Abstract
Efficient methods for delivery of antisense DNA or small interfering RNA (siRNA) are highly needed. Cationic materials, which are conventionally used for anionic oligonucleotide delivery, have several drawbacks, including aggregate formation, cytotoxicity and a low endosome escape efficiency. In this report a bio-reactive mask (i.e., disulfide unit) for cationic amino groups was introduced, and the mask was designed such that it was removed at the target cell surface. Insolubility and severe cellular toxicity caused by exposed cationic groups are avoided when using the mask. Moreover, the disulfide unit used to mask the cationic group enabled direct delivery of oligonucleotides to the cell cytosol. The molecular design reported is a promising approach for therapeutic applications.
Introduction
Antisense DNA and small interfering RNA (siRNA) are promising therapeutics against various diseases because of their large target repertoire and effectiveness.1–5) However, delivery of these therapeutic oligonucleotides (ONs) into target cells remains a significant challenge because of poor permeability.6) ONs have poor permeability because of the negative charge of the phosphate backbone, which repulses with negative charge of cell membranes. Thus, various cationic materials have been used for delivering these anionic ONs to cells,7) and cationic liposomes are an example of a cationic material used.8–11) Generally, the electric charge ratio is between five and ten.12) Excessive amounts of cationic liposomes electrostatically interact with ONs to form large nano-complexes that promote the delivery of ONs to cells.8–11) This cationic complex, however, has several drawbacks: (1) the formed liposomes display heterogeneity in molecular size, which makes these species unsuitable for clinical application; and (2) the large complex enters cells via endocytosis with low endosome escape efficiency, which decreases the gene silencing effect.13) Recently, cationic cell penetrating peptide (CPP) conjugates (abundant with amino groups or guanidyl groups) have been used.14,15) Controlling the molecular weight of these conjugates is straightforward and more stable structures are formed when compared with that of cationic liposomes. However, the exposed cation will interact with anionic ONs during manipulation and form insoluble complexes, which greatly reduces ease of manipulation or administration.16) In addition, cytotoxicity is associated with these cationic conjugates.17)
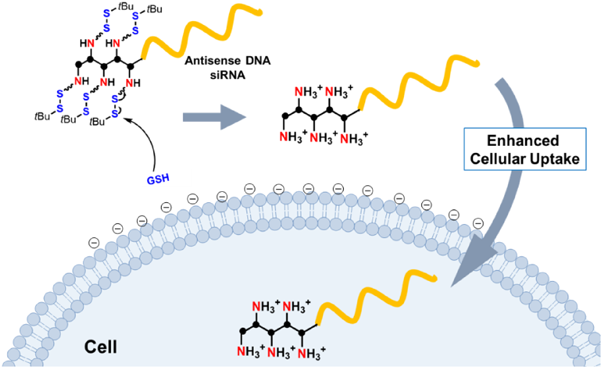
In this report, we have used a bio-cleavable disulfide-protecting group to mask the cationic charge of the amino group, and thus address the abovementioned issues. We hypothesized that with this disulfide protecting group ONs would adopt suitable physical properties that prevent ONs aggregation or precipitation during manipulation. After administration and under the reductive environment close to the target cell surface, the amino group is released to enhance ON cellular uptake (Fig. 1). The protected amino (abbreviated as proamino) molecular unit can be prepared as the phosphoramidite form (Table 1a) to conjugate readily with antisense DNA/siRNA on a DNA synthesizer with desired repeats.
