2014 Volume 20 Issue 3 Pages 563-569
2014 Volume 20 Issue 3 Pages 563-569
The article demonstrates a method of simultaneous determination for four pesticide residues (phoxim, chlorpyrifos, imidacloprid and chlorantraniliprole) in bamboo shoot using quick, easy, cheap, effective, rugged and safe (QuEChERS)-matrix solid phase dispersion (MSPD) cleanup and liquid chromatography-mass/mass spectrometry (LC-MS/MS). Bamboo shoot sample (5.0 g) was extracted with 20 mL acetonitrile and cleanup with 2.0 g dispersive primary secondary amine (PSA). The results showed the developed QuEChERS-MSPD-LC-MS/MS method is simple, rapid and effective. Average recoveries ranged between 87.5% and 107.2% with RSD values from 5.2% to 12.4% at two concentration levels (20 and 200 μg/kg). The method limit of detection (LOD) below the regulatory maximum residue limits for the pesticides were achieved. The sample preparation time is only 30 min, which is faster than the application of the traditional standard method (at least 400 min). In addition, the new developed method is more environmental-friendly due to the less solvent consumption.
Bamboo shoot is an important non-timber forest product (NTFP) in the edible forest foods of Asia areas, and is also exported in large quantities to Japan, EU and USA. It is rich in nutrients, and its major components are carbohydrate, protein, lipid and dietary fiber (Chang et al., 2013). Other functional components include vitamins, amino acids and trace elements (Singhal et al., 2013). Currently, it is conventional practice for bamboo shoot growers to apply pesticides to control diseases and pests. The presence of pesticide residues in bamboo shoot is one possible risk source for consumers, due to their possible long adverse health effects (Eddleston 2013; Hernandez et al., 2013). So it is necessary to monitor and control residual levels in bamboo shoot in order to meet regulatory requirements and protect the consumers. Bamboo shoot is planted in forestland and suffers little effect from other plants (different from the agricultural products in farmland). So the kinds of pesticide residues in bamboo shoot could be concluded from the cultivation procedure. Phoxim and chlorpyrifos are traditional insecticides for insect control in bamboo shoot; however, the application of imidacloprid and chlorantraniliprole are developed fast in last few years. In this context, it is important to note that there are many published papers about analytical methodologies for different kinds of pesticide residues determination in vegetables (Arienzo et al., 2013), fruits (Banerjee et al., 2013; Malhat et al., 2013) and animal muscles (Park et al., 2013). In contrast, scientific reports about sample preparation or development of analytical methodologies for pesticide residues quantification in bamboo shoot are scarce (Guo et al., 2011).
The practical needs for an appropriate pesticide control are mainly focused on simple and fast sample treatment methods that may be easily implemented in routine laboratories. The QuEChERS (quick, easy, cheap, effective, rugged and safe) method is well known for its applicability in simultaneous analysis of a large number of pesticides in a variety of matrices, and has received a worldwide acceptance because of its simplicity and high throughput (Miao et al., 2013). This method is based on an acetonitrile extraction and an induced partition by addition of anhydrous MgSO4 and NaCl. Traditionally, a commercial solid-phase extraction (SPE) column is often used for the next clean-up step to remove the co-extracts. The QuEChERS-SPE method has been applied for many kinds of pesticides in different matrices (Forsberg et al., 2011; Kowalski et al., 2011).
In our previous paper (Ding et al., 2013), a method based on SPE and LC-MS/MS (liquid chromatography-tandem mass spectrometry) for seven pesticides residues determination in bamboo shoot was developed. The sample was extracted with acetonitrile. However, the commercial SPE column is high cost and the matrix effect still exists. Although the matrix-matched calibration method was used to avoid matrix effect with certain matrixes, some problems occurred in the large scale samples analysis. When the samples came from different regions, the matrixes were also variable, which weakened the accuracy and precision of the results (due to the only matrix-matched calibration). There is impossible to build many calibrations to fit different samples, especially in real samples analysis. In addition, the previous procedure had a bit large of sample amount (25 g sample) and high solvent consuming (at least 60 mL acetonitrile), many co-extracts were also extracted and might decrease the chromatography stability and sensitivity. Therefore, a new environment-friendly method should be developed to overcome the matrix effect. And the pesticide residues in bamboo shoot could be determined by standard calibration.
