2020 Volume 68 Issue 2 Pages 150-154
2020 Volume 68 Issue 2 Pages 150-154
Singlet oxygen (1O2) is highly oxidative and exerts strong cytotoxic effects. We tried to establish the best combination of a singlet oxygen generation system and a detection method with ESR, for measurement of the quenching activities of various substances. The photosensitizing reaction of rose bengal or thermal decomposition of 4-methyl-1,4-etheno-2,3-benzodioxin-1(4H)-propanoic acid (endoperoxide, EP) was used for the generation of 1O2, and a sterically hindered secondary amine, 2,2,6,6-tetramethyl-4-piperidone (TEMPD) or 2,2,6,6-tetramethyl-4-piperidinol (TEMP-OH), was used as the 1O2 detection probe. These secondary amines were oxidized by 1O2 to form stable nitroxide radicals, which were detectable by ESR. TEMPD was found to be readily oxidized by air, causing large background signals in comparison with TEMP-OH. The ESR signal obtained by the irradiation of rose bengal with visible light in the presence of TEMP-OH consisted of two kinds of nitroxide radical overlapping. In contrast, only a single nitroxide signal was observed when TEMP-OH was reacted with 1O2 generated from EP. Therefore, the best combination should be EP as the 1O2 generator and TEMP-OH as the detection probe. When using this combination, we found that the concentrations of some organic solvents such as dimethyl sulfoxide and acetonitrile should be kept constant for reliable quantification, because the concentrations of organic solvents affect the ESR signal intensity.
Singlet oxygen (1O2), a reactive oxygen species (ROS), is highly oxidative and exerts strong cytotoxic effects. Singlet oxygen is thought to be a cause of some disorders, such as photo-aging, skin damage and erythropoietic porphyria.1–4) On the other hand, the cytotoxicity of 1O2 is valuable in cancer treatment, which is called photodynamic therapy.5,6)
It is not easy to quantify 1O2, because it has very short lifetime (about 3 µs). In water, 1O2 molecules collide rapidly with H2O molecules, causing the 1O2 molecules at a higher energy state return to ground state oxygen molecules. There are several methods to detect 1O2: 1) near-infrared (NIR) time-resolved spectroscopy, 2) ESR spectroscopy with sterically hindered secondary amine probes, 3) spectrophotometric analysis with diphenylbenzofuran, and 4) fluorescence measurement of a probe such as Singlet Oxygen Sensor Green. In biological and biochemical studies, NIR time-resolved spectroscopy has been used for 1O2 detection.7,8) However, the required equipment is very specialized and is not commercially available. Spectrophotometric or fluorescent methods with some probes are easy, but colored chemicals including antioxidants sometimes interfere with the spectrophotometric or fluorescent analysis. An alternative method that has been applied for the measurement of 1O2 is ESR in combination with sterically hindered secondary amine probes. The 1O2 detector probe, 2,2,6,6-tetramethylpiperidine (TEMP) was introduced in 1976.9) As shown in Fig. 1, TEMP reacts with 1O2 to produce a stable nitroxide radical, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). The reaction product, TEMPO, can be quantified by ESR spectroscopy. Similarly, 2,2,6,6-tetramethyl-4-piperidone (TEMPD) and 2,2,6,6-tetramethyl-4-piperidinol (TEMP-OH), also sterically hindered secondary amines, have been used as 1O2 detector probes10–12) (Fig. 1).
In order to measure the 1O2 quenching activity of various substances, artificial generation of 1O2 is required. Two methods are available: 1) photosensitization using a photosensitizer such as rose bengal or hematoporphyrin, and 2) thermal decomposition of 4-methyl-1,4-etheno-2,3-benzodioxin-1(4H)-propanoic acid (endoperoxide, EP). 1O2 is generated from triplet oxygen by energy transfer from photosensitizers excited by light of a specific wavelength. However, it has been reported that this method produces other radical species in addition to 1O2.12) Recently, Mukai et al. reported the singlet oxygen absorption capacity (SOAC) assay method for measuring antioxidant activity.13–15) In the SOAC method, 1O2 is measured using 2,5-diphenyl-3,4-benzofuran (DPBF) as an UV-Vis absorption probe and EP as a 1O2 generator.
In the present study, to determine the most appropriate method to measure the 1O2 quenching activities of various substances, combinations of 1O2 generating systems and sterically hindered secondary amine probes with ESR spectroscopy were compared. In addition, some factors affecting the detection system were examined.