Results and Discussion
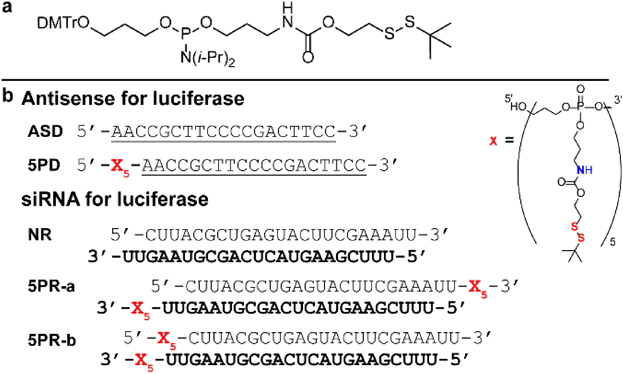
Proamino phosphoramidite 8 was synthesized from bis(diisopropylamino)chlorophosphine and was coupled with alcohol 6 and subsequently with 2-(tert-butyldisulfanyl)ethan-1-ol 4 in a one-pot reaction (Chart S1). Next, proamino phosphoramidite was attached to the 5′ terminus of antisense DNA (phosphorothioate backbone, named ASD) and five repeats of the proamino unit were introduced (5PD) (Table 1b). In our previous study of ONs with repeated disulfide unit,18) we evaluated molecular property of three types of disulfide-modified ONs, i.e., ON with one, five or ten repeats of disulfide unit. It was the revealed that ten repeats of disulfide unit induced the micelle-forming property to ONs at high concentration (> 50 µM), which was not suitable for broad application. The repeat number of disulfide unit was hence set to be five. The molecular weight of the five repeats of the proamino unit is only about one third of the antisense DNA, and the conjugate is completely charge-neutral due to the phosphotriester backbone. The 19-mer antisense DNA sequence was designed to target firefly luciferase (961–979 site), which enables monitoring of the gene-silencing effect by the dual-luciferase assay.19) All the ONs used in this study were synthesized by using an automatic DNA synthesizer and purified by HPLC, and the purity was confirmed by analytical HPLC (Figs. S1–3, Table S2).
Table 1. (a) Structure of Proamino Phosphoramidite; (b) Sequences of the Antisense DNA and siRNA
Underlined parts are the phosphorothioate DNA. The guide strands of siRNA are shown in bold. (Color figure can be accessed in the online version.)
Initially, the physical properties of 5PD aggregation or micelle formation was investigated with the addition of 2-p-toluidinylnaphthalene-6-sulfonate (TNS) and measured by a fluorophotometer. TNS is an environmental-responsive fluorescent molecule, which is used for measuring the critical micelle concentration.20–22) 5PD between 1 and 75 µM showed no significant fluorescence enhancement (Fig. S4). These results showed that 5PD did not form micelles over the concentration examined. The mono-dispersity of 5PD enables easy manipulation of samples and this conjugate is suitable for clinical applications.
