2025 Volume 73 Issue 3 Pages 227-233
2025 Volume 73 Issue 3 Pages 227-233
The wearing of medical gowns during anticancer drug preparation is recommended for the prevention of drug exposure. Non-breathable and breathable gowns (gown− and gown+, respectively) are both available. However, anticancer drugs may permeate “gown+.” In the present study, water, hydrophilic and lipophilic dyes, and aqueous solutions of several model chemicals with different physical properties (pyridoxine, antipyrine, ethyl p-hydroxybenzoate, and butyl p-hydroxybenzoate) were applied to four types of gowns and their chemical permeabilities were measured. The permeability of gowns to vaporized ethanol was also investigated because several volatile anticancer drugs are currently used in the treatment of cancer. The results obtained showed that the hydrophilic chemical, pyridoxine, did not permeate any of the gowns tested. Furthermore, gowns became more permeable as the lipophilicity of chemicals increased. No significant changes were observed in the chemical permeability between “gown−” and “gown+,” suggesting that the protective efficacy of the gowns against permeation by anticancer drugs was similar regardless of breathability. On the contrary, “gown + ” was permeable to vaporized ethanol, whereas “gown−” was not. The present study demonstrates the need for safety measures in lipophilic or volatile anticancer drug handling and the importance of developing medical gowns that are highly resistant to chemical permeation.
Malignant neoplasms (cancer) are one of the three major diseases in Japan, along with heart disease and cerebrovascular disease, and crude mortality and morbidity rates have both shown yearly increases.1) Although surgery used to be the mainstay of treatment, chemotherapy, along with radiation therapy and immunotherapy, is now widely used to treat malignant neoplasms,2–4) and depending on the type of malignant neoplasm, mortality rates have decreased.5) However, anticancer drugs are associated with strong side effects, including carcinogenic, teratogenic, and mutagenic effects.6–8) In addition, some patients develop second cancers years or decades after receiving chemotherapy.8–10) The International Agency for Research on Cancer (IARC) evaluates substances and factors related to chemical carcinogenicity and classifies them into four groups: “Group 1; Carcinogenic to humans,” “Group 2A; Probably carcinogenic to humans,” “Group 2B; Possibly carcinogenic to humans,” and “Group 3; Not classifiable as to its carcinogenicity to humans.” Anticancer drugs classified in Group 1 include busulfan, melphalan, etoposide, and cyclophosphamide. Those in Group 2A include doxorubicin. Anticancer drugs in Group 2B include bleomycin, mitomycin C, dacarbazine, and mitoxantrone. Finally, drugs in Group 3 include fluorouracil, prednimustine, vinblastine, and actinomycin.11) In addition, the National Institute for Occupational Safety and Health (NIOSH) created a peer review plan for the 2019 update of its List of Hazardous Drugs in Healthcare Settings due to the potential workplace exposure of healthcare workers who prepare or administer hazardous drugs (e.g., certain cancer therapies, antivirals, hormone therapies, and bioengineered agents).12)
Anticancer drugs are highly potent and may lead to health hazards not only for patients undergoing treatment, but also for medical staff. Medical staff are exposed to anticancer drugs in many settings, including during drug dispensing and preparation, drug transport and storage, the disposal of leftover drugs, the disposal of patient excrement and vomit, and contact with the drugs given or used by patients, all of which are unavoidable when anticancer drugs are used to treat cancer patients. Pharmacists and nurses working in clinical settings must perform activities that carry a high risk of occupational exposure. Therefore, medical staff wear protective clothing, such as medical gowns, masks, and gloves, to protect against occupational exposure.
A previous study reported that physiological responses, such as heart rate and body temperature, during gown-wearing operations differed depending on the environmental temperature and humidity.13) For example, the risk of physiological responses is known to increase during anticancer drug preparation when gowns are worn. Some medical gowns are breathable, whereas others are not (gown+ and gown−, respectively). Although “gown + ” has been shown to decrease the risk of physiological responses,13) it may be less protective against drug exposure.14,15) In previous studies, the use of polyethylene or vinyl-coated gowns has been recommended.14,15) Therefore, gowns that are both protective against drug exposure and breathable are expected to prevent the risk of both physiological responses and cancer drug exposure in medical staff.
