2020 Volume 68 Issue 2 Pages 167-172
2020 Volume 68 Issue 2 Pages 167-172
A heterogeneous metal catalyst enabled the intramolecular oxidative coupling of diarylamines to form carbazoles with molecular oxygen as the sole oxidant. Rh/C had efficient catalytic activity and allowed the catalyst loading to be reduced to 0.1 mol% while maintaining excellent yields of carbazoles. This reaction is operationally simple in an open-to-air setup, and provides a green and atom-economical process for an efficient synthetic approach to N-substituted carbazoles.
Carbazoles have widespread applications in both the pharmaceutical and materials sciences.1–4) Many biologically active natural products consisting of a carbazole framework have also been isolated in past decades.5) As a result, significant efforts have been applied toward the development of efficient methods for the synthesis of carbazoles.6–8) Among them, direct oxidative cyclization reactions have emerged as one of the most powerful approaches for carbazole synthesis because preactivation of the substrates can be avoided, in addition to the atom- and step-economy of the reaction.9–13) Ever since Åkermark et al. reported the oxidative cyclization of diphenylamine via a two C–H bond activation process using a stoichiometric amount of palladium acetate,14) the palladium-catalyzed intramolecular oxidative coupling reaction of diarylamines in the presence of various oxidants such as cupric acetate,15–17) tert-butyl hydroperoxide,18) silver oxide,19) and oxygen20–22) has been further developed and the potential synthetic utility of this type of transformation has been significantly improved (Chart 1A). In recent years, the photochemical cyclization23,24) and molybdenum pentachloride mediated25) homogeneous reactions of triarylamines and diarylamines under mild conditions have been successfully developed. In contrast, relatively little progress has been made in heterogeneous catalysis for the synthesis of carbazoles from diarylamines, despite the high efficiency, robustness, practicality, and facile reusability of heterogeneous catalysts.26–28) Matsubara et al. reported the first heterogeneous catalysis of the intramolecular oxidative coupling of diphenylamine using platinum on carbon under hydrothermal conditions29) (Chart 1B). However, these heterogeneous reactions still suffered from high temperatures, harsh reaction conditions, and the formation of only 9H-carbazoles; N-substituted carbazoles could not be accessed. Therefore, the heterogeneous metal-catalyzed synthesis of carbazoles via direct aryl–aryl bond formation reactions that are amenable to environmentally sustainable chemistry would be valuable.30)
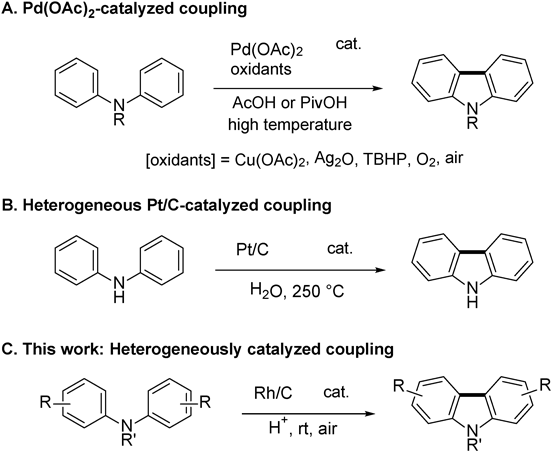
We previously developed aerobic oxidative homo-coupling and selective cross-coupling reactions of aryl amines catalyzed by heterogeneous metal catalysts.31–34) These reactions are the first examples of catalytic intermolecular oxidative coupling reactions of aryl amines under mild conditions using oxygen as a co-oxidant.35–38) Recently, we reported the heterogeneously catalyzed aerobic oxidative intramolecular coupling reaction of aromatic compounds to provide triphenylenes and carbocyclic biaryl compounds in good yields.39) These results encouraged us to develop an efficient method for the synthesis of carbazoles via the intramolecular oxidative aryl–aryl coupling of diarylamines. Herein, we report the heterogeneously catalytic oxidative cyclization of triarylamines and diarylamines under aerobic conditions (Chart 1C).