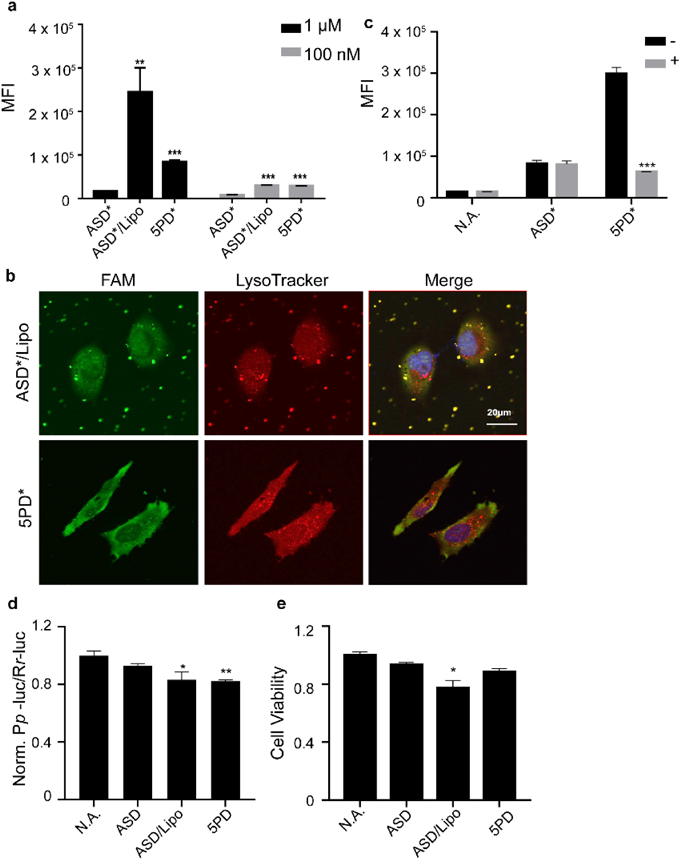
Next, cellular uptake was evaluated by flow cytometry with fluorescein labeled ONs (ASD*, 5PD*, Table S1) in HeLa cells (Fig. 2a). ASD* transfected by cationic liposomes (ASD*/Lipo) was measured as a positive control. The results showed that at 1 µM, 5PD* showed significantly higher cellular uptake when compared with that of ASD* and even showed comparable cellular uptake with the ASD*/Lipo at 100 nM. Confocal laser scanning microscopy (CLSM) was used to observe the intracellular distribution of 5PD* and ASD*/Lipo (Fig. 2b) using LysoTracker for staining endosomes and lysosomes (indicated as a red signal). After 1 h incubation, only yellow colocalization spots were seen for ASD*/Lipo, indicating that almost all of the ASD* was trapped inside endosomes. And trace amount of ASD* was observed in nucleus, which may be due to the transport along the microtubles.23) In contrast, green fluorescence signals of 5PD* were observed distinctively from red spots, indicating that 5PD* was not trapped inside endosomes but distributed efficiently in the cytosol. This efficient cytosolic distribution should afford effective gene silencing, thus making its clinical application promising. Moreover, 5PD* did not localize to some specific region in cells but was distributed evenly, which provides further evidence that 5PD* does not aggregate.
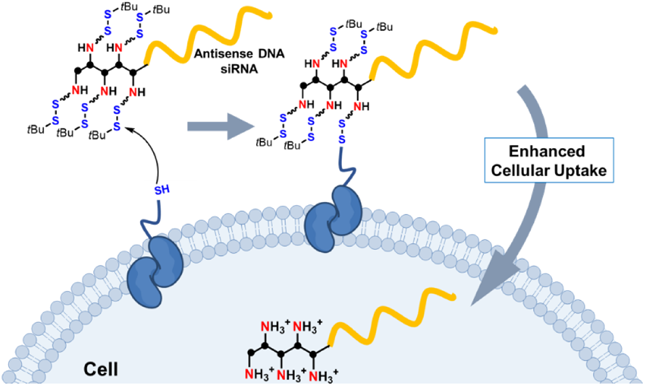
According to our recent results, disulfide modification enables ONs to achieve rapid cytosolic distribution via a thiol-disulfide exchange reaction on cellular membranes.18) To clarify whether such a disulfide-exchange reaction is responsible in this case, we evaluated cellular uptake following pretreatment with sodium iodoacetate, which blocks free thiol groups present on cellular membranes (Fig. 2c). The cellular uptake of 5PD* was significantly (around 80%) inhibited by iodoacetate pretreatment, whereas no inhibition was observed for ASD*. This result proved that the cellular uptake of 5PD* depends mainly on the thiol-disulfide exchange reaction, which was unexpected. Rather, we anticipated that exposed amino cations would play a role in permeability enhancement, but this experiment proved that the disulfide moiety is more important for cellular uptake than cations (Fig. 3).