The present study assessed the protective gown’s ability to prevent exposure to anticancer drugs in relation to the issue of healthcare workers being exposed during the preparation of anticancer drugs. Four types of medical gowns (three types of gown− and one of gown+) used in the preparation of anticancer drugs were selected and the permeation of the gowns by water (moisture), hydrophilic and lipophilic dyes, and chemicals of different polarities was assessed. In addition, the permeation and diffusion routes of chemicals were considered in gown penetration experiments.
Anticancer drugs such as cyclophosphamide, ifosfamide, and bendamustine are known to evaporate at room temperature.16–18) Inhalation is a recognized route of exposure to vaporized anticancer drugs.16,17) However, since the preparation of anticancer drugs is typically conducted inside safety cabinets, it is considered unlikely that inhalation occurs during the preparation process. On the contrary, vaporized anticancer drugs may permeate through the gown (“gown + ”) and adhere to clothing or skin, particularly on the arms placed inside the safety cabinet. Therefore, the permeability of gowns to ethanol gas (EtOH gas) was also investigated.
The following medical gowns worn during the preparation of anticancer drugs were obtained from Taketora Holding (Yokohama, Japan), Morain Co. (Tokyo, Japan), and Nikka Micron (Misato, Saitama, Japan): the Ferlack No. 100 gown® (FL gown), DuPon™ Tyvek® Chemotherapy gown (DTC gown), and Chemo light gown® (CL gown), respectively. In addition, the Proshare Half Lamination Gown® (PHL gown) worn during anticancer drug administration was obtained from As One Co. (Osaka, Japan). The FL, CL, and PHL gowns are non-woven fabrics coated with polyethylene (all gown−), whereas the DTC gown does not have a polyethylene coating. The DTC gown is characterized by an ultra-dense polyethylene fiber consisting of many layers of intricately layered ultra-fine long fibers (gown+). Table 1 shows a summary of the properties of these gowns.
| Gown name | Ferlack No. 100 gown® | Chemo light gown® | Proshare Half Lamination gown® |
Chemotherapy gown® |
|---|---|---|---|---|
| Abbreviation | FL gown | CL gown | PHL gown | DTC gown |
| Gown category | Gown− | Gown− | Gown− | Gown+ |
| Gown property | Polyethylene coating on non-woven fabric | Ultra-dense polyethylene fiber | ||
| Gown thickness (mm) | 0.186 ± 0.003* | 0.116 ± 0.005* | 0.123 ± 0.008* | 0.135 ± 0.011* |
| SEM image of the gown surface | 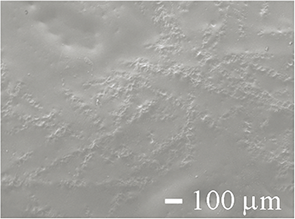 |
 |
 |
 |
| SEM image of the gown back |  |
 |
 |
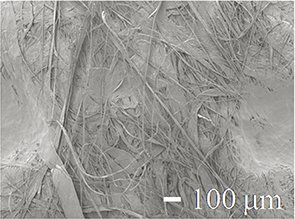 |
| SEM image of a cross-section of the gown |  |
 |
 |
 |
*Each value represents the mean ± standard deviation (S.D.) (n = 10).
