Abstract
In order to investigate the relationship between the chemical composition of essential oils and haplotypes of the psbA-trnH intergenic spacer region of chloroplast DNA (psbA-trnH) in Valerianae Fauriei Radix (Japanese Valerian; JV), we analyzed the DNA sequence and GC-MS metabolome of JV from Japanese markets and of herbal specimens from related species. DNA analysis revealed that JV products from Japan consisted of three haplotypes, namely AH-1, -2 and -5 reported in our previous study. The GC-MS metabolome revealed five chemotypes (J1, J2, C, K and O), of which J1, J2 and C were detected in the JV products from Japan. Chemotypes J1 and J2, with kessyl glycol diacetate (KGD) as the main volatile component, were found in the products of Japanese origin whereas chemotype C, with 1-O-acetyl-2,10-bisaboladiene-1,6-diol (ABD), was found in the products of Chinese and Korean origin. The haplotypes were correlated with the chemotypes: haplotype AH-1 for chemotype J1, AH-2 for chemotype J2 and AH-5 for chemotype C, suggesting that the chemical diversity of JV is not attributed to the environmental factors rather to the genetic factors. Since KGD and ABD were reported to have sedative effects and nerve growth factor (NGF)-potentiating effects, respectively, understanding the chemotypes and selecting an appropriate one would be important for the application of JV. The psbA-trnH haplotypes could be useful DNA markers for the quality control and standardization of JV.
Introduction
Valerianae Fauriei Radix (Japanese Valerian; JV) has been defined as the root and rhizome of Valeriana fauriei Briquet (Valerianaceae, which is classified into Caprifoliaceae in Angiosperm phylogeny group (APG) IV) in the Japanese Pharmacopoeia (JP) 18th ed.1) In Japan, the drug was originally used as a domestic alternative to Western Valerian (WV; root of V. officinalis L.), which was introduced to Japan from the West in the 19th century due to its sedative effect.2) Although both JV and WV had been listed in the 2nd ed. and 3rd ed. of JP, only JV is included since 4th ed., mainly owing to its predominant use.2) JV has a sedative effect and is used as a component of various conventional crude drug products (non-Kampo crude drug products). Approximately 20 t of JV are consumed annually in Japan.3) Not only is the V. fauriei from Japan used for JVs, but those imported from China have also been used in recent years to meet the increasing demand for JVs in Japan.3)
Takamura et al. had reported that CH2Cl2 extracts of roots of Valerian from Hokkaido, China, and Europe (botanical origin not shown) prolonged the sleeping time in mice after hexobarbital injection. The order of their pharmacological intensity is as follows: Hokkaido > China > Europe; moreover, a volatile compound, kessyl glycol diacetate (KGD) was identified as an active component in the Hokkaido sample.4) Furthermore, Yoshitomi et al. had compared the sedative effect of two Japanese cultivars of V. fauriei, the original plant of JV, named Kameba-kisso and Hokkai-kisso on the sleeping time after pentobarbital injection and on the spontaneous motor activity in an open field test and reported both of them to have similar sedative effects.5)