Recently, matrix solid-phase dispersion (MSPD) has been found, in many cases, to provide equivalent or superior results to older official methods conducted by more classical countercurrent extraction and/or SPE techniques (Sobhanzadeh et al., 2011; Villaverde et al., 2012). MSPD achieves sample homogenization, extraction, and cleanup simultaneously by using a relatively small sample size, low solvent consumption, and a minimum amount of solid phase. MSPD has been shown to be applicable to pesticide residue analysis of oil (Liu et al., 2013), onion (Rodrigues et al., 2010), and milk (Mu et al., 2012), etc. Further, it has been rather consistently observed that MSPD requires approximately 95% less solvent and can be performed in 90% less time when compared to such classical methods. The use of smaller sample sizes, combined with lower solvent consumption, purchase and disposal, which make MSPD competitive with such methods on several levels and should be considered as an alternative when developing new analytical protocols. The aim of this work is to develop and validate a QuEChERS-MSPD method for the simultaneous determination phoxim, chlorpyrifos, imidacloprid and chlorantraniliprole in bamboo shoot with LC-MS/MS.
Reagents and solutions HPLC-grade acetonitrile and formic acid were obtained from Merck (Darmstadt, Germany). Phoxim, chlorpyrifos, imidacloprid and chlorantraniliprole were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The stock solutions (200 mg/L) of four insecticides were prepared in acetonitrile. Six mixed working standard solutions (5, 10, 100, 200, 500 and 1000 (μg/L) were prepared by diluting stock standard solution with acetonitrile.
A Milli-Q-Plus ultrapure water system from Millipore (Milford, MA, USA) was used throughout the study to obtain the HPLC- grade water used during the analyses. Other solvents were from Shanghai Sanying Chemical Reagents (Shanghai, China), pesticide residue analysis quality. Primary secondary amine (PSA) was from Agela (Agela, USA). Graphitized carbon black (GCB) was from Sinopharm Chemical Reagents (Shanghai, China).
LC Conditions The LC system consisted of a High performance liquid chromatography (Waters, Milford, MA). Chromatographic separations of pesticides were performed on a HSS T3 column (5 μ, 100 mm × 2.1 mm, i.d., Waters). A gradient program was used with mobile phase, consisting of solvent A (0.1% formic acid +5 mmol/L ammonium acetate, in water) and solvent B (acetonitrile) as follows: 80:20 A:B (initial), 10 – 90% A with 90 – 10% B (0 – 5 min), 10:90 A:B (5 – 10 min), 10 – 80% A with 90 – 20% B (10 – 14 min), 80:20 A:B (14 – 15 min). A subsequent re-requilibration time (3 min) was performed before next injection. The flow rate was 0.3 mL/min, the injection volume was 10 μL, and the column and sample temperatures were maintained at 35°C.
MS/MS conditions MS/MS was performed on a Waters Quattro Premier triple-quadruple mass spectrometer equipped with an ESI source (Waters, Milford, MA). The parameters used for the mass spectrometry under the ESI+ mode were as follows: capillary voltage 3.50 kV, cone voltage 45 V, source block temperature 80°C, cone gas 50 L/h, desolvation temperature 450°C, desolvation gas (nitrogen gas) 550 L/h. The parameters of the m/z and collision energy of precursor ions and quantitative product ions from four pesticides are shown in Table 1.
Sample extraction by QuEChERS A portion (5.0 g) of prehomogenized sample was weighed in a 50 mL plastic centrifuge tube. The sample was extracted one time with 20 mL of acetonitrile by homogenization with the high speed blender (Ultra-Turrax T18, IKA, Germany) for 2 min. After the addition of 3 g MgSO4 and 2 g NaCl, each mixture was shaken intensively for 1 min and centrifuged for 2 min at 8000 rpm. An aliquot of the organic phase (10.0 mL) was transferred and concentrated by a rotatory evaporator to about 1 mL at 40°C.