Photoactivation of rose bengal, a photosensitizer, and thermal decomposition of EP were used for 1O2 generation. EP is a reagent used for the SOAC method, in which 1O2 is generated in ethanol/chloroform/D2O (50 : 50 : 1, v/v/v) solution at 35°C.13–15) In the present study, we dissolved EP in ethanol because some other solvents may affect the lifetimes of ROS.
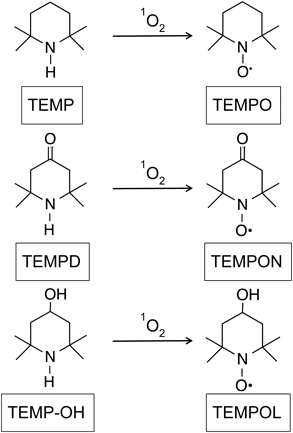
Figure 2A shows a representative spectrum obtained by the reaction of TEMPD in rose bengal solution irradiated with visible light. The three-line ESR spectrum is attributable to the stable nitroxide, 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPON). However, the spectral pattern suggests that some nitroxides other than TEMPON is present in the solution. A similar spectrum was obtained by the reaction of TEMPD in EP-containing solution incubated at 35°C (data not shown). TEMPD showed a comparatively high-intensity ESR signal even when 1O2 was not produced at time 0 of the incubation, and little change was observed in the intensity by extending the incubation time from 5 min to 30 min or by increasing the concentration of EP from 0.3 to 3 mM (Fig. 2B). As shown in Fig. 2C, the signal intensity increased with time, suggesting that a small amount of 1O2 may be generated. This observation means that the background signal of TEMPD is too large for the detection of 1O2 generated from EP. In contrast, irradiation of rose bengal with visible light in the presence of TEMPD produced a three-line ESR spectrum corresponding to TEMPON. The ESR signal intensity increased with irradiation time (Fig. 2D).
(A) A representative ESR spectrum of TEMPD solution (1 mM) after the reaction with photoactivated rose bengal (0.1 mM). (B) Time course of the ESR signal intensity. The mixed solution of TEMPD (20 mM) and EP was incubated at 35°C, and the ESR spectrum was measured at various times. EP concentrations were as follows: 0.3 mM (triangle), 1.5 mM (closed circle), 3 mM (diamond). (C) The change in the ESR signal intensity (Δ Signal intensity) is plotted vs. time (3 mM EP). (D) The reaction of TEMPD (10 mM) with 1O2 generated from photo-activated rose bengal (0.05 or 0.1 mM) irradiated with visible light for various times. The change in the ESR signal intensity is plotted vs. irradiation time.
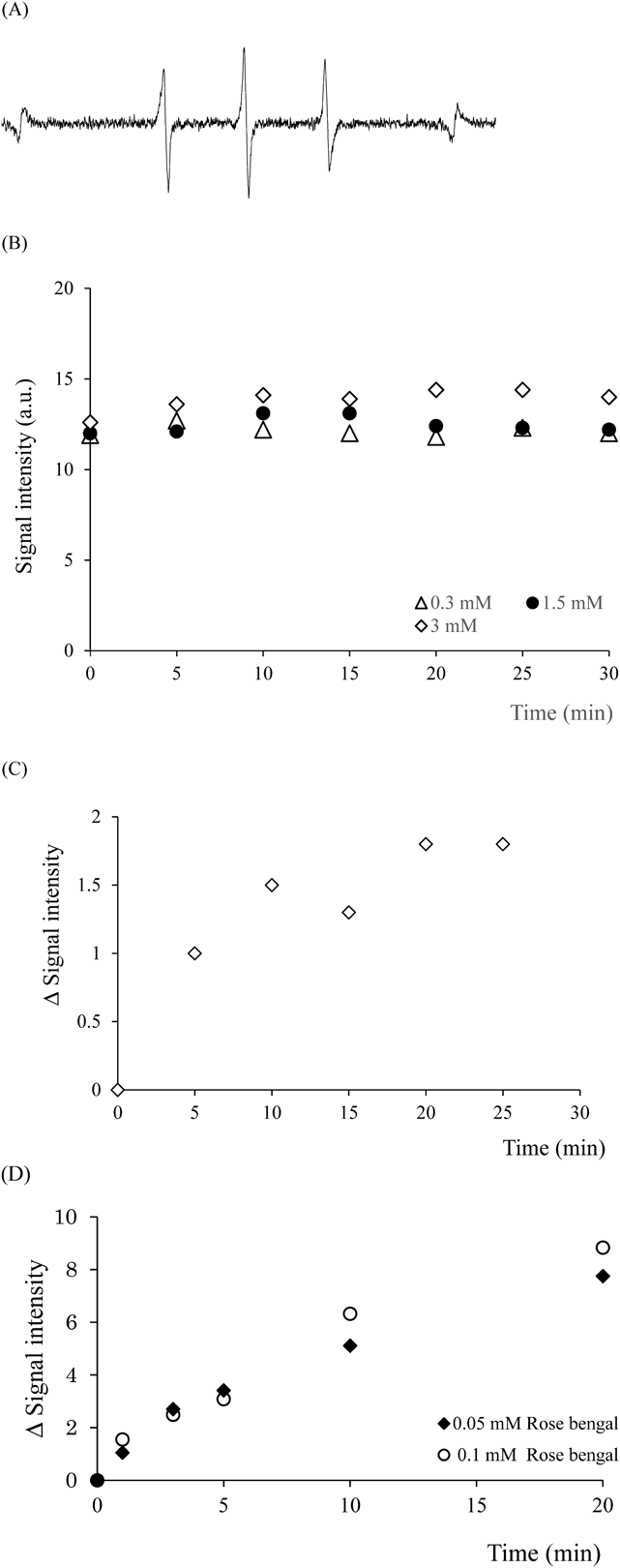