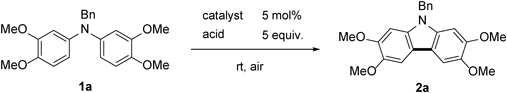
To test whether the heterogeneous metal-catalyzed intramolecular coupling reaction of diarylamines would proceed as anticipated, N-benzyldiarylamine 1a was reacted in the presence of 5 mol% Rh/C catalyst (Table 1). Fortunately, the reaction proceeded efficiently in trifluoroacetic acid (TFA) under open-air conditions at room temperature to afford benzocarbazole 2a in 76% yield (entry 1). When dichloroethane was used as a co-solvent, the amount of TFA was decreased to 5 equiv. and 2a was obtained in 99% yield (entry 2). The cyclization reaction of 1a in benzotrifluoride proceeded more smoothly to afford 2a in high yield (entry 3). We next examined the effect of various acids in benzotrifluoride, and found TFA to be the most efficient under the standard conditions (entries 4–7). In the absence of acid, the reaction did not proceed, even after heating at 50°C overnight (entry 8). We then examined various catalysts for the coupling reaction. The use of Rh/Al2O3 delivered 2a in high yield despite the slightly longer reaction time (entry 9). Catalysts such as Pd/C, Pd/Al2O3, Pt/C, and Pt/Al2O3 showed high reactivities, and these reactions were completed in shorter reaction times with good product yields (entries 10–13). In contrast, Ru/C and Ru/Al2O3 were found to be less active (entries 14 and 15). Despite the higher activity of the palladium and platinum catalysts, the slightly lower yields meant that the best conditions were achieved by a combination of Rh/C and TFA at room temperature. When the reaction was performed under an argon atmosphere, a reduced yield of 2a was obtained (entry 17). This indicates that oxygen plays an important role as a terminal oxidant.40)
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Acid | Solvent | Time (h) | Yields (%) |
| 1b) | 5% Rh/C | CF3CO2H (TFA) | — | 1 | 76 |
| 2 | 5% Rh/C | TFA | CHCl2CHCl2 | 3 | 99 |
| 3 | 5% Rh/C | TFA | CF3C6H5 | 2 | 92 |
| 4 | 5% Rh/C | MsOH | CF3C6H5 | 4 | 29 |
| 5 | 5% Rh/C | CHF2CO2H | CF3C6H5 | 8.5 | 26 |
| 6 | 5% Rh/C | CHCl2CO2H | CF3C6H5 | 54 | 9 |
| 7 | 5% Rh/C | CH3CO2H | CF3C6H5 | 31 | No reaction |
| 8c) | 5% Rh/C | — | CF3C6H5 | 30 | No reaction |
| 9 | 5% Rh/Al2O3 | TFA | CF3C6H5 | 5 | 87 |
| 10 | 5% Pd/C | TFA | CF3C6H5 | 1 | 73 |
| 11 | 5% Pd/Al2O3 | TFA | CF3C6H5 | 1 | 80 |
| 12 | 5% Pt/C | TFA | CF3C6H5 | 0.5 | 81 |
| 13 | 5% Pt/Al2O3 | TFA | CF3C6H5 | 0.5 | 74 |
| 14 | 5% Ru/C | TFA | CF3C6H5 | 87 | 23 |
| 15 | 5% Ru/Al2O3 | TFA | CF3C6H5 | 87 | 4 |
| 16 | — | TFA | CF3C6H5 | 39 | No reaction |
| 17d) | 5% Rh/C | TFA | CF3C6H5 | 2 | 15 |
a) Reaction conditions: catalyst (5 mol%), acid (5 equiv.), room temperature (r.t.), air. b) TFA (excess) was used as solvent. c) 50°C. d) Under argon.