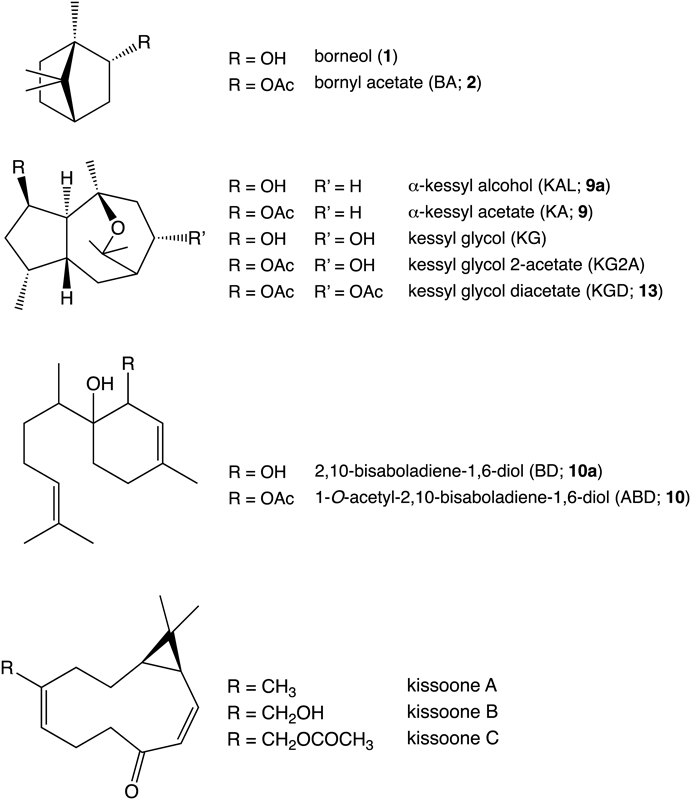
JV is known to have a wide variety of chemical compositions of essential oils. Chemical structures of the principal volatile compounds reported in JV are shown in Fig. 1. Kameba-kisso, used as a raw material for JV in the past, mainly contains α-kessyl acetate (KA)6) while Hokkai-kisso, mainly used as JV now, contains KGD as the main volatile compound.6) Wild plant samples of V. fauriei collected from various places in Japan have also been reported to include KGD, KA and related sesquiterpenes in various ratios.7) However, V. fauriei cultivated in Korea has been reported to contain the other sesquiterpenes, such as 1-O-acetyl-2,10-bisaboladiene-1,6-diol (ABD).8) Relationship between the chemical components and the intensity of the sedative effect found by Takamura et al.4) still remains unclear.
Our previous study on Eurasian medicinal valerian species had revealed that the partial sequences of the psbA-trnH intergenic spacer in chloroplast DNA (psbA-trnH) were useful for the correct identification of V. fauriei and the other Eurasian medicinal valerian species, and that V. fauriei had five different haplotypes (AH-1 to AH-5).9) However, the relationship across the haplotypes and chemical diversity in V. fauriei remains unclear.
The current study aimed to determine the psbA-trnH haplotypes of the commercial JV available in Japanese markets and the herbal specimens of Valeriana plants, including V. fauriei and its cultivars, and analyzed their chemical composition of essential oils by using the GC-MS to clarify the relationship between the psbA-trnH haplotypes and chemical profiles of V. fauriei.
Results and Discussion
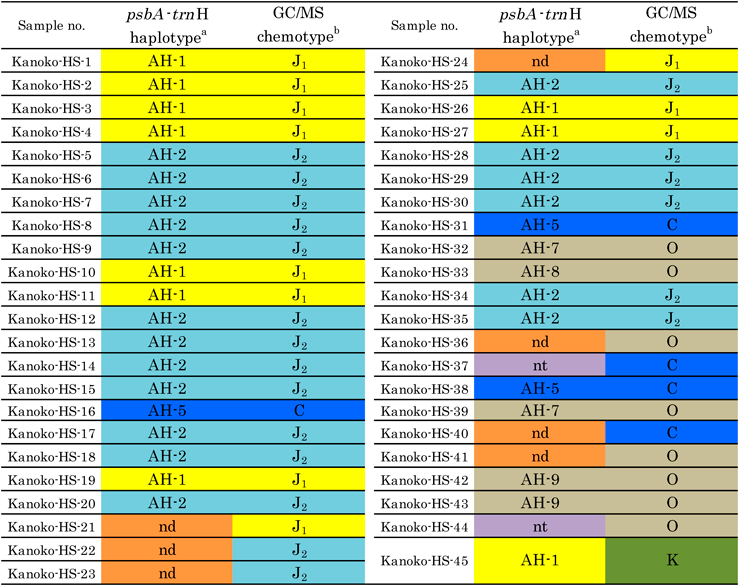
Haplotypes of Commercial Japanese Valerian and Herbal SpecimenAmong a total of 45 samples (Table 1), 43 samples (excluding Kanoko-HS-37 and -44), were subjected to genetic analysis, and the nucleotide sequences of 36 samples were determined. The DNA sequence of each sample was classified into seven haplotypes (AH-1, -2 and AH-5–9, shown in Tables 2, 3). All JV samples of Japanese origin had either AH-1 or AH-2. The JV samples of Chinese origin had AH-5. One of the two JV herbal specimens of JV from Korea showed AH-7 (Kanoko-HS-39), and the haplotype of the other was not determined due to its complex sequence pattern. Haplotypes AH-3 and -4, found in Okayama and Hokkaido wild samples in our previous study, were not observed in the present study since it targeted only Japanese market samples. The results together indicated that JVs available in Japanese markets are derived from V. fauriei, as defined in JP, and that they consist of three haplotypes, AH-1, -2 and -5.