Next, TEMP-OH was used as a sterically hindered secondary amine probe for 1O2 detection. The ESR signal obtained by mixing TEMP-OH and EP solutions is shown in Fig. 3A. The spectrum has a triple-line signal derived from a nitroxide radical. However, the signal obtained by the light irradiation of rose bengal in the presence of TEMP-OH was clearly different in shape from the signal obtained in the presence of TEMPD (Fig. 2A). The ESR signal obtained when light was irradiated to the mixed solution of TEMP-OH and rose bengal showed obvious splitting, suggesting the overlap of two similar ESR signals (Fig. 3B). ESR spectra of authentic TEMPON and 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) were measured for comparison with the ESR spectrum described above. This comparison confirmed that the signal split (Fig. 3B) is caused by overlapping of TEMPOL and TEMPON spectra (data not shown). Nakamura et al. reported a similar ESR spectrum derived from TEMP-OH in rose bengal solution after irradiation of laser light at 532 nm for 60 s.12)
(A) A representative ESR spectrum derived from the mixed solution of TEMP-OH (50 mM) and EP (3 mM) at 40°C for 40 min. (B) A representative ESR spectrum derived from the reaction of TEMP-OH (5 mM) with 1O2 generated from photo-activated rose bengal (0.1 mM) irradiated with visible light for 3 min.
The irradiation of xanthine dyes such as rose bengal generates both 1O2 and superoxide anion radicals by energy transfer and electron transfer processes.16) In addition, generation of hydrogen peroxide by the irradiation of visible light to rose bengal, and generation of hydroxyl radical by the irradiation of laser light have been reported.12,17) Next, we examined the possibility of generation of ROS other than 1O2 as the cause of the signal split observed from the reaction of TEMP-OH under photo-activation of rose bengal. When mannitol, which is a scavenger of hydroxyl radicals, was added to the reaction system, the split was suppressed, suggesting that hydroxyl radicals are produced in the system. Indeed, it is reported that the exposure of TEMPOL to hydroxyl radical led to the appearance of a new triplet ESR signal attributable to TEMPON, along with a reduction in the intensity of the TEMPOL signal.18,19) Although TEMPOL is produced by the reaction of TEMP-OH with 1O2, it is possible that the splitting of the signal appeared because some TEMPOL was converted to TEMPON in the presence of hydroxyl radical. In contrast, the signal splitting was not observed in the reaction of TEMP-OH with 1O2 generated from EP. It is plausible that 1O2 abstracts only hydrogen atoms of amine groups, while not oxidizing 4-hydroxy groups. So it is possible that when rose bengal is irradiated with visible light, ROS such as hydroxyl radicals in addition to 1O2 are generated, and the 4-hydroxyl group of TEMP-OH is oxidized.