The hydrophilic dye Patent Blue (PB) and the lipophilic dye Sudan II (SII) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan) and Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), respectively, and were used in visual measurements of the penetration of chemicals into gowns. Pyridoxine (vitamin B6; VB6) was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.), antipyrine (ANP) from FUJIFILM Wako Pure Chemical Corporation, and 4-hydroxybenzoic acid ethyl ester (ethyl paraben; EP) and 4-hydroxybenzoic acid butyl ester (butyl paraben; BP) from Tokyo Chemical Industry Co. Ltd., and the permeability of each gown to these chemicals was assessed. Kanto Chemical Co., Inc. (Tokyo, Japan) supplied 99.5% EtOH. Other reagents and solvents were of HPLC quality or special grade and were used without purification. Table 2a shows the molecular weight and c logP (a measure of polarity) of the model chemicals used to evaluate gown permeability.
| (a) Model Chemicals | |||
|---|---|---|---|
| Model drug | Abbreviation | M.W. | c logP* |
| Patent blue | PB | 566.7 | −4.97 |
| Sudan II | SII | 276.3 | 6.12 |
| Water | H2O | 18.0 | – |
| Ethanol gas | EtOH (g) | 46.1 | – |
| Pyridoxin | VB6 | 169.2 | −0.35 |
| Antipyrine | ANP | 188.2 | 0.20 |
| 4-Hydroxybenzoic acid ethyl ester | EP | 166.1 | 2.51 |
| 4-Hydroxybenzoic acid butyl ester | BP | 194.2 | 3.57 |
| (b) Representative Anticancer Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Anticancer drug | M.W. | c logP* | IARC classification*** | Anti-cancer drug | M.W. | c logP * | IARC classification*** |
| Levofolinate calcium | 601.58 | −10.498 | – | Prednimustine | 272.69 | 0.475 | 3 |
| Bleomycin | 1415.55 | −7.676 | 2B | Dacarbazine | 182.18 | 0.477 | 2B |
| Goserelin | 1269.43 | −2.880 | – | Cyclophosphamide | 279.10 | 0.803 | 1 |
| Arsenic trioxide | 197.84 | −2.603 | – | Ifosfamide | 261.09 | 0.923 | – |
| Cytarabine | 243.22 | −2.195 | – | Eribulin | 729.90 | 1.235 | – |
| Oxaliplatin | 397.29 | −1.600** | – | Pirarubicin | 627.64 | 1.756 | – |
| Mitomycin C | 334.33 | −1.352 | 2B | Mitoxantrone | 444.48 | 2.299 | 2B |
| Leuprorelin | 1269.45 | −0.993 | – | Irinotecan | 677.19 | 2.428 | – |
| Gemcitabine | 263.20 | −0.712 | – | Degarelix | 1632.30 | 2.750 | – |
| Busulfan | 246.30 | −0.529 | 1 | Vindesine | 753.93 | 3.422 | – |
| Fluorouracil | 130.08 | −0.577 | 3 | Aclarubicin | 811.88 | 3.425 | – |
| Methotrexate | 454.44 | −0.529 | 3 | Vincristine | 824.96 | 4.037 | – |
| Pemetrexed | 427.41 | −0.217 | – | Docetaxel | 807.89 | 4.080 | – |
| Melphalan | 305.20 | −0.207 | 1 | Paclitaxel | 853.91 | 4.730 | – |
| Etoposide | 588.56 | −0.112 | 1 | Vinblastine | 810.96 | 5.230 | 3 |
| Amrubicin | 483.46 | 0.031 | – | Cabazitaxel | 894.01 | 5.440 | – |
| Doxorubicin | 543.52 | 0.317 | 2A | Vinorelbine | 778.93 | 5.730 | – |
| Nogitecan | 421.45 | 0.430 | – | Actinomycin | 1255.42 | 8.048 | 3 |
*c logP was calculated by Chem Draw Professional. **The n-octanol/water partition coefficient of oxaliplatin at 25°C from the interview form of elplat® in Japan was shown instead of c logP. ***“Group 1; Carcinogenic to humans,” “Group 2A; Probably carcinogenic to humans,” “Group 2B; Possibly carcinogenic to humans,” and “Group 3; Not classifiable as to its carcinogenicity to humans.”
The surface, back, and cross-sectional structures of the four gowns were gold-coated and then observed using SEM (S-3000N; Jeol, Tokyo, Japan).