Table 1. Details of Valerian Roots Used in This Study
| Sample No. | Crude drug Namec) | Locality | Collection year | Sample No. | Crude drug Namec) | Locality | Collection year |
|---|
| Kanoko-HS-1a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-24a) | JV | Hokkaido, Japan | 2015 |
| Kanoko-HS-2a) | JV | Hokkaido, Japan | 2018 | Kanoko-HS-25a) | JV | Hokkaido, Japan | 2016 |
| Kanoko-HS-3a) | JV | Hokkaido, Japan | 2017 | Kanoko-HS-26a) | JV | Hokkaido, Japan | 2016 |
| Kanoko-HS-4a) | JV | Hokkaido, Japan | 2016 | Kanoko-HS-27a) | JV | Hokkaido, Japan | 2016 |
| Kanoko-HS-5a) | JV | Ibaraki, Japan | 2015 | Kanoko-HS-28a) | JV | Hokkaido, Japan | 2017 |
| Kanoko-HS-6a) | JV | Aomori, Japan | 2019 | Kanoko-HS-29a) | JV | Hokkaido, Japan | 2018 |
| Kanoko-HS-7a) | JV | Aomori, Japan | 2019 | Kanoko-HS-30a) | JV | Hokkaido, Japan | 2018 |
| Kanoko-HS-8a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-31a) | JV | Liaoning, China | 2016 |
| Kanoko-HS-9a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-32 | JatV | China | Unknown |
| Kanoko-HS-10a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-33 | WV | Unknown | Unknown |
| Kanoko-HS-11a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-34b) | JV | Hokkaido, Japan | 2016 |
| Kanoko-HS-12a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-35b) | JV | Hyogo, Japan | 2017 |
| Kanoko-HS-13a) | JV | Aomori, Japan | 2019 | Kanoko-HS-36b) | JV | Sichuan, China | Unknown |
| Kanoko-HS-14a) | JV | Fukushima, Japan | 2019 | Kanoko-HS-37b) | JV | Liaoning, China | 2015 |
| Kanoko-HS-15a) | JV | Hokkaido, Japan | 2018 | Kanoko-HS-38b) | JV | Liaoning, China | 2015 |
| Kanoko-HS-16a) | JV | Northeast, China | 2018 | Kanoko-HS-39b) | JV | Korea | Unknown |
| Kanoko-HS-17a) | JV | Hokkaido, Japan | 2019 | Kanoko-HS-40b) | JV | Korea | 2012 |
| Kanoko-HS-18a) | JV | Hokkaido, Japan | Unknown | Kanoko-HS-41b) | WV | Sichuan, China | 1986 |
| Kanoko-HS-19a) | JV | Hokkaido, Japan | 2009 | Kanoko-HS-42b) | JatV | Sichuan, China | 2008 |
| Kanoko-HS-20a) | JV | Hokkaido, Japan | 2010 | Kanoko-HS-43b) | JatV | Korea | Unknown |
| Kanoko-HS-21a) | JV | Hokkaido, Japan | 2011 | Kanoko-HS-44b) | Valeriana sp. | Korea | Unknown |
| Kanoko-HS-22a) | JV | Hokkaido, Japan | 2012 | Kanoko-HS-45b) | JV (Kameba-kisso) | Toyama Univ. | Oct. 2013 |
| Kanoko-HS-23a) | JV | Hokkaido, Japan | 2013 |
a) Commercial product in Japan. b) Herbal specimen. c) JV, VJ, and WV are the roots and rhizomes of Valeriana fauriei, V. jatamansi and V. officinalis, respectively.