Sample clean-up by MSPD The extracting solution was transferred to the mortar, where it was gently blended and homogenized together with dispersant until obtaining a fine powder. This mixture was then transferred to a commercially available minicolumn. The minicolumn was connected to a vacuum system for solid phase extraction adjusting the flow to 1 mL/min. The elution step was carried out with 5 mL of eluting solution. The final extract was evaporated to dryness by nitrogen, then was dissolved in 0.5 mL acetonitrile. Prior to analysis, the obtained extract was filtered through a 0.22 μm PTFE filter (Milford, MA, USA).
| pesticide | precursor ion (m/z) | quantitative product ion (m/z) | collision energy (eV) | confirmation product ion (m/z) | collision energy (eV) |
|---|---|---|---|---|---|
| phoxim | 298.9 | 129.0 | 11 | 76.8 | 29 |
| chlorpyrifos | 349.5 | 96.5 | 27 | 197.8 | 20 |
| imidacloprid | 255.9 | 209.0 | 17 | 175.0 | 16 |
| chlorantraniliprole | 483.6 | 453.0 | 15 | 286.0 | 18 |
Evaluation of matrix effect Matrix effect (%ME) is that the measurement of an analyte concentration or mass is influenced by one or more undetected components from the sample. In order to evaluate the influence of matrix components in the detector response, two sets of standard solutions containing the four pesticides at three concentration levels of 10, 100 and 1000 μg/L were prepared. The first series was obtained by diluting the working standard solutions in simple solvent solutions (triplicate). The second was prepared by diluting the same working standard solutions in extracts of the matrix (triplicate) obtained from pesticide-free samples. The evaluation of the influence of co-extracts on chromatographic responses of pesticides was performed using the following equation:
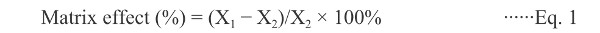 |
where X1 is the average of the areas of analytical solution of each pesticide prepared in matrix extract and X2 is the average of the areas of the solutions of these pesticides prepared in simple solvent solutions.
Method optimization All data were the mean recoveries of triplicate analyzing. Two parameters, including sorbent material and eluting solution were optimized to produce the best results for pesticides analysis. The comparison experiments were carried out using bamboo shoot samples spiked with pesticides at 100 μg/kg.
Comparison with the traditional standard method Unfortunately, there are not any existing standard methods that could be applied for the selected pesticides analysis. The selected four pesticides should be analyzed by different standard methods. The process was depicted simply as following.
Phoxim and chlorpyrifos analysis. Bamboo shoot sample (25.0 g) was extracted with 50 mL of acetonitrile by homogenization with the high speed blender for 2 min. The filtrate was collected into 100 mL mixing cylinder with stopper (7 g NaCl included). The mixture was shaken by hand for 1 min. After 30 min, 10 mL of the top layer (acetonitrile) was transferred and then concentrated to nearly dry by nitrogen. The final extract was dissolved in 5 mL acetone. Prior to analysis (GC-FPD), the obtained extract was filtered through a 0.22 μm PTFE filter.
Imidacloprid analysis. The method was based on liquid-liquid extraction (LLE). Bamboo shoot sample (20.0 g) was transferred into a 250 mL flat conical flask followed by 100 mL methanol. After 30 min, the mixture was extracted by homogenization with the high speed blender for 2 min. The filtrate was collected and the residue was extracted by 80 mL methanol for homogenization once again. All the filtrates were transferred into a 500 mL separatory funnel (100 mL 5% NaCl solution included) and extracted with 50, 50 mL petroleum ether. The bottom layer (water) was collected. After addition of 0.5 mL HCl, the mixture was extracted with 40, 30, 20 mL dichloromethane. The organic phase was collected and then concentrated to about 2 mL at 40°C. Finally, the extract was concentrated nearly to dry by nitrogen and a SPE column was applied for the purification process. The final extract was dissolved in 10 mL methanol. Prior to analysis (HPLC), the obtained extract was filtered through a 0.22 μm PTFE filter.
Chlorantraniliprole analysis. Because chlorantraniliprole is a new type insecticide, it has not been included in any standard methods. We used the same extraction method as phoxim analysis in this comparison study.
QuEChERS extraction As shown in Table 2, the extraction efficiencies of QuEChERS for the four pesticides were from 58.5% to 86.1%. However, during the concentration process, some white particles were precipitated out, which might be the co-extracts of polysaccharides from bamboo shoot. And the co-extracts had a notable effect on the stability of the method, which was confirmed by the RSD% data (>16%, even up to 27.9% for imidacloprid). To compare the matrix effect, responses of matrix matched standards were compared with responses of the solvent ones. Matrix matched standards were acquired by added standard solution into the final blank sample solution. As shown in Table 2, the matrix effects were obviously decreased for the four pesticides, even down to −64.7% for imidacloprid. Therefore, a clean-up procedure was necessary.