It was reported that mannitol, superoxide dismutase, and catalase did not affect the yield of nitroxide radical generated by TEMP-OH and 1O2 from rose bengal.12) In the present study, however, we observed that the signal splitting was suppressed by mannitol. The discrepancy might be due to differences in the experimental conditions (e.g., the concentrations of TEMP-OH and rose bengal, the wavelength, and the quantity of the visible light), which could change the quantitative balance between 1O2 and other ROS such as hydroxyl radical.
Comparison of TEMP-OH and TEMPD as the 1O2 Detection ProbeComparing the background signals of TEMP-OH and TEMPD solutions in the absence of 1O2, the ESR signal of TEMPD solution was much larger. Also, when both aqueous solutions were left at room temperature, the ESR signal of TEMPD solution increased with time, but the signal intensity of TEMP-OH solution did not (Fig. 4), meaning that TEMPD is more easily oxidized in air. From this observation, we can say that TEMP-OH is a better probe than TEMPD for detecting 1O2.
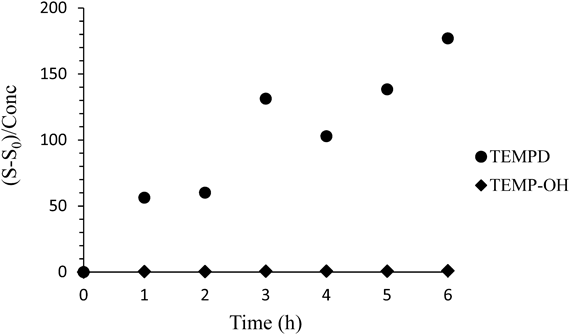
An aqueous solution of TEMPD (10 mM) and TEMP-OH (200 mM) was left at room temperature. The ordinate is the signal intensity per unit concentration, (S–S0)/concentration, where S and S0 are the intensities of the ESR signal obtained at each time and 0 min, respectively.
Although we can detect 1O2 caused by the photoactivation of rose bengal using TEMPD as a sterically hindered secondary amine probe, we cannot ignore the formation of TEMPON by air oxidation. When 1O2 is generated by the irradiation of rose bengal with visible light, the ESR signal derived from TEMP-OH is composed of two species, TEMPOL and TEMPON. Therefore, the best combination of 1O2 generation system and sterically hindered secondary amine probe is the thermal decomposition of EP and TEMP-OH.
Confirmation of the Detection of EP-Derived Singlet Oxygen by TEMP-OHNext, we tried to confirm whether the ESR signal detected in the mixed solution of EP and TEMP-OH was truly derived from 1O2. The signal intensity obtained immediately after TEMP-OH was added to the EP solution was very small, but increased with incubation time (Fig. 5A). An increase in the intensity of the ESR signal was also observed with increasing incubation temperature (data not shown). When D2O was added to the solution, the ESR signal intensity was enhanced (Fig. 5A). This enhancement of the signal intensity was dependent on the D2O concentration (Fig. 5B). Since D2O extends the lifetime of 1O2,20–23) this effect explains the observed result. When EP concentration was changed, the signal intensity increased linearly with the increase of EP concentration at least to 4 mM, suggesting that the signal intensity increased with the increase of 1O2 concentration. When histidine or NaN3, a scavenger or quencher of 1O2, was added to the EP and TEMP-OH mixture solution, the ESR signal intensity was suppressed in a dose-dependent manner (Figs. 5D, 5E). It is reasonable to assume that any increase or decrease of the ESR signal intensity corresponds to the quantity of 1O2, because the signal intensity decreased by addition of histidine and increased with increasing D2O concentration. From these results, EP can be used as a 1O2 generator not only for the SOAC method but also for the ESR method.

(A) Time course of the ESR signal intensity in the presence or absence of D2O. EP (1 mM) and TEMP-OH (30 mM) were mixed at 35°C and incubated for 40 min. Closed circles represent the intensity of the ESR signal observed in the absence of D2O, and open diamonds represent the intensity observed in the presence of D2O (33.3%). (B) Effect of D2O concentration on the ESR signal intensity. EP (3 mM) and TEMP-OH (30 mM) were mixed at 35°C and incubated for 40 min. (C) Effect of EP concentration on the ESR signal intensity. Various concentrations of EP was mixed with TEMP-OH (30 mM) at 35°C in the presence of D2O (33.3%) and incubated for 40 min. (D) Effect of the concentration of histidine on the ESR signal intensity. (E) Effect of the concentration of NaN3 on the ESR signal intensity. In the experiments for D and E, the ESR signal was derived from the reaction of TEMP-OH (30 mM) with 1O2 generated from EP (1 mM) at 35°C for 40 min in the presence of D2O (16.7%) and various concentrations of histidine or NaN3. Error bar shows standard deviation (S.D.) (n = 3).