Table 2. Comparison of the DNA Sequences of
psbA-
trnH Intergenic Spacer for the Identification of Haplotypes Found in This Study
| Nucleotide positiona) |
|---|
Species/samples
(haplotype or accession No.) | 5 | 14 | 32 | 37 | 38 | 39 | 44 | 45 | 51 | 52 | 53 | 55 | 56 | 57 | 58 | 59 | 63 | 65 | 66 | 67 | 68 | 69 | 71 | 72 | 73 | 96 | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 111 | 112 | 113 | 114 | 115 | 116 | 117 | 118 | 226 | 243 | 256 |
|---|
| Valeriana fauriei (AH-1) | T | T | — | A | A | A | A | A | A | A | A | A | T | T | T | C | A | — | — | A | G | C | — | — | T | — | T | T | T | C | T | T | A | T | A | A | T | G | A | A | T | — | C | |
| V. fauriei (AH-2) | . | . | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
| V. fauriei (AH-3) | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
| V. fauriei (AH-4) | . | . | . | — | — | — | . | . | . | . | . | . | . | . | . | C | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
| V. fauriei (AH-5) | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
| V. officinalis (AH-6)b) | . | . | . | — | . | . | . | . | . | C | . | . | C | . | . | C | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
| V. officinalis (LR861814) c) | . | . | . | — | . | . | . | . | . | C | . | . | C | . | . | C | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | xd) |
| KANOKO-HS-32, 39 (AH-7) | . | . | A | — | — | . | T | . | C | . | . | . | . | G | C | C | . | . | — | — | G | A | T | G | G | A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | . | — | . |
| KANOKO-HS-33 (AH-8) | . | . | A | — | — | . | . | . | C | . | . | . | . | G | C | C | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | — | — | — | — | — | — | — | — | . | — | . |
| KANOKO-HS-42, 43 (AH-9) | . | . | A | — | — | . | T | . | C | . | . | . | . | G | C | C | . | . | C | C | . | A | . | — | — | A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | . | — | . |
| V. jatamansi (LR861815)c) | . | . | A | — | — | . | T | . | C | . | . | . | . | G | C | C | . | . | C | C | . | A | . | — | — | A | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | . | — | xd) |
| V. wallrothii (AY794305)c) | A | A | . | — | . | . | . | . | . | C | . | C | C | . | . | C | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . |
| V. dioica (AY794255)c) | . | — | A | A | . | . | . | . | C | . | . | . | . | . | C | C | T | G | — | — | . | . | . | — | — | . | C | . | . | . | . | A | . | — | — | — | — | — | — | — | — | G | T | A |
| V. hardwickii (KT020987)c) | . | . | A | — | — | — | . | G | T | . | T | . | . | G | C | C | . | G | — | — | . | A | . | — | — | . | — | . | . | . | . | . | . | — | — | — | — | — | — | — | — | . | T | . |
| V. fauriei (KJ025054)c) | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | G | — | — | . | . | . | — | — | . | — | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | — | . |
a) Variable site positions are denoted from the 5′ end of the psbA–trnH intergenic spacer. b) Cultivar from Tsukuba Division, Research Center for Medicinal Plant Resources, NIBIOHN and Medicinal Botanic Garden, Health Sciences University of Hokkaido, Japan (ref. 9). c) Data in the International Nucleotide Sequence Database (INSD; DDBJ/EMBL/GenBank) d) No data. Dots (.) and dashes (—) denote nucleotides identical to those in the top row of the table and the alignment gaps, respectively.
Table 3. The
psbA-
trnH Intergenic Spacer Haplotype and Chemotype of Valerian Roots Used in This Study
a) Corresponds to Table 2. b) Corresponds to Fig. 3. nd, not determined; nt, not tested.