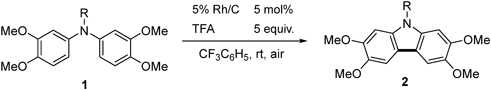
With the optimized conditions in hand, we explored the substrate scope of the intramolecular biaryl coupling reaction (Table 2). In addition to N-benzyldiarylamine, the reaction of N-propyl- and cyclohexyl-substituted compounds 1b and 1c also proceeded efficiently to afford carbazoles 2b and 2c in 85% and 98% yields, respectively (entries 1 and 2). The Rh/C-catalyzed reaction of N-tert-butyl compound 1d was unsuccessful, resulting in a complex mixture. When Pd/Al2O3 was used as the catalyst under oxygen atmosphere, the cyclization reaction proceeded to provide 38% of carbazole 2d and 60% of the tert-butyl group deprotected carbazole as a result of the instability of the product under acidic conditions (entry 3). Triarylamines 1e, 1f, and 1g also underwent the cyclization reaction to provide N-aryl carbazoles 2e, 2f, and 2g, respectively, in good to moderate yields (entries 4–6). The reaction tolerated both electron-rich and electron-poor substituents on the benzene ring. The reaction of 1f possessing the 3,4,5-trimethoxyphenyl group did not proceed cleanly to result in the low yielding of 2f (entry 5). Because diarylamines with electron-withdrawing groups are less reactive, the cyclization reaction of 1g was conducted under an oxygen atmosphere to provide the ester group-substituted carbazole 2g in 81% yield (entry 6). In contrast, a secondary amine possessing an N-H group (1h) was sluggish, and acetamide 1i was inert to this reaction (entries 7 and 8).
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | 1 | Air/O2 | Time (h) | 2 | Yields (%) |
| 1 | n-C3H7 | 1b | Air | 2 | 2b | 85 |
| 2 | c-Hex | 1c | Air | 6 | 2c | 98 |
| 3b) | tert-Bu | 1d | O2 | 2 | 2d | 38 c) |
| 4 | 4-tert-Bu-C6H4 | 1e | Air | 2 | 2e | 94 |
| 5 | 3,4,5-(MeO)3-C6H2 | 1f | Air | 3 | 2f | 38 |
| 6 | 4-(MeO2C)-C6H4 | 1g | O2 | 21 | 2g | 81 |
| 7 | H | 1h | Air | 10 | 2h | Complex mixture |
| 8 | Ac | 1i | O2 | 94 | 2i | No reaction |
a) Reaction conditions: 5% Rh/C (5 mol%), TFA (5 equiv.), CF3C6H5, r.t. b) 5% Pd/Al2O3 was used. c) 60% of 2h was obtained along with 2d.
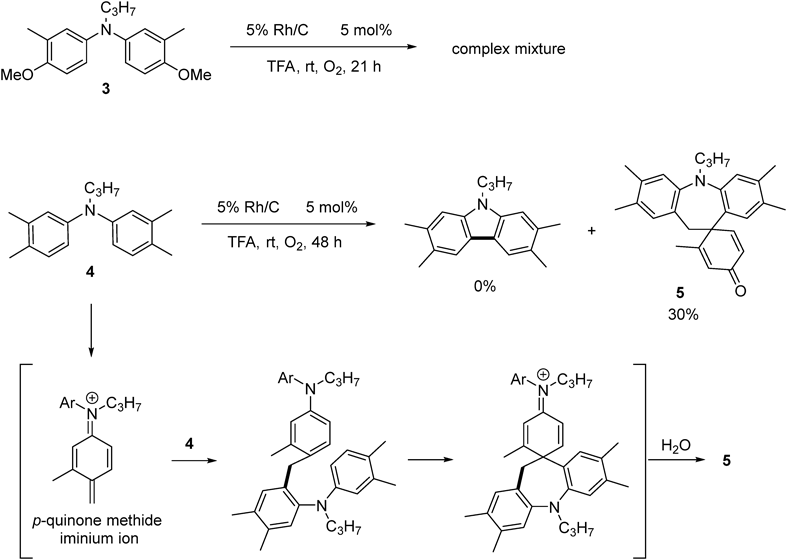
Since tetramethoxydiphenylamines 1 were excellent substrates for the intramolecular biaryl coupling because of their electron-rich nature, as seen in Table 2, we next examined the cyclization of differentially substituted diarylamines (Chart 2). When 3,3′-dimethyl substituted substrate 3 was used, the carbazole was not produced because side reactions such as intermolecular coupling occurred. In addition, tetramethyldiphenylamine 4 was not cyclized to the corresponding carbazole, but 30% of dihydroazepine 5 was obtained instead, probably due to an addition-cyclization reaction involving the in-situ generated p-quinone methide imine intermediate.41,42) These results indicated that tetramethoxylated compounds were preferred for the Rh/C-catalyzed aerobic intramolecular oxidative coupling to obtain carbazoles in high yields.