Measurement of Dye and Moisture Absorption into Medical GownsBy applying the hydrophilic dye PB and the lipophilic dye SII to the gown, it can be inferred that if the gown becomes stained with the dye, the dye has permeated through it. In other words, if the gown is not stained with the hydrophilic dye PB but is stained with SII, it can be concluded that the gown does not allow the permeation of hydrophilic chemicals. Dye and water (moisture) absorption experiments were conducted on the gowns using a vertical diffusion cell, as shown in Fig. 1a. In the penetration experiment of the hydrophilic dye PB and the lipophilic dye SII, one of the four gown sheets was initially set in a vertical diffusion cell (effective permeation area of 3.14 cm2) with cyanoacrylate adhesive. The gown was set in the diffusion cell with the surface or back surface on the chemical application side (donor side), and 2.0 mL of 0.2% PB aqueous solution or 0.2% SII soybean oil was applied to the donor side. No solvent was applied to the receiver side, which was left empty. After 8 h, the dye solution was removed and the presence or absence of staining on the surface and back surface of the gown was evaluated as no staining (−), slight staining (+), or staining (++).

(a) Gown permeation experiments on dye and moisture absorption, (b) model chemicals, and (c) vaporized and permeated EtOH.
When purified water is applied and the gown membrane contains purified water, it is considered that the chemicals are also incorporated into the gown membrane (permeated) along with the purified water. In the water (moisture) absorption experiment, four different gown sheets were set in a vertical diffusion cell with the surface and back surface of the sheet on the donor side, and 2.0 mL of purified water was applied to the donor side (Fig. 1a). No solvent was applied to the receiver side, which was left empty. After 8 h, purified water was removed from the surface, and the amount of water absorbed into the gown sheet was evaluated by a gravimetric measurement (XS205DU, Mettler Toledo, Tokyo, Japan). The application time of the dye and purified water were set assuming that the gown would be used for 8 h, which corresponds to the standard working hours in Japan.
Gown Permeation Experiment on Model Chemicals19–21)In the gown permeation experiment on model chemicals, gown sheets were set in a similar vertical diffusion cell using cyanoacrylate adhesive (Fig. 1b). The back surface of the gown was set in the receiver cell. Phosphate-buffered saline (pH 7.4, PBS, maximum volume: approx. 15 mL) was added to the receiver cell. Gowns were not pretreated for hydration, and permeation experiments were initiated by adding 2.0 mL of each model chemical (VB6; 100 mg/mL, ANP; 10 mg/mL, EP; 700 µg/mL, BP; 100 µg/mL) dissolved in PBS in the donor cell. The receiver solution was stirred with a magnetic stirrer and heated to 37°C. Receiver liquid samples (1.0 mL) were collected periodically, and the same amount of PBS was returned to maintain the sink condition of the receiver solutions.
Gown Permeation Experiment on Vaporized EthanolIn the vaporized EtOH permeation experiment, each gown was set in the same vertical diffusion cell as described above (Fig. 1c). A total of 10 mL of 10% EtOH solution was applied to the bottom of the vertical diffusion cell (maximum volume approximately 15 mL) and heated to 37°C. The gas concentration of vaporized EtOH permeating upwards (approximately 8.5 mL in volume) was periodically measured using XP-3160 (New Cosmos Electric Co., Osaka, Japan).
Analytical Method for Each ChemicalVB6, ANP, EP, and BP concentrations in samples were measured using HPLC (Shimadzu Co., Kyoto, Japan). The column temperature was maintained at 40°C and the injection volume was 20 µL. The column used to assess the concentration of VB6 was Inertsil® NH2, 5 µm 4.6 × 250 mm (GL Sciences, Tokyo, Japan) and the mobile phase was acetonitrile: 0.1% phosphoric acid solution = 50 : 50. The flow rate was 1.0 mL/min and the measurement wavelength was 290 nm. The column used to measure ANP, EP, and BP concentrations was TSKgel ODS-80Ts QA, 5 µm 4.6 × 250 mm (Tosoh, Tokyo, Japan). The mobile phase for ANP was acetonitrile: 0.1% phosphoric acid solution = 30 : 70 containing tetrabutylammonium hydrogen sulfate at a concentration of 5 mM. The mobile phase for EP was acetonitrile: 0.1% aqueous phosphoric acid = 45 : 55. The mobile phase for BP was acetonitrile: 0.1% aqueous phosphoric acid = 50 : 50. Flow rates and measurement wavelengths were 1.0 mL/min and 290 nm for ANP, 1.2 mL/min and 260 nm for EP, and 1.0 mL/min and 260 nm for BP.