In terms of congeners, one WV sample (Kanoko-HS-33) showed AH-8 haplotype and two of three samples of Jatamans Valeriana Rhizome (JatV; Rhizome and root of V. jatamansi Jones in Chinese Pharmacopoeia; Kanoko-HS-42, and -43) indicated AH-9, the third one (Kanoko-HS-32) showing AH-7. Although the botanical origin of AH-9 was presumed to be from V. jatamansi based on its sequence similarity, those of AH-7 and -8 could not be identified owing to the absence of a similar sequence in the International Nucleotide Sequence Database (INSD; DDBJ/EMBL/GenBank).
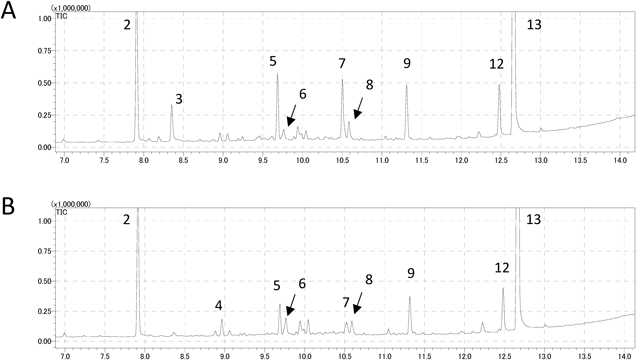
Chemical Profile of Essential Oil in Commercial Japanese Valerian and Herbal SpecimenRepresentative GC-MS chromatograms obtained in this study are shown in Fig. 2. Fifteen peaks were detected over 7–14 min of retention time in all the samples. JV samples of Japanese origin showed peaks 13 and 2 as the main components and peaks 3, 5–9, and 12 as the minor components (Fig. 2-A). Peaks 2 and 13 were identified as bornylacetate (BA) and KGD, respectively based on the comparison of retention times and mass spectra with those of authentic standards. The Chinese and Korean JV samples mainly contained 2 and 10, whereas the Korean samples contained an additional compound 11 (Figs. 2-B, C). Kameba-kisso (Kanoko-HS-45) contained 2 and 9 as the main compounds together with 1, 3, 4, 5, 7–9a, 11 and 12 as the minor ingredients (Fig. 2-D). Peaks 1, 3, and 8 were identified as borneol, α-terpinyl acetate, and valeranone, respectively, using the National Institute of Standard Technology (NIST) mass library search. This chemical profile was well consistent with that reported by Takeuchi et al.7) Peaks 9 and 10 were identified as KA and ABD by comparison with compounds isolated from the roots of Kameba-kisso and Chinese JV, respectively. The retention times and mass spectra of 9a and 10a were identical to those of α-kessyl alcohol (KAL) and 2,10-bisaboladiene-1,6-diol (BD), which were prepared by hydrolysis of KA and ABD, respectively. Kessyl glycol 2-acetate (KG2A) prepared by hydrolysis of KGD showed the same retention time as peak 12, but its mass spectrum did not match with that of the peak; therefore, we could not identify 12. A similar observation had been reported by Suzuki et al.6) The other peaks, 4, 5, 6, 7, 11 were also unidentified due to their small content and no similarity to the data in NIST library. On the other hand, neither WV nor JatV showed the characteristic peaks (Figs. 2-E, F). Kanoko-HS-41 from V. officinalis was collected in 1986, but Takeuchi et al. reported similar result.7) Therefore, we do not think it is attributed to the oldness of the sample.