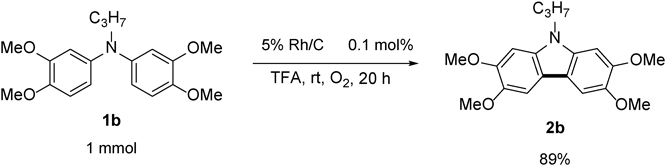
The reaction of 1b on a 1 mmol scale also proceeded efficiently (Chart 3). Furthermore, even when using only 0.1 mol% of 5% Rh/C, the intramolecular oxidative coupling afforded carbazole 2b in excellent yield, with a turnover number of up to 890.
We have developed a catalytic aerobic intramolecular oxidative coupling of N-alkyldiarylamines and triarylamines using a heterogeneous metal catalyst, providing an efficient route to N-substituted 2,3,6,7-tetramethoxycarbazoles in high yields. Further studies on synthetic applications are underway in our laboratory.
NMR spectra were recorded on Varian 400-MR (1H-NMR at 400 MHz, 13C-NMR at 100 MHz) and Bruker AVANCE III HD-500 (1H-NMR at 500 MHz, 13C-NMR at 125 MHz) spectrometers, using tetramethylsilane (TMS) or CDCl3 as the reference standard (1H-NMR at 0.00 ppm (TMS), 13C-NMR at 77.0 ppm (CDCl3)). Chemical shifts are reported in ppm. Peak multiplicities use the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartet; quin, quintet; sept, septet; m, multiplet; br, broadened. IR spectra were recorded on a JASCO FT/IR-4200 spectrometer. Mass spectra and high-resolution mass spectra (HRMS) were obtained on JEOL JMS-700 and Waters SYNAPT G2-Si HDMS mass spectrometers, respectively. Melting points (mp) were recorded on Yanaco MP-500D and SRS Opti Melt MPA 100 apparatuses and were uncorrected. Analytical TLC was performed on precoated plates (0.25 mm, Merck silica gel 60 F254). Column chromatography was performed with silica gel (40–50 µm, Kanto Chemical Co., Inc., Japan). Preparative HPLC was performed on a recycling system utilizing LC-9210 II Next and LC-5060 instruments (JAI Co., Inc., Japan). Commercially available chemicals were used as purchased. Diarylamines and triarylamines were prepared according to the method reported in the literature.43)
General Procedure for Intramolecular Oxidative Coupling of Diarylamines: N-Benzyl-2,3,6,7-tetramethoxycarbazole 2a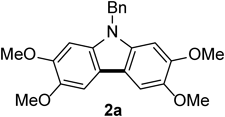
To a solution of N-benzyldiarylamine (1a, 156 mg, 0.40 mmol) and trifluoroacetic acid (0.15 mL, 2.0 mmol) in benzotrifluoride (2 mL) was added 5% Rh/C (42 mg, 0.020 mmol) and stirred at room temperature under open-air conditions for 2 h. After addition of a saturated NaHCO3 solution, the resulting mixture was extracted with AcOEt and the organic layer was washed with brine, dried over MgSO4, filtered, and concentrated in vacuo to give the crude product, which was purified by silica gel column chromatography (30% AcOEt–hexane) to afford 142 mg (92%) of the title compound. Recrystallization from AcOEt–hexane afforded 2a as colorless needles (mp 176–178°C). 1H-NMR (500 MHz, CDCl3) δ: 7.47 (2H, s), 7.30–7.21 (3H, m), 7.14–7.08 (2H, m), 6.78 (2H, s), 5.43 (2H, s), 4.01 (6H, s), 3.89 (6H, s). 13C-NMR (125 MHz, CDCl3) δ: 148.3 (s), 144.1 (s), 137.2 (s), 135.1 (s), 128.8 (d), 127.4 (d), 126.2 (d), 115.1 (s), 102.1 (d), 93.0 (d), 56.6 (q), 56.2 (q), 46.7 (t). IR (ATR): 1473, 1242, 1152, 1049 cm−1. MS (EI) m/z: 91 (Bn), 362 (M+ − CH3), 377 (M+, 100%), 378 (M+ + H). HRMS (EI) m/z Calcd for C23H23NO4 (M+): 377.1627. Found: 377.1625.