Table 1 shows SEM photographs of the surface, back surface, and cross-sectional structure of each medical gown. The surface, back surface, and cross-sectional structures of the FL, CL, and PHL gowns (gown−) were very similar. Every “gown−” surface had a polyethylene coating, and no pores were observed from the surface. The back surfaces of each “gown−” comprised fibers and were crimped to prevent unraveling, and two layers (a polyethylene coating layer and fiber layer) were noted in cross-sections. On the contrary, the surface of the DTC gown (gown+) numerous pores was observed on the surface. The reverse side of the DTC gown consisted of fibers, whereas the cross-section showed a very thin coated or crimped layer and a fiber layer. The fibers of the DTC gown (gown+) were finer and denser than those of the FL, CL, and PHL gowns (gown−).
Table 3 shows the extent of staining on the gown surface or back surface when the hydrophilic and lipophilic dyes, PB and SII, respectively, were applied. After the application of PB to the gown surface for 8 h, no staining was observed on the FL or CL gowns (“gown−”), whereas slight staining was noted on the PHL gown (“gown−”). Regarding the DTC gown (gown+), staining was observed on the surface of the PB-applied side, but not on the opposite side (back surface). After the application of PB to the back surface of the gown for 8 h, slight staining was detected on the FL and CL gowns (gown−). The PHL gown (gown−) showed clear staining. The DTC gown (gown+) showed staining on the surface (back) of the PB-applied side, but not on the opposite side (surface). Furthermore, when SII was applied to the gown surface for 8 h, slight staining was noted on the FL, CL, and PHL gowns (gown−). When SII was applied to the surface and back surface of the DTC gown (gown+), clear staining was detected in both cases.
| Applied dye and gown surface | Observed gown surface | FL gown | CL gown | PHL gown | DTC gown |
|---|---|---|---|---|---|
| PB application on the surface | Observation from the surface | — | — | +* | ++ |
| Observation from the back surface | — | — | +* | — | |
| PB application on the back surface | Observation from the surface | +* | +* | ++* | — |
| Observation from the back surface | +* | +* | ++* | ++ | |
| SII application on the surface | Observation from the surface | +* | +* | +* | ++ |
| Observation from the back surface | +* | +* | +* | ++ | |
| SII application on the back surface | Observation from the surface | ++* | +* | ++* | ++ |
| Observation from the back surface | ++* | +* | ++* | ++ |
*Dyed fibers were observed in the gown because the coated polyethylene layer was transparent.
Figure 2 shows the amount of water absorbed by the gown. The amount of water absorbed when purified water was applied to the surfaces of the FL, CL, and PHL gowns (gown−) was negligible (0.004, 0.011, and 0.016 mg/cm2, respectively). On the contrary, when purified water was applied to the back surfaces of the FL, CL, and PHL gowns (“gown−”), water absorption was higher than when it was applied to their surfaces (0.786, 0.042, and 0.032 mg/cm2, respectively). The amount of water absorbed from the back surface of the FL gown was 185-fold higher than that from the surface. On the contrary, no significant difference was observed in water absorption between the surface and back surface of the DTC gown (gown+). Water absorption from the surface of the DTC gown was slightly higher than that from the surfaces of the FL, CL, and PHL gowns.

■ and □: Purified water was applied to the surface and back surface. Data are shown as mean ± S.D. (n = 3).
Figure 3 shows the time course of the cumulative amount of each chemical that permeated through the FL, DTC, CL, and PHL gowns. Values were shown as %/cm2 normalized to the applied drug concentration. The hydrophilic chemical VB6 did not penetrate any gown (Fig. 3a). However, model chemicals with a c log P value ≥0.2 penetrated all gowns. The FL gown was slightly more permeable to the model chemicals than the other gowns. Permeability to the model chemicals did not significantly differ among the CL, PHL, and DPT gowns (Figs. 3b–3d).