Multivariate Analysis of GC-MS ProfileTo investigate the similarity of chemical profiles from each sample, GC-MS data were subjected to multivariate analysis, hierarchical cluster analysis (HCA), and principal component analysis (PCA). A dendrogram of the HCA and three-dimensional (3D)-score plot of PCA are shown in Fig. 3. Principal component (PC) 1 to PC3 reflected 72.6% of all data (contribution ratio was 46.2% for PC1, 18.2% for PC2 and 8.2% for PC3) in PCA. In HCA, five clusters were found, namely chemotype K (kameba), C (China), J1, J2 (Japan), and O (others). The chemotype of each sample, together with its psbA-trnH haplotypes is shown in Table 3. Chemotype K (green in Fig. 3) consisted of only one sample, namely Kameba-kisso. Chemotype C (blue) contained all Chinese JV products, herbal specimens, and a Korean specimen, whose main essential oil components were BA and ABD. All JV products and herbal specimens from Japan, except for Kameba-kisso, were classified into either chemotype J1 (yellow; 11 samples) or J2 (light blue; 20 samples). Chemotype O included 8 samples that showed no characteristic peaks. Representative GC-MS chromatograms of chemotypes J1 and J2 are shown in Fig. 4. Although all of the Japanese JV samples contained KGD as the main ingredient, the differences occurred in the content of minor components, such as 3, 4, 5 and 7. Chemotype J1 contained higher amounts of compounds 3, 5 and 7 than chemotype J2, whereas the latter had more of compound 4 than chemotype J1. This was also supported by PCA of the data matrix ranging from 8 to 11 min, where the data derived from major peaks such as BA, ABD and KGD were excluded (Supplementary Fig. S1). The PCA score plot (Supplementary Fig. S1-A) provided a classification similar to that of the PCA of all data, and the loading plot (Supplementary Fig. S1-B) indicated the variables corresponded to peaks 3–5.
As shown in Table 3, psbA-trnH haplotypes were well correlated with chemotypes, except for Kameba-kisso (Kanoko-HS-45); haplotypes AH-1, -2 and -5 corresponded to chemotypes J1, J2, and C, respectively. This suggested that the chemical diversity of essential oils in V. fauriei cannot be attributed to the environmental factors but rather to the genetic factors. Similar observations were reported for intraspecific variation of Perilla frutescens (L.) Britton (Labiatae) and Acorus calamus L. (Araceae/Acoraceae in APG IV)10–13)P. frutescens has various chemotypes and is controlled by a series of multiple alleles such as G, H, R, Fr1, and Fr2 (Supplementary Fig. S2). The presence or absence of allele G regulates the biosynthesis of monoterpenes or phenylpropanoids. As one of the substantial genes, DNA encoding l-limonene synthase was cloned from P. frutescens.12) A. calamus also has multiple chemotypes, containing sesquiterpenes and phenylpropanoids in various ratios, which correlate with the genotypes of the 5S ribosomal DNA (rDNA) spacer region of nuclear DNA.13)
The putative biosynthetic pathway of the sesquiterpenes in Valeriana roots is shown in Supplementary Fig. S3. KGD and ABD are biosynthesized from a common starting substance, farnesyl pyrophosphate (FPP), by different enzymes responsible for cyclization to their carbon skeletons in the initial step.14) In contrast, the biosynthetic route for KGD and KA includes a common cyclase and different enzymes for the oxidation step after the construction of the carbon skeleton. DNA polymorphisms in haplotypes AH-1, -2 and -5 reflect the differences of biosynthetic pathway. Haplotypes AH-1 and -2 linked with guaian-type sesquiterpenes are more similar each other in their DNA sequence than AH-5 linked with bisabolene-type sesquiterpenes, supporting that the chemical diversity of essential oils in V. fauriei is caused by the differences in their genetic background.
As mentioned above, the guaiane-type sesquiterpenes such as KGD are active compounds for sedative effect.4,5) In addition, JV has been reported to exert a nerve growth factor (NGF)-potentiating effect, with BD as an active compound together with three-membered ring sesquiterpenes such as kissoones A–C15,16) (Fig. 1). These compounds are expected to be useful for the medical treatment of dementia. Therefore, selection of an appropriate chemotype depending on the symptoms such as hysteria, irritation and dementia will ensure more effective medical application of JV. As suggested in our previous report, the psbA-trnH intergenic spacer sequence of V. fauriei may be a useful DNA marker for this purpose.
