2020 Volume 68 Issue 9 Pages 895-898
2020 Volume 68 Issue 9 Pages 895-898
We have developed a catalytic aerobic oxidative dimerization reaction of benzofuranones using a Pd(II)-µ-hydroxo complex. Radical-radical cross-coupling of the resulting dimers with azo compounds enabled the one-pot synthesis of structurally congested benzofuranones having two distinct vicinal all-carbon quaternary centers.
Oxidation is a key methodology to increase the molecular complexity of simple starting materials,1) but there is still a need for an efficient, selective, economical and environmentally friendly approach. In this context, the development of catalytic aerobic oxidation with the use of molecular oxygen (O2) as an oxidant has been attracting attention.2–5) A key to achieving selective aerobic oxidation is to suppress undesired pathways involving reactive oxygen species (ROS). Furthermore, in order to ensure safe operation, we require mild reaction conditions that avoid the use of high temperatures and pressures.6)
We recently reported the development of a radical-radical cross-coupling between the persistent (long-lived) radical 1•7) and the transient (short-lived) radical 4•, affording 5 with two contiguous all-carbon centers.8,9) The persistent radical 1• is readily generated from 2, which contains an elongated C(sp3)–C(sp3) bond, simply by heating without the assistance of redox-active transition metals.8–16) However, the requisite dimers 2 were synthesized from the corresponding monomers 1 using a stoichiometric amount of K3[Fe(CN)6] as an oxidant under basic conditions at 80°C according to the literature procedure.16–19) In this Note, we describe a mild protocol for aerobic oxidative dimerization of 1 to provide the dimers 2 by using Pd-µ-hydroxo complex I, previously employed as an acid-base catalyst20,21) (Chart 1, i). We also successfully applied this catalytic aerobic dimerization to a formal oxidative cross-coupling (cross-dehydrogenative coupling) reaction,22–27) comprising dimerization of 1/cross-coupling of 2 with azo compounds 3 (Chart 1, ii). This reaction sequence enables the one-pot synthesis28) of 5 starting from 1.
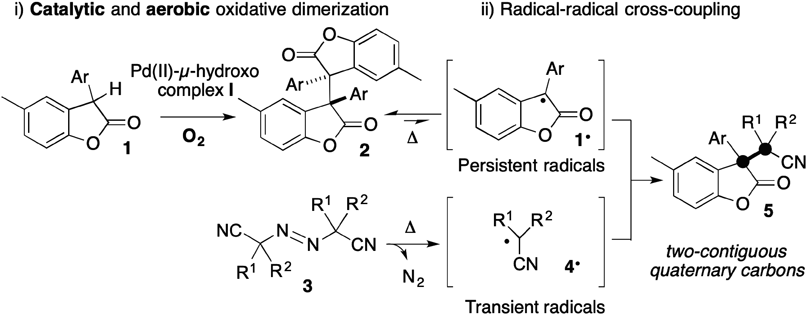
To establish milder conditions for preparing 2, we planned to apply our Pd-μ-hydroxo catalyst I to catalytic aerobic oxidative dimerization of 1,29) focusing on the functional inertness of I to O2, ROS and water. We postulated that the highest occupied molecular orbital (HOMO)-raising activation of 1 with catalyst I would enhance the generation of 1• via single-electron transfer to O2, allowing the homo-coupling to proceed smoothly to give the corresponding dimer 2 under aerobic conditions (Chart 1, step i). We also anticipated that cross-coupling of the resulting dimers 2 with azo compounds 3, corresponding to a formal aerobic oxidative cross-coupling reaction, would afford 5 with single-flask operation (Chart 1, step ii).
We began by focusing on aerobic dimerization of benzofuranones 1 (Table 1). Although 1 has been widely used in acid-base catalysis,30) its use in radical reactions is relatively rare.12–14) We initiated this study with use of racemic Pd complex I as a catalyst for the proposed aerobic dimerization of 1 because the C(sp3)–C(sp3) bond in 2 is readily cleavable even at room temperature, providing thermodynamically stable meso-2, rather than dl-2, as the major product.8) We found that the use of CH3CN as the solvent is critical to obtain a high yield of meso-2a (Table 1, entry 1 vs. entry 2). When we examined the reaction of 1a in the presence of Pd complex I (5 mol%) under an oxygen atmosphere in tetrahydrofuran (THF), which is a standard solvent for acid-base catalysis with I,21) benzofuranone 1a was recovered in 94% yield (Table 1, entry 1). In contrast, the reaction in CH3CN under the same conditions gave meso-2a in 77% yield (Table 1, entry 2). Pd complexes II and III are not effective under the conditions (Table 1, entries 3 and 4), suggesting that the rather basic character of I is an important factor to promote the aerobic oxidative dimerization of 1a. In a control experiment without I, we recovered 1a in 99% yield (Table 1, entry 5), and this result also indicates a key role of I in the aerobic oxidative dimerization of 1a. Benzofuranones having different substituents on the aromatic group at the C(3) position in 1 also reacted under these aerobic conditions, giving the corresponding dimers (Table 1, entries 6–8). The electronic properties of the substituent on the aromatic ring in 1 influence the yield of dimers 2. For example, 1b (R = F) gave the best yield of the corresponding dimer 2b (85%). Meanwhile, 1d (R = MeO) was less reactive under our conditions, and the dimer 2d was obtained in only 28% yield, with recovery of 1d in 9% yield.31) Generally the yields of 2 in the developed reactions are higher than those in the previous method using stoichiometric amounts of K3[Fe(CN)6] and KOH (see Table S1).