Data were normalized by the amounts of model chemicals applied. (a) VB6, (b) ANP, (c) EP, (d) BP. ●: FL gown, ▲: CL gown, ■: PHL gown, and ○: DTC gown. Each data point represents the mean ± S.D. (n = 3). *N.D.: not detected.
Figure 4 shows the relationship between the c logP of the model chemicals and normalized data on the cumulative amount of permeation (%/cm2) through each gown after 8 h. All gowns became more permeable as the lipophilicity of chemicals increased. Permeability to BP, which is highly lipophilic, was the highest for the FL gown. On the contrary, no significant difference was observed in BP permeability among the DTC, CL, and PHL gowns.

Data were normalized by the amount of model chemicals applied. ●: FL gown, ▲: CL gown, ■: PHL gown, ○: DTC gown. Each data point represents the mean ± S.D. (n = 3). *N.D.: not detected.
Figure 5 shows the permeability of vaporized EtOH through the FL, CL, PHL, and DTC gowns. A permeant may be in a gas, liquid, or gel form in membrane permeation experiments using diffusion cells.19) In the present study, the membrane permeation experiment using gas molecules was performed by assuming that molecules diffuse according to a pressure gradient. The permeation of vaporized EtOH was not detected in the non-breathable FL, CL, and PHL gowns. On the contrary, the DTC gown was permeated by vaporized EtOH 1 min after the application of 10% EtOH and a constant value was noted from 45 min. This permeation behavior indicates that gas molecules permeate a membrane faster than molecules in liquids and that the permeation behavior of chemicals from gases and liquids differs.

●: FL gown, ▲: CL gown, ■: PHL gown, ○: DTC gown. Each data point represents the mean ± S.D. (n = 3). *N.D.: not detected.
The rate (amount) of chemical permeation through a membrane by simple diffusion generally depends on the molecular weight and polarity of the applied (exposed) chemical.22–25) Table 2b shows the molecular weights and c logP of anticancer drugs commonly used in clinical practice. The molecular weights and c logP ranges of representative anticancer drugs range between 130.08 and 1632.30 and between −10.50 and 8.05, respectively. Since the membrane permeation of drugs by simple diffusion depends on the physicochemical properties of the drug as described above,22–25) the present results obtained from the gown permeability evaluation using model chemicals may be used to estimate the gown permeability of anticancer drugs. Regarding the model chemicals tested, generic c log P values between −0.77 and 3.57 were selected, which represent the center of the c logP range for generic anticancer drugs (Table 2a). Drugs with c log P values higher than 3.57 were not selected because of their extremely low solubility in water, which leads to difficulties in making aqueous solutions.