The gene silencing effect of ASD/Lipo and 5PD was evaluated by a dual luciferase assay (Fig. 2d). Cells stably expressing both firefly luciferase/renilla dual luciferase (HeLa-LucRluc) were treated with 100 nM 5PD, ASD or ASD/Lipo. Firefly luciferase expression was measured 24 h after administration and was normalized to the renilla luciferase expression. The results showed that 5PD suppressed firefly luciferase gene expression by 30%, which is not significantly different to that of the positive control ASD/Lipo (Fig. 2d). Since the affinity to the target RNA strand is an important factor in antisense mechanism, we investigated the binding affinity of 5PD or 5AD (antisense DNA with five repeats of amino unit) to the target RNA sequence (senseR, firefly luciferase 961–979 site) by melting temperature measurements (Fig. S5a). 5AD was produced by treating 5PD with dithiothreitol and releasing those amino groups. The melting temperature of 5PD and 5AD with their target RNA strand did not show significant difference (Fig. S5b), which means the above gene silencing effect mainly reflects ONs cellular uptake, not the difference in affinity to the target RNA. In addition, cell toxicity was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with a 1 µM ONs sample. After 24 h incubation, ASD/Lipo decreased cell viability by 23% when compared with that of the no administration group, whereas 5PD did not show additional cellular toxicity when compared with that of ASD (Fig. 2e). These results demonstrated that 5PD functions like lipofectamine but without severe cell toxicity.
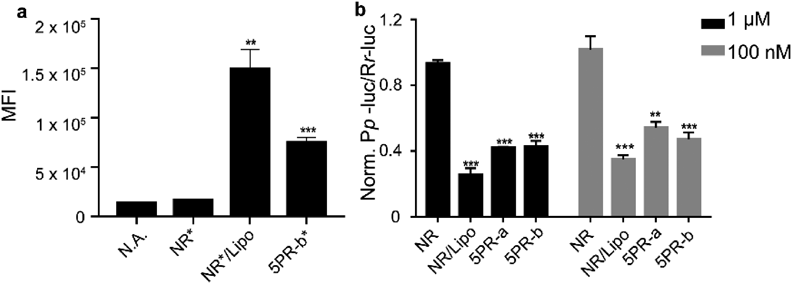
Next, we evaluated whether the proamino design would be effective for siRNA. Since the 5′ terminus of the guide strand is very important for formation of the RNA induced silencing complex (RISC), we kept this site unmodified and introduced modifications to the other three terminus.14,24) Accordingly, we designed the following two combinations of modified siRNA (Table 1b): (1) 5PR-a: proamino unit attached to the 3′ terminus of both guide and passenger strands; and (2) 5PR-b: proamino unit attached to the 3′ terminus of the guide strand and the 5′ terminus of the passenger strand. The sequence of the siRNA used targeted the firefly luciferase gene (867–885 site). Initially, we evaluated the permeability of 5PR-b*, NR* and NR*/Lipo. 5PR-b* showed five times higher permeability than NR*, which is half that of NR*/Lipo (Fig. 4a). In addition, we compared their knockdown efficiency by the dual-luciferase assay. 5PR-b showed 60% knockdown efficiency, which was slightly higher than 5PR-a (Fig. 4b) at 1 µM and about 55% knockdown efficiency at 100 nM. From these results, further investigations are necessary for improving the permeability of siRNA with different molecular designs.