Although we clarified the relationship between the psbA-trnH haplotype and chemotypes in essential oils in this study, Kameba-kisso (Kanoko-HS-45) has haplotype AH-1 despite its chemotype K. The survey on the genetic region linked with the chemical differences between Kameba-kisso and Hokkai-kisso will find more useful DNA marker and reveal the developing process of these two cultivars.
Conclusion
DNA sequence analysis of the psbA-trnH region and GC-MS metabolome of commercial Valerianae Fauriei Radix (Japanese Valerian) revealed the JV products from Japan to have been derived from V. fauriei with three haplotypes, AH-1, -2, and -5, and three chemotypes, J1, J2, and C, respectively. JV products of Japanese origin had either chemotype J1 or J2. They had a common main volatile component, KGD, and different minor components such as α-terpinyl acetate. Chemotype C was found in JV products of Chinese and Korean origin, with BA and ABD as their main components. Each haplotype was correlated with a chemotype, such as haplotype AH-1 for chemotype J1, AH-2 for chemotype J2 and AH-5 for chemotype C, suggesting that the chemical diversity in JV is not attributed to the environmental factors but rather to the genetic factors. Since KGD and ABD were reported to have sedative effect and nerve growth factor (NGF)-potentiating effects, respectively, understanding the chemotypes of JV and selecting an appropriate one for use against individual symptoms would be important. As suggested in our previous report, the psbA-trnH haplotype of V. fauriei may be a useful DNA marker for this purpose.
Experimental
MaterialsA total of 45 commercial JV samples and their related ones were obtained from Japanese crude drug manufacturers. The root sample of Kameba-kisso was prepared from a cultivated plant by the third author, Takao et al. Details of all the samples are shown in Table 1. Among the 38 JV samples, 31 samples were produced in Japan, 5 in China and 2 in Korea. The related crude drug samples consisted of WV and JatV; the latter drug is listed in the Chinese Pharmacopoeia.17) All samples were deposited in the medicinal plant cultivation facility of the National Institute of Health Sciences. KGD was kindly provided by Mr. Kenji Takebayashi of the Toyama Prefectural Institute for Pharmaceutical Research. BA was purchased from Tokyo Chemical Industry (Tokyo, Japan). The other reagents used were of research grade.
Screening for DNA Polymorphisms of psbA-trnHDNA sequence analysis was performed according to our previous report.9)
GC-MS AnalysisAt first, we investigated extraction solvents using methanol, dichloromethane and ether. Although the chromatographic pattern of all solvents is similar in our interest range between 5–22 min, dichloromethane is superior to the others in its handling. Therefore, we selected dichloromethane as extraction solvents.
To 100 mg of powdered samples, 5 mL of dichloromethane was added and shaken at 5 Hz for 20 min. After centrifugation at 1000 × g for 10 min, the supernatant was passed through a membrane filter (0.45 µm) and 1 µL of the filtrate was used for GC-MS.
GC-MS analysis was performed by using GCMS-QP2010Ultra (Shimadzu, Kyoto, Japan) equipped with DB-5MS (30 m length, 0.25 mm ID, 0.25 µm film thickness; Agilent J&W Scientific, Santa Clara, CA, U.S.A.) according to the following conditions: injection temperature, 250 °C; split mode (6 : 1); oven temperature program, 60 °C (0–2 min) –Δ12°C/min–300 °C (22–32 min); carrier gas, helium; flow rate, 36.4 cm/s (linear velocity mode); ionization mode, EI; ionization energy, 1.04 kV. Peak annotation was performed using the NIST Mass Spectral Library or by comparison of retention time and mass spectra with those of authentic samples.
Isolation and Preparation of Volatile CompoundsThe hydrolysis of KGD under mild and strongly alkaline conditions yielded KG2A and kessyl glycol (KG), respectively. KA (9) and ABD (10) were isolated from ether extracts of Kameba-kisso (Kanoko-HS-45) and Chinese JV (Kanoko-HS-31), respectively. KA and ABD were hydrolyzed to obtain α-kessyl alcohol (KAL; 9a) and 2,10-bisaboladiene-1,6-diol (BD; 10a), respectively. The fractionation scheme, hydrolysis conditions, and spectral data are shown in the Supplementary Materials.
Multivariate AnalysisThe GC-MS chromatogram of each sample was aligned and the peak was extracted using Profiling solution (Shimadzu) with ion alignment method to obtain a data matrix for multivariate analysis. HCA and PCA were conducted using SIMCA-14 (Umetrics) with Pareto scaling.
Acknowledgments
This study was supported by a research grant from the Japan Agency for Medical Research and Development, AMED [Grant No. JP17ak0101074], and a Grant-in-Aid for the Cooperative Research Project from the Institute of Natural Medicine, University of Toyama, in 2020 and 2021.
Conflict of Interest
Dr. Takuro Maruyama received research grants from Kobayashi Pharmaceutical Co., Ltd. (Osaka, Japan), Nippon Funmatsu Yakuhin Co., Ltd. (Osaka, Japan), and Tsumura & Co. (Tokyo, Japan)
Supplementary Materials
This article contains supplementary materials.
References
- 1) Ministry of Health, Labour and Welfare, “Japanese Valerian,” 18th ed. The Japanese Pharmacopoeia, 2021, p. 1900.
- 2) Yanagisawa K., Jpn. J. History Pharm., 48, 63–74 (2013).
- 3) Yamamoto Y., Kasahara R., Taira M., Higuchi Y., Yamaguchi Y., Shiratori M., Sasaki Y., Shoyakugaku Zasshi, 75, 89–105 (2021).
- 4) Takamura K., Kakimoto M., Kawaguchi M., Iwasaki T., Yakugaku Zasshi, 93, 599–606 (1973).
- 5) Yoshitomi S., Watanabe M., Kawanishi F., Satake M., Nat. Med., 54, 55–60 (2000).
- 6) Suzuki H., Zhang B. C., Harada M., Iida M., Satake M., Shoyakugaku Zasshi, 47, 305–310 (1993).
- 7) Takeuchi M., Suzuki Y., Suzuki S., Fujino H., Yoshizaki M., Kohda H., Nat. Med., 55, 225–230 (2001).
- 8) Nishiya K., Kimura T., Takeya K., Itokawa H., Phytochemistry, 36, 1547–1548 (1994).
- 9) Fujii T., Mori T., Tatsuo Y., Takao Y., Fujino H., Tsuchida T., Minami M., J. Nat. Med., 75, 699–706 (2021).
- 10) Koezuka Y., Honda G., Tabata M., Phytochemistry, 25, 859–863 (1986).
- 11) Ito M., “Phylogenetic and chemotaxomic study on genus Perilla in Japan (Provisional translation),” Ph. D. thesis, Kyoto University, 1999, ‹https://doi.org/10.11501/3156164›.
- 12) Yuba A., Yazaki K., Tabata M., Honda G., Croteau R., Arch. Biochem. Biophys., 332, 280–287 (1996).
- 13) Sugimoto N., Kiuchi F., Mikage M., Mori M., Mizukami H., Tsuda Y., Biol. Pharm. Bull., 22, 481–485 (1999).
- 14) Cane D. E., Sesquiterpene Biosynthesis: Cyclization Mechanisms, “Comprehensive Natural Products Chemistry,” Vol. 2, ed. by Barton S. D., Nakanishi K., Elesevier, Oxford, U.K., 1999, pp. 155–200.
- 15) Guo Y., Xu J., Li Y., Yamakuni T., Ohizumi Y., Planta Med., 72, 373–375 (2006).
- 16) Guo Y., Xu J., Li Y., Watanabe R., Oshima Y., Yamakuni T., Ohizumi Y., Chem. Pharm. Bull., 54, 123–125 (2006).
- 17) Chinese Pharmacopoeia Commission, Jatamans Valeriana Rhizome, “Pharmacopoeia of the People’s Repulic of China 2015 (English Edition),” Vol. 1, China Medical Science Press, Beijin, China, 2015, p. 474.