When the EP and TEMP-OH mixture solution was reacted at 40°C for 40 min, the ESR signal intensity was smaller than with irradiation of rose bengal with visible light for 3 min (Fig. 3), meaning that the 1O2 production from EP is smaller.
Effect of Organic Solvents in the Assay SystemWe investigated the effects of organic solvents on the ESR signal intensity in the 1O2 detecting system using EP and TEMP-OH. When dimethyl sulfoxide (DMSO) was added to the solution, the ESR signal intensity was enhanced (Fig. 6A). Similarly, the ESR signal intensity was enhanced by acetonitrile (Fig. 6B). For measurement of the 1O2 scavenging activity of lipophilic substances, both DMSO and acetonitrile are often used to dissolve the substances in water. Because both organic solvents affect the ESR signal intensity of the detection system, in this assay they would affect the results for 1O2 quenching ability of test substances. It is reported that the reaction rate constants of TEMP and 1O2 in H2O and ethanol are different.24) Further, it is reported that the lifetime of 1O2 varies greatly with different solvents.25) These organic solvents may thus affect the quantities of 1O2 produced from EP and/or the lifetime of 1O2. Although the exact reason for the enhancement of signal intensity in the presence of these organic solvents is unknown, the concentration of organic solvent should at least be kept constant when using this assay system.

(A) Effect of DMSO on the ESR signal intensity. (B) Effect of CH3CN on the ESR signal intensity. The ESR signal was obtained from the sample after the reaction of TEMP-OH with 1O2 generated from EP at 35°C for 40 min in the presence of D2O (16.7%). Error bar shows S.D. (n = 3).
The results obtained in the present study confirmed that 1O2 generated from EP can be detected by ESR with TEMP-OH. However, one important consideration is that, although the amount of 1O2 generated from EP is small, it is possible to test the 1O2 scavenging activity of substances in a reaction of 40 min in the presence of D2O using TEMP-OH as a detector. If we use rose bengal as the 1O2 generation system, colored substances would interfere with the light absorption of rose bengal, and 1O2 generation would consequently be suppressed. In addition, the light irradiation might decompose the test compound, leading to a decrease in the 1O2 scavenging activity. Therefore, EP is more suitable than photo-sensitized rose bengal as the 1O2 generating system. As a detection probe, TEMP-OH is more suitable than TEMPD, because TEMP-OH is less readily air-oxidized than TEMPD. Thus, the combination of EP and TEMP-OH is the best among the combinations examined. The concentrations of organic solvents should be kept constant when using this system for the measurement of 1O2.
EP was obtained from Wakentech Co., Ltd., Japan. TEMP-OH was obtained from Sigma-Aldrich Co., Ltd., U.S.A. D2O was obtained from Cambridge Isotope Laboratories, Inc., U.S.A. Other reagents were obtained from Wako Pure Chemical Corporation, Japan. All the reagents were of the highest grade available and were used without further purification.
Generation of Singlet OxygenThe EP solution was warmed at 35°C to generate 1O2. Alternatively, 1O2 was generated by irradiation of visible light to rose bengal solution (0.1 mM) from a distance of 3 cm at 194 klux, using a Schott Megalight 100 with halogen lamp (Moritex Corporation) as the light source.
ESR MeasurementsSamples were loaded in a flat cell made of quartz, and the X-band ESR spectra were recorded using a JES-FA100 ESR spectrometer (JEOL Ltd., Japan). Mn2+ was used as the external standard. The signal intensity was calculated by dividing the signal height at the lowest magnetic field side of the nitroxide radical by the signal height of Mn2+. ESR spectra were analyzed with a WinRad ESR Data Analyzer (Radical Research Co., Ltd., Japan).
This work was supported in part by JSPS KAKENHI Grant Number 25450178 to KA.
The authors declare no conflict of interest.