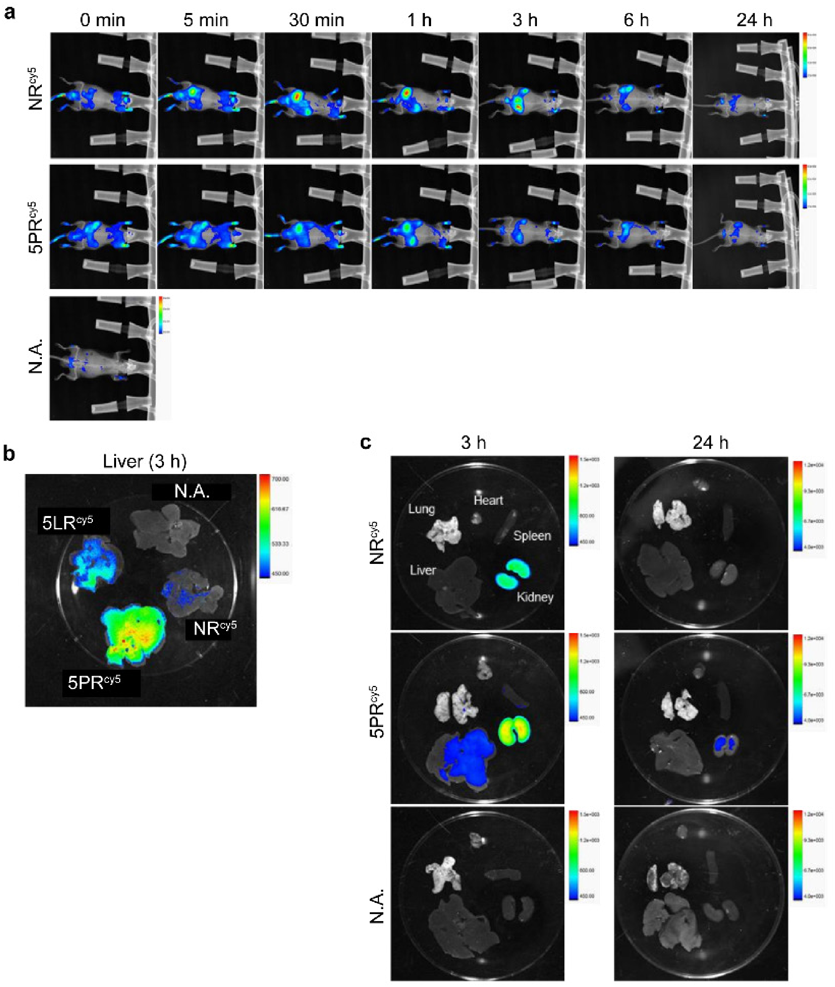
Finally, we performed animal experiments with mice to observe how the proamino unit affected the distribution of ONs in vivo. NRcy5 and 5PRcy5 at 0.25 mg/kg (Table S1) were injected into 5-week old male ICR mice by tail intravenous injection. At several time points (5, 30 min, 1, 3, 6 and 24 h) after administration, the in vivo distribution was observed by an In-Vivo Xtreme imaging system. NRcy5 showed a strong fluorescence signal in the kidney and bladder immediately after administration. In contrast, 5PRcy5 showed lower levels of renal excretion with distribution to other organs (Fig. 5a). Mice were sacrificed and the distribution of the ONs in each organ was examined at 3 h and 24 h. At 3 h administration, 5PRcy5 showed stronger intensity in liver than ASDcy5 (Figs. 5b, c). After 24 h, most of the ONs were metabolized and excreted out of the body (Fig. 5a). These results indicate that 5PR should be suitable for silencing disease-related genes in liver.
Conclusion
In summary, we developed a new delivery method for ONs based on the proamino unit, which is composed of amino units masked with a disulfide unit. The disulfide unit was used here to mask the cationic amino groups, and a novel phosphoramidite compound with the proamino group was synthesized. The phosphoramidite form makes it easy to modify ONs on a DNA synthesizer and should facilitate industry applications. These conjugated ONs showed permeability enhancement, efficient cytosolic distribution and minimal cell toxicity. This may be a result of the thiol-disulfide exchange reaction on cell membranes, which occurs because of the disulfide-protecting group on the amino units. In addition, our proamino modification did not affect gene-silencing effects and promoted ONs delivery to liver in vivo. Efforts to improve the molecular design for greater gene silencing and in vivo applications are ongoing in our group.
Acknowledgments
This work was supported by AMED (JP19fk0310102, JP19ck0106368, and JP19am0401008 to H.A.), and by JST-CREST (JPMJCR18S1 to H.A.).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Materials
The online version of this article contains supplementary materials.
References
- 1) Burnett J. C., Rossi J. J., Chem. Biol., 19, 60–71 (2012).