Figures 3 and 4 show that VB6, a hydrophilic chemical, did not penetrate any gowns. Therefore, even if an aqueous solution of a hydrophilic anticancer drug comes into contact with a polyethylene-coated gown or a gown made of ultradense polyethylene fibers, it will not penetrate the gown. However, the present results showed that gowns became more permeable as the lipophilicity of chemicals increased, which indicates that the gown permeation route of the model chemicals from aqueous solutions was not through the pores between the fibers, where water and other liquids penetrate, but via the hydrophobic regions of the fibers. If the permeation route is through pores that are permeated by water or other substances, the model chemicals are expected to have the same permeation coefficient regardless of their polarity. In other words, the route of permeation of the chemicals is the gown itself. This suggests that the permeation route of chemicals is in the hydrophobic region of a gown and that permeation behavior may be expressed by Fick’s diffusion equation, which is commonly used to assess membrane rates. The result showing that gowns became more permeable as the lipophilicity of chemicals increased was supported by negligible water (moisture) permeation. In other words, the permeation of water (moisture) (not only water, but also hydrophilic chemicals) through the surface did not occur in “gown−,” which did not have small pores through which air may pass. In addition, when the hydrophilic dye PB was applied to the DTC gown, it did not penetrate to the opposite side of the dye-applied surface (Table 3). These results suggest that even the DTC gown (gown+) with small pores prevented the permeation of very highly hydrophilic chemicals. In addition, since moisture did not permeate the gown, it may have accumulated inside the gown. In the FL, CL, and PHL gowns (gown−), gown permeability increased for more lipophilic chemicals. The weight change in gowns was negligible after the application of purified water to the gown surface. These results indicate that water infiltration into the gown was negligible due to the pore-free polyethylene coating on the gown surface. Furthermore, gowns became more permeable as the lipophilicity of chemicals increased, similar to the DTC gown (gown+). The hydrophilic dye did not penetrate to the reverse side, whereas the lipophilic dye penetrated to the back surface. This result supports the lipophilic portion of the gown being the primary route for chemical permeation. Since the gown permeability of lipophilic chemicals is high, it is desirable to prepare hydrophilic anticancer drugs first and lipophilic anticancer drugs last in any gown in order to limit anticancer drug exposure. Furthermore, it is important to frequently change into a new gown when preparing lipophilic anticancer drugs for a long time. The protective ability of the DTC gown (gown+) against anticancer drugs did not markedly differ from those of polyethylene-coated gowns (gown−) (Fig. 4). Since the DTC gown (gown+) has been shown to prevent physiological responses, even under high-temperature conditions,13) the use of “gown + ” with good breathability is useful for drug preparation under these conditions, such as in summer.
The present results showed that vaporized EtOH immediately permeated the DTC gown and that the gown permeation of gas molecules was related to the presence of pores in the gowns (Fig. 5). The permeation of water molecules is prevented when fiber pores are water-repellent, whereas that of gas molecules is not. Gas molecules permeate via a pressure gradient. In addition, it was considered that EtOH could permeate due to the airflow inside the safety cabinet. Vaporized chemicals may permeate through the gown and adhere to areas such as the arms placed inside the safety cabinet.
Therefore, the DTC gown may not be suitable for the preparation of volatile anticancer drugs. In other words, when preparing anticancer agents, such as cyclophosphamide, ifosfamide, and bendamustine,16–18) which are known to vaporize at room temperature, polyethylene-coated “gowns–,” such as the FL, CL, and PHL gowns, are suitable. When wearing a DTC gown, it is desirable to use a Closed-System Drug Transfer Device (CSTD)26–29) that prevents the dispersal or leakage of liquids or volatile organic compounds.
In this study, regarding the model chemicals tested, generic c log P values between −0.77 and 3.57 and small molecular weight were selected. For anticancer drugs with a large molecular weight, the permeability may be lower based on the results of this test. In addition, the model chemicals are dissolved in purified water for application. In actual formulations, various additives such as EtOH and surfactants are included in the base formulation.30) Since EtOH and surfactants are known to affect membrane permeability,31,32) it is believed that future tests should also examine the impact of additives on the gown permeation of chemicals.
The present study suggests that a detailed understanding of gown characteristics and the physicochemical properties of anticancer agents is needed before drug preparation to protect healthcare workers from anticancer drug exposure. In addition, as a common point for all gowns, the exposure of medical staff to anticancer drugs may be prevented by creating firm operation manuals, such as the preparation of hydrophilic anticancer drugs first and changing gowns after preparing highly lipophilic anticancer drugs. The use of CSTD is recommended when preparing volatile organic compounds, which prevents the dispersal and leakage of liquids or volatile organic compounds. The use of non-breathable gowns for the preparation of anticancer drugs will help prevent exposure.
It is also important to collect detailed information, such as the classification by the IARC, regarding substances with and without carcinogenicity to humans, in order to protect healthcare workers from exposure to antineoplastic drugs.
Collectively, the present results suggest that continued safety efforts are needed in the preparation of lipophilic or volatile organic compounds as well as in the development of medical gowns that are virtually impervious to chemicals.
The authors declare no conflict of interest.