N-Propyl-2,3,6,7-tetramethoxycarbazole 2b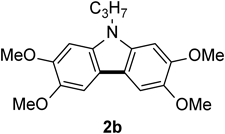
The title compound (2b, 111 mg, 85%) was prepared according to General procedure (conditions: 2 h) using N-propyldiarylamine (1b, 132 mg, 0.40 mmol). Pale brown prism (mp 168–170°C; AcOEt-hexane). 1H-NMR (500 MHz, CDCl3) δ: 7.44 (2H, s), 6.86 (2H, s), 4.19 (2H, t, J = 7.0 Hz), 4.00 (12H, s), 1.89 (2H, sext, J = 7.0 Hz), 098 (3H, t, J = 7.0 Hz). 13C-NMR (125 MHz, CDCl3) δ: 148.2 (s), 143.8 (s), 134.9 (s), 114.9 (s), 102.1 (d), 92.8 (d), 56.6 (q), 56.3 (q), 44.8 (t), 22.4 (t), 11.8 (q). IR (ATR): 1479, 1254, 1200, 1153, 1059, 839 cm−1. MS (EI) m/z: 314 (M+ − CH3), 329 (M+, 100%), 330 (M+ + H). HRMS (EI) m/z Calcd for C19H23NO4 (M+): 329.1627. Found: 329.1620.
N-Cyclohexyl-2,3,6,7-tetramethoxycarbazole 2c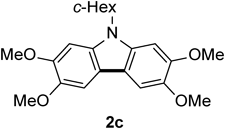
The title compound (2c, 146 mg, 98%) was prepared according to General procedure (conditions: 6 h) using N-cyclohexyldiarylamine (1c, 148 mg, 0.40 mmol). Brown solids (mp 190–191°C; CHCl3-hexane). 1H-NMR (500 MHz, CDCl3) δ: 7.42 (2H, s), 7.01 (2H, s), 4.35–4.26 (1H, m), 4.01 (6H, s), 4.00 (6H, s), 2.35–2.25 (2H, m), 2.05–1.84 (5H, m), 1.61–1.51 (2H, m), 1.42–1.31 (1H, m). 13C-NMR (125 MHz, CDCl3) δ: 147.8 (s), 143.8 (s), 134.1 (s), 115.6 (s), 101.9 (d), 94.7 (d), 56.6 (q), 56.5 (q), 55.5 (d), 31.0 (t), 26.5 (t), 25.8 (t). IR (ATR): 1477, 1155, 1064, 836 cm−1. MS (EI) m/z: 354 (M+ − CH3), 369 (M+, 100%), 370 (M+ + H). HRMS (EI) m/z Calcd for C22H27NO4 (M+): 369.1940. Found: 369.1944.