| Pesticide | Extraction efficiency (%) | RSD (%) | Matrix effect (%) |
|---|---|---|---|
| phoxim | 78.2 | 20.0 | −33.8 |
| chlorpyrifos | 86.1 | 16.5 | −39.5 |
| imidacloprid | 58.5 | 27.9 | −64.7 |
| chlorantraniliprole | 70.3 | 16.5 | −38.5 |
Selection of sorbent PSA and GCB were often used to remove interferences and pigments. However, they may also interact with target compounds or cause the loss of analytes. To evaluate possible adverse effects of PSA and GCB on determination the four pesticides, a series of experiments were conducted with different amount of PSA and GCB combined with 1 mL of acetonitrile extracting solution at 100 μg/kg fortification level (Table 3). With the increase of the amount of PSA from 0.5 g to 2.0 g, the average recoveries of the four pesticides were increased from 30.7% to 73.3%. When the amount of PSA was up to 3.0 g, the recoveries was decreased, which might result from the insufficient dosage of eluting solvent. When the dosage was improved from 5 mL to 10 mL, the data could be increased. However, the recoveries of imidacloprid were unsatisfactory (below 59%) from PSA analysis. In comparison, the average recoveries for the four pesticides from GCB analysis were all smaller than the PSA analysis. So 2.0 g PSA was chosen in the next study.
| Pesticide | Mass PSA (g) | Mass GCB (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| phoxim | 34.3 | 51.1 | 78.2 | 54.3 | 37.6 | 49.7 | 71.7 | 45.6 |
| chlorpyrifos | 42.5 | 46.9 | 86.1 | 67.9 | 42.1 | 76.5 | 65.3 | 43.2 |
| imidacloprid | 15.6 | 27.9 | 58.5 | 55.3 | 25.7 | 30.2 | 43.7 | 52.1 |
| chlorantraniliprole | 30.3 | 46.6 | 70.3 | 42.1 | 47.9 | 65.6 | 72.4 | 71.2 |
| Average recover | 30.7 | 43.1 | 73.3 | 54.9 | 38.3 | 55.5 | 63.3 | 53.0 |
Selection of elution solvent The selection of elution solvent is critical, as well as the choice of sorbent and the chemically bonded phase, since it affects the efficiency of the extraction by MSPD. All components of the system interact simultaneously with the elution solvent. These interactions involve the analyte with the sorbent, with the chemically bonded phase (PSA) and with the dispersed matrix, and the matrix with the sorbent and with the chemically bonded phase. During the insufficient elution ability of acetonitrile for imidacloprid, we optimized the eluant with different levels of acetonitrile concentrations in dichloromethane. Acetonitrile from 0% to 100% (volume percent) in dichloromethane were used to test the recoveries of the pesticides from bamboo shoot. Fig. 1 showed the results of recovery (R%) for the compounds under evaluation using different elution solvents (The spiked level was 100 μg/kg). According the R% values, when the ratio of dichloromethane was more than 40%, a notable overestimation performance from most of the tested pesticides (except imidacloprid) appeared. However, the ratio of acetonitrile in dichloromethane between 70% – 90% resulted in satisfactory R% for all of the pesticides (R% between 70% – 110%). Obviously, the addition of dichloromethane could improve the elution ability of the elution solvent (compared with the single acetonitrile). The mixture acetonitrile: dichloromethane (7:3, V/V) was chosen as the optimal elution solvent owing to the satisfactory results for all the tested pesticides (R ranged 96.7% – 102.4%, with RSD < 10%).
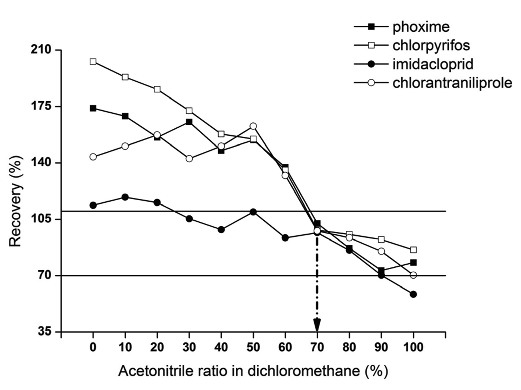
Recovery of four pesticides as determined by mixed solution of acetonitrile in dichloromethane. The two horizontal lines represented the recovery values were 70% and 110%, respectively.