- 2) Farooqi A. A., Rehman Z. U., Muntane J., Onco Targets Ther., 7, 2035–2042 (2014).
- 3) Ozcan G., Ozpolat B., Coleman R. L., Sood A. K., Lopez-Berestein G., Adv. Drug Deliv. Rev., 87, 108–119 (2015).
- 4) Sharma V. K., Rungta P., Prasad A. K., Rsc Adv., 4, 16618–16631 (2014).
- 5) Wittrup A., Lieberman J., Nat. Rev. Genet., 16, 543–552 (2015).
- 6) Dowdy S. F., Nat. Biotechnol., 35, 222–229 (2017).
- 7) Juliano R. L., Nucleic Acids Res., 44, 6518–6548 (2016).
- 8) Buyens K., De Smedt S. C., Braeckmans K., Demeester J., Peeters L., van Grunsven L. A., de Mollerat du Jeu X., Sawant R., Torchilin V., Farkasova K., Ogris M., Sanders N. N., J. Control. Release, 158, 362–370 (2012).
- 9) Ewert K. K., Zidovska A., Ahmad A., Bouxsein N. F., Evans H. M., McAllister C. S., Samuel C. E., Safinya C. R., Top. Curr. Chem., 296, 191–226 (2010).
- 10) Lin Q., Chen J., Zhang Z., Zheng G., Nanomedicine (Lond), 9, 105–120 (2014).
- 11) Xue H. Y., Guo P., Wen W. C., Wong H. L., Curr. Pharm. Des., 21, 3140–3147 (2015).
- 12) Sakurai F., Inoue R., Nishino Y., Okuda A., Matsumoto O., Taga T., Yamashita F., Takakura Y., Hashida M., J. Control. Release, 66, 255–269 (2000).
- 13) Radler J. O., Koltover I., Salditt T., Safinya C. R., Science, 275, 810–814 (1997).
- 14) Bendifallah N., Rasmussen F. W., Zachar V., Ebbesen P., Nielsen P. E., Koppelhus U., Bioconjug. Chem., 17, 750–758 (2006).
- 15) Turner J. J., Ivanova G. D., Verbeure B., Williams D., Arzumanov A. A., Abes S., Lebleu B., Gait M. J., Nucleic Acids Res., 33, 6837–6849 (2005).
- 16) Meade B. R., Dowdy S. F., Adv. Drug Deliv. Rev., 59, 134–140 (2007).
- 17) Saar K., Lindgren M., Hansen M., Eiriksdottir E., Jiang Y., Rosenthal-Aizman K., Sassian M., Langel U., Anal. Biochem., 345, 55–65 (2005).
- 18) Shu Z., Tanaka I., Ota A., Fushihara D., Abe N., Kawaguchi S., Nakamoto K., Tomoike F., Tada S., Ito Y., Kimura Y., Abe H., Angew. Chem. Int. Ed. Engl., 58, 6611–6615 (2019).
- 19) Xu Y., Zhang H. Y., Thormeyer D., Larsson O., Du Q., Elmen J., Wahlestedt C., Liang Z., Biochem. Biophys. Res. Commun., 306, 712–717 (2003).
- 20) Liang J. N., Curr. Eye Res., 6, 351–355 (1987).
- 21) McClure W. O., Edelman G. M., Biochemistry-Us, 6, 559–566 (1967).
- 22) Mousset E., Oturan N., van Hullebusch E. D., Guibaud G., Esposito G., Oturan M. A., Agron Sustain. Dev., 33, 839–846 (2013).
- 23) Cardarelli F., Digiacomo L., Marchini C., Amici A., Salomone F., Fiume G., Rossetta A., Gratton E., Pozzi D., Caracciolo G., Sci. Rep., 6, 25879 (2016).
- 24) Carthew R. W., Sontheimer E. J., Cell, 136, 642–655 (2009).