N-tert-Butyl-2,3,6,7-tetramethoxycarbazole 2d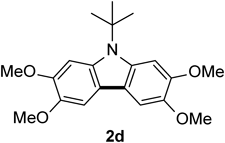
The title compound (2d, 52 mg, 38%) was prepared according to General procedure (conditions: O2, 2 h) using 5% Pd/Al2O3 (43 mg, 0.020 mmol) and N-tert-butyldiarylamine (1d, 138 mg, 0.40 mmol). Pale yellow prism (mp 195–199°C; AcOEt–hexane). 1H-NMR (500 MHz, CDCl3) δ: 7.38 (2H, s), 7.34 (2H, s), 4.00 (6H, s), 3.97 (6H, s), 1.97 (9H, s). 13C-NMR (125 MHz, CDCl3) δ: 147.0 (s), 143.7 (s), 134.6 (s), 117.1 (s), 101.0 (d), 98.7 (d), 58.5 (s), 56.5 (q), 56.4 (q), 31.0 (q). IR (ATR): 1483, 1464, 1139, 1021 cm−1. MS (EI) m/z: 287 (M+ + H − tert-Bu, 100%), 343 (M+), 344 (M+ + H). HRMS (EI) m/z Calcd for C20H25NO4 (M+): 343.1784. Found: 343.1781.
N-(4-tert-Butyphenyl)-2,3,6,7-tetramethoxycarbazole 2e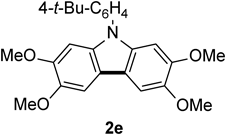
The title compound (2e, 157 mg, 94%) was prepared according to General procedure (conditions: 2 h) using triarylamine (1e, 169 mg, 0.40 mmol). Colorless needles (mp 161–164°C; AcOEt–hexane). 1H-NMR (500 MHz, CDCl3) δ: 7.61 (2H, d, J = 8.5 Hz), 7.47 (2H, d, J = 8.5 Hz), 7.46 (2H, s), 6.90 (2H, s), 4.03 (6H, s), 3.89 (6H, s), 1.44 (9H, s). 13C-NMR (125 MHz, CDCl3) δ: 150.1 (s), 148.3 (s), 144.6 (s), 135.33 (s), 135.30 (s), 126.9 (d), 126.1 (d), 115.5 (s), 101.7 (d), 93.9 (d), 56.6 (q), 56.3 (q), 34.8 (s), 31.4 (q). IR (ATR): 1487, 1470, 1194, 1120 cm−1. MS (EI) m/z: 404 (M+ − Me), 419 (M+, 100%), 420 (M+ + H). HRMS (EI) m/z Calcd for C26H29NO4 (M+): 419.2097. Found: 419.2095.
N-(3,4,5-Trimethoxyphenyl)-2,3,6,7-tetramethoxycarbazole 2f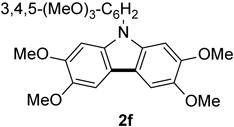
The title compound (2f, 69 mg, 38%) was prepared according to General procedure (conditions: 3 h) using triarylamine (1f, 182 mg, 0.40 mmol). Pale pink needles (mp 147–148°C; Et2O). 1H-NMR (500 MHz, CDCl3) δ: 7.46 (2H, s), 6.89 (2H, s), 6.75 (2H, s), 4.03 (6H, s), 3.99 (3H, s), 3.90 (6H, s), 3.88 (6H, s). 13C-NMR (125 MHz, CDCl3) δ: 154.2 (s), 148.3 (s), 144.7 (s), 137.1 (s), 135.4 (s), 133.7 (s), 115.5 (s), 104.3 (d), 101.9 (d), 93.8 (d), 61.0 (q), 56.7 (q), 56.33 (q), 56.29 (q). IR (ATR): 1597, 1474, 1451, 1426, 1160, 1124, 1041, 1016, 994 cm−1. MS (EI) m/z: 438 (M+ − Me), 453 (M+, 100%), 454 (M+ + H). HRMS (EI) m/z Calcd for C25H27NO7 (M+): 453.1788. Found: 453.1795.