Method validation Linearity of the method was tested with standard mixtures at seven concentration levels (n = 3) in the range 5 – 1000 μg/L for each compound. Correlation coefficients above 0.999 were obtained for all of the compounds.
The accuracy of the method was evaluated regarding the recovery assays. Blank bamboo shoot samples were fortified by adding a known volume of standard solution containing a mixture of pesticides in 5.0 g sample at the beginning of the process. The levels were 20 and 200 μg/kg. Three replicates were carried out at each spiking level to determine the mean recovery and RSD. The observed RSDs of the analyzed samples were in general lower than 13%. Obtained results from mean recoveries and RSD (%) for all pesticides at two concentration levels are shown in Table 4. For all compounds in all samples, mean recoveries lie within an acceptable range, from 87.5% to 107.2% with RSD values from 5.2% to 12.4%.
| Pesticide | Spiked | In tra-assay (%) | Inter-assay (%) | MRL (μg/kg) | ||
|---|---|---|---|---|---|---|
| (μ/kg) | Recovery (%) | RSD | Recovery (%) | RSD | CN/US/JPa | |
| Phoxim | 20 | 97.2 | 8.7 | 96.7 | 10.1 | 50 / None/20 |
| 200 | 93.6 | 6.1 | 102.9 | 6.3 | ||
| Chlorpyrifos | 20 | 90.4 | 5.2 | 102.5 | 12.4 | 100/50/500 |
| 200 | 105.8 | 7.4 | 100.2 | 6.5 | ||
| Imidacloprid | 20 | 90.2 | 8.3 | 87.5 | 10.5 | 500/500/500 |
| 200 | 95.7 | 6.7 | 98.2 | 11.5 | ||
| Chlorantraniliprole | 20 | 102.4 | 8.6 | 98.7 | 9.3 | None/None/None |
| 200 | 100.5 | 5.3 | 107.2 | 7.1 | ||
Repeatability of the developed analytical method in order to obtain precision were calculated by running three extractions of bamboo shoot samples in five replicate in single day and in three different days, as intra-day and inter-day precision study. Table 4 shows the recovery values and the RSDs obtained from these assays. The limit of detection (LOD) for the four pesticides was 20 μg/kg. The method LOD levels are considerably low since they are far below the maximum residue level regulations established for selected pesticides in this study. The method validation studies for spiked samples indicated that the present method provides good recoveries and reasonable precision for selected pesticides.
Comparison with traditional standard method As shown in Table 5, all the pesticides displayed a decrease in signal from the standard methods. The presence of matrix co-extractives (notable white particles precipitated out during the concentration process) suppressed the response of the four pesticides from −15.5% to −32.7%. However, the transparent and colorless solution from the developed MSPD method could slightly enhance the signal, which might be beneficial for the pesticides analysis. In addition, the developed MSPD method could be finished within 30 min for the four pesticides analysis simultaneously, which is faster and simpler than the standard methods (70 – 240 min for each pesticide, at least 400 min to obtain the results for the four pesticides and many laborious steps included). Moreover, the sample and solvent consumption are notable smaller than the standard ones. Especially there is not using any high toxic chlorinated solvent in the developed MSPD method. The results demonstrated the advantages of the developed method over the standard ones.
| Pesticide | Operation time (min) | Solvent consumption (mL) | Sample usage (g) | Matrix effect (%) |
|---|---|---|---|---|
| phoxim | 70/30 | 60/30 | 25/5 | −25.2/+10.3 |
| chlorpyrifos | 70/30 | 60/30 | 25/5 | −30.3/+5.3 |
| imidacloprid | 240 / 30 | 400 / 30 | 20/5 | −15.5/40.7 |
| chlorantraniliprole | 70/30 | 60/30 | 25/5 | −32.7/+3.5 |
Applications of the method Twenty bamboo shoot samples available from the local market and production base were analyzed for the four pesticides using the above method. The results from the real samples showed that only chlorpyrifos was detected in one sample (Fig. 2), but it was found below the maximum residue levels allowed by the regulation.
An efficient, fast, and easy to perform analysis method based on QuEChERS-MSPD followed by LC-MS/MS was successfully applied to determine four selected pesticides in bamboo shoot. The results demonstrated that the accuracy, linearity, and selectivity of the proposed method are acceptable with good recoveries and low LOD (20 μ/kg) allowing application of the procedure for detection below the levels imposