
- 6) Bauvois B., Dauzonne D., Med. Res. Rev., 26, 88–130 (2006).
- 7) Santos A. N., Langner J., Herrmann M., Riemann D., Cell. Immunol., 201, 22–32 (2000).
- 8) Petrovic N., Schacke W., Gahagan J. R., O’Conor C. A., Winnicka B., Conway R. E., Mina-Osorio P., Shapiro L. H., Blood, 110, 142–150 (2007).
- 9) Mina-Osorio P., Trends Mol. Med., 14, 361–371 (2008).
- 10) Haraguchi N., Ishii H., Mimori K., Tanaka F., Ohkuma M., Kim H. M., Akita H., Takiuchi D., Hatano H., Nagano H., Barnard G. F., Doki Y., Mori M., J. Clin. Invest., 120, 3326–3339 (2010).
- 11) Wang X., Zhang L., Yang K., Zhang C., Zhang J., Fang H., Xu W., Biol. Pharm. Bull., 33, 1658–1665 (2010).
- 12) Jiang Y., Li X., Hou J., Huang Y., Wang X., Jia Y., Wang Q., Xu W., Zhang J., Zhang Y., Eur. J. Med. Chem., 143, 334–347 (2018).