N-(4-Methoxycarbonylphenyl)-2,3,6,7-tetramethoxycarbazole 2g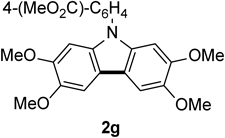
The title compound (2g, 136 mg, 81%) was prepared according to General procedure (conditions: O2, 21 h) using triarylamine (1g, 168 mg, 0.40 mmol). Pale brown needles (mp 226–227°C; AcOEt). 1H-NMR (500 MHz, CDCl3) δ: 8.30 (2H, d, J = 8.5 Hz), 7.66 (2H, d, J = 8.5 Hz), 7.45 (2H, s), 6.94 (2H, s), 4.03 (6H, s), 3.99 (3H, s), 3.88 (6H, s). 13C-NMR (125 MHz, CDCl3) δ: 166.4 (s), 148.4 (s), 145.1 (s), 142.5 (s), 134.5 (s), 131.5 (d), 128.4 (s), 126.0 (d), 116.2 (s), 101.8 (d), 93.8 (d), 56.6 (q), 56.3 (q), 52.4 (q). IR (ATR): 1719, 1606, 1280, 1198, 1166, 1106 cm−1. MS (EI) m/z: 406 (M+ − Me), 421 (M+, 100%), 422 (M+ + H). HRMS (EI) m/z Calcd for C24H23NO6 (M+): 421.1525. Found: 421.1534.
2,3,6,7-Tetramethoxycarbazole 2h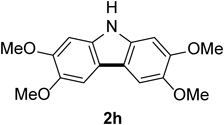
Colorless needles (mp 231–233°C; AcOEt). 1H-NMR (500 MHz, CDCl3) δ: 7.73 (1H, br s), 7.40 (2H, s), 6.93 (2H, s), 4.00 (6H, s), 3.96 (6H, s). 13C-NMR (125 MHz, CDCl3) δ: 148.2 (s), 144.3 (s), 134.0 (s), 115.8 (s), 101.9 (d), 94.5 (d), 56.6 (q), 56.2 (q). IR (ATR): 3400, 1491, 1473, 1196, 1128, 1001, 838 cm−1. MS (EI) m/z: 272 (M+ − Me), 287 (M+, 100%), 288 (M+ + H). HRMS (EI) m/z Calcd for C16H17NO4 (M+): 287.1158. Found: 287.1151.
N-Propyldihydrodibenzoazepine 5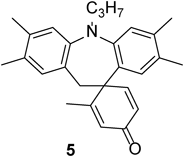
The title compound (5, 11 mg, 30%) was prepared according to General procedure (conditions: O2, 48 h) using diarylamine (4, 54 mg, 0.20 mmol). Pale brown oil. 1H-NMR (500 MHz, CDCl3) δ: 6.93 (1H, s), 6.90 (1H, s), 6.88 (1H, s), 6.79 (1H, d, J = 10 Hz), 6.39 (1H, s), 6.18 (1H, d, J = 10 Hz), 6.14 (1H, s), 3.74–3.62 (2H, m), 2.20 (3H, s), 2.19 (6H, s), 2.01 (3H, s), 1.88 (3H, s), 1.61 (2H, sext, J = 7.5 Hz), 0.95 (3H, t, J = 7.5 Hz). 13C-NMR (125 MHz, CDCl3) δ: 186.7 (s), 166.4 (s), 157.0 (d), 148.5 (s), 145.4 (s), 136.5 (s), 135.8 (s), 132.1 (d), 131.9 (s), 130.7 (d), 130.2 (s), 130.1 (s), 126.1 (d), 124.5 (d), 121.7 (d), 120.4 (d), 52.0 (t), 50.9 (s), 40.0 (t), 21.2 (t), 20.5 (q), 19.8 (q), 19.7 (q), 19.2 (q), 18.6 (q), 11.9 (q). IR (ATR): 1661, 1498, 879, 730 cm−1. MS (EI) m/z: 342 (M+ − C3H7), 356 (M+ − C2H5), 385 (M+, 100%). HRMS (EI) m/z Calcd for C27H31NO (M+): 385.2406. Found: 385.2410.
This work was partly supported by JSPS KAKENHI Grant Nos. 17K15430 and 19K06985 from Ministry of Education, Culture, Sports, Science and Technology (MEXT), The Futaba foundation, the Takahashi Industrial and Economic Research foundation, and the Cooperative Research Program of the Network Joint Research Center for Materials and Devices.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.