2024 Volume 72 Issue 3 Pages 309-310
2024 Volume 72 Issue 3 Pages 309-310
The inhibition mode of a retro-inverso (RI) inhibitor containing a hydroxyethylamine dipeptide isostere against the human T-cell leukemia virus type-1 (HTLV-1) protease was examined. Enzymatic evaluation of the RI-modified inhibitor containing a D-allo-Ile residue revealed that HTLV-1 was competitively inhibited. IC50 values of the RI-modified inhibitor and pepstatin A, a standard inhibitor of aspartic proteases, were nearly equivalent.
Retro-inverso (RI) modification involves a reversion of the amino acid sequence of the target peptide accompanied by replacing each L-amino acid with the corresponding D-amino acid.1) The resulting topography of the RI-modified peptide is expected to retain that of the native L-peptide because of the double conversions, the N- to C-terminal sequence and L to D stereo conversion.2) Thus, RI-modified peptides should interact with their native target using a similar interaction mode as the parent peptide.3,4) However, few applications of RI modification for protease inhibitors have been reported because of the challenges in estimating the necessary conversion of the stereo-structure at the scissile site.
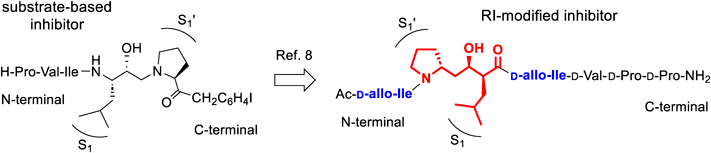
In a previous study on transition state mimic inhibitors of human T-cell leukemia virus type 1 (HTLV-1), which is isolated from patients with adult T-cell leukemia/lymphoma,5) we have demonstrated that simple RI-conversions, including L- to D-amino acid conversions, are not effective in protease inhibitors containing transition state mimics.6,7) The results from these studies showed that a unique RI modification at a specific hydroxyethylamine dipeptide isostere, a key site of the protease inhibitor, is required for the inhibitory activity of the RI-modified inhibitor, which suggests that a specific RI modification is required to retain the topography of the parent inhibitor. Additionally, conversion of the Ile side-chain configuration is required to mimic the total topology of the original inhibitor as well as the RI-conversion of the backbone structure to improve the inhibitory activity8) (Fig. 1). On the basis of these results, in the present study, an inhibition mode of the resulting RI-modified inhibitor was examined to confirm that the RI-modified inhibitor functions as a competitive inhibitor similar to that of the original inhibitor and the affinity of the RI-modified inhibitor toward the protease was comparable to a reference competitive inhibitor.
The docking simulation of the RI-modified inhibitor with HTLV-1 protease, an aspartic protease, indicated that conversion of the side-chain configuration at Ile residues combined with the RI-conversion of the main-chain configuration, including the scissile site configuration, ensured the entire topology of the RI-modified inhibitor was similar to that of the original ligand.8) The results suggested that a combined RI-based conversion of a transition state mimic inhibitor should yield the same mode of inhibition as the original inhibitor. The inhibitory mechanism of the resulting RI-modified inhibitor was further examined by constructing a Lineweaver–Burk plot. The rate of cleavage for different amounts of substrate (H-KGPPVILPIQAP-NH2) [S] by the recombinant HTLV-1 protease in the absence or presence of the RI-modified inhibitor (0, 25, 50, or 100 µM) was monitored during the initial 15-min reaction period using HPLC, as described previously.8,9) The enzymatic reaction rate (v, µM/min) was obtained by monitoring a reduction in the peak area corresponding to the substrate, and the resulting 1/v was plotted against 1/[S]. The plots resulted in four straight lines with the same y-axis intercept reflecting competitive inhibition toward the protease (Fig. 2, each plot obtained at each inhibitor concentration (0, 25, 50, or 100 µM) is shown in Supplementary Fig. S1).

Since an IC50 value of the RI-modified HTLV-1 inhibitor was confirmed as 85 µM in our previous study, the level of inhibitory potency as a competitive inhibitor was evaluated by comparing the IC50 value of the RI-modified HTLV-1 inhibitor with that of the IC50 value of pepstatin A, a well-known competitive inhibitor of aspartic proteases. From the sigmoidal curve of pepstatin A obtained using the same procedure for the RI-modified inhibitor (Supplementary Fig. S2), the IC50 value was estimated to be 60 µM, suggesting the inhibitory activity of the RI-modified inhibitor is comparable to pepstatin A.
Attempt to Estimate the Affinity of the RI-Modified Inhibitor Using Surface Plasmon Resonance (SPR)The IC50 values described above were calculated by monitoring the UV absorbance of the substrate peptide (i.e., peak area) using a conventional HPLC. UV detection of peptides has a sensitivity of approximately micromolar. Thus, surface plasmon resonance (SPR) analysis was used as another possible measure to estimate the affinity of the RI-modified inhibitor because SPR is a reproducible and sensitive technique for measuring molecular interactions in real-time.10,11)
The interaction responses indicate binding at the sub-µM level. However, the kinetic constants for this interaction were not determined because dissociation following the conventional washing step with buffer was barely observed. The experimental details are included in “Experimental” in Supplementary Materials.
We showed that a RI-modified HTLV-1 protease inhibitor that contains a specific transition-mimic structure combined with main- and side-chain RI-conversions is an effective competitive inhibitor. Enzyme kinetic evaluation using Lineweaver–Burk analysis clearly showed that the RI-modified inhibitor is a competitive inhibitor, which is similar to that of pepstatin A. In conventional HPLC using UV detection, IC50 values of the RI-modified inhibitor and pepstatin A were very similar. The SPR results showed that the RI-modified inhibitor displays a similar sensorgram as pepstatin A. These results confirmed that the configuration at the scissile site and RI modification at main- and side-chain configurations are essential for developing this RI-modified protease inhibitor. The D-configuration of the components enhances the stability of RI-modified derivatives, thus making these compounds potentially suitable for physiological digestion. Thus, the results indicate that retro-inverso conversion of a transition state mimic inhibitor is a possible approach to designing protease inhibitors for oral administration.
The authors thank Dr. Kenta Teruya (Tohoku University) for supplying the recombinant HTLV-1 protease.
The authors declare no conflict of interest.
This article contains supplementary materials. Each Lineweaver–Burk plot obtained at each inhibitor concentration, sigmoidal curves for pepstatin A and experimental details describing enzymatic evaluations (procedures for Lineweaver–Burk analysis and SPR measurement) are included therein.