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Ar | Yield of 2 (%)b) | Recovery of 1 (%)c) |
| 1 | I | THF | Ph | 2a: n.d. | 1a: 94 |
| 2 | I | CH3CN | Ph | 2a: 77 | 1a: n.d. |
| 3 | II | CH3CN | Ph | 2a: n.d. | 1a: 99 |
| 4 | III | CH3CN | Ph | 2a: 1 | 1a: 98 |
| 5 | None | CH3CN | Ph | 2a: n.d. | 1a: 99 |
| 6 | I | CH3CN | 4-F-C6H4- | 2b: 85 | 1b: n.d. |
| 7 | I | CH3CN | 4-Me-C6H4- | 2c: 57 | 1c: 1 |
| 8 | I | CH3CN | 4-MeO-C6H4- | 2d: 28 | 1d: 9 |
a) Reactions were run on a 0.1 mmol scale. b) Isolated yield. c) NMR yield determined by using CH2Br2 as an internal standard.
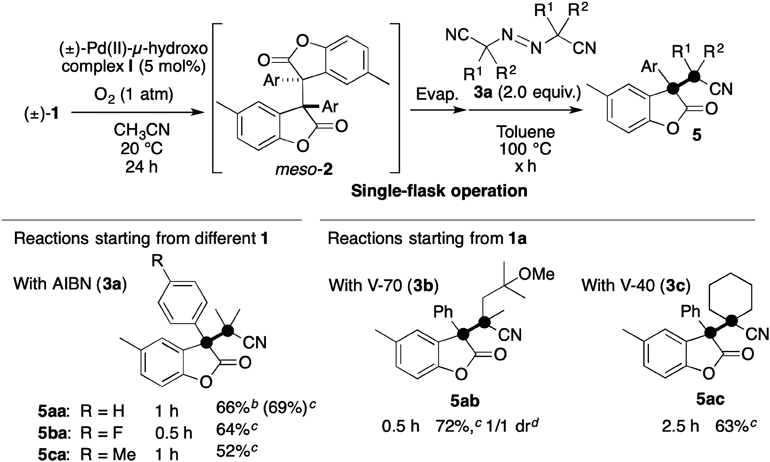
With suitable conditions in hand for the Pd-catalyzed aerobic oxidative dimerization of 1, we examined the feasibility of sequential reactions to achieve a formal one-pot oxidative cross-coupling synthesis of benzofuranone 5 having two-contiguous all-carbon quaternary centers (Chart 2). In the developed procedure, CH3CN is evacuated after the reaction mixture has been stirred for 24 h under aerobic conditions. The second reaction was then performed by adding 3 (2 equiv. to 1) in toluene after replacing O2 with Ar, according to a previously developed procedure.8,9) As we expected, upon heating the reaction mixture to 100°C, we obtained the cross-coupling adducts 5 in reasonable yields. Benzofuranones 1a, 1b and 1c are efficiently transformed to the corresponding cross-coupling adducts (5aa: 69%, 5ba: 64%, 5ca: 52%) with the use of AIBN (3a: azobisisobutyronitrile)32) as a precursor of the transient radical. Azo compounds V-70 [3b: 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile)]32) and V-40 [3c: 1,1′-azobis(cyclohexane-1-carbonitrile)]32) are also available for this one-pot procedure starting from 1a, affording 5ab (72%, 1/1 diastereomeric ratio)33) and 5ac (63%), respectively. Importantly, the differences in yields of 5 between the developed one-pot protocol and the previous protocol using isolated 29,34,35) were small, suggesting that the presence of (±)-Pd catalyst I does not interfere with the cross-coupling of 2 with 3.
a) Reactions were run on a 0.1 mmol scale. b) Isolated yields in 2 steps from 1a. c) NMR yields in 2 steps from 1 determined by using CH2Br2 as an internal standard. d) Diastereomeric ratio (dr) was determined by 1H-NMR analysis of the crude reaction mixture.
In conclusion, we have developed a formal aerobic oxidative cross-coupling reaction of benzofuranones 1 with azo compounds 3. The key to success in this sequential single-flask transformation is the chemoselectivity of Pd catalyst I. This catalyst selectively activates benzofuranones 1 to form the corresponding dimer 2 under aerobic conditions, whereas it is functionally inert to radical species, including O2. Further studies on catalyst-controlled aerobic oxidations are underway.
This work was supported in part by RIKEN’s Strategic Programs and KAKENHI (JP17H02213, 18K19156 and JP18H04277 in Precisely Designed Catalysts with Customized Scaffolding) from JSPS and MEXT. RIKEN HOKUSAI GreatWave (GW) provided the computer resources for the DFT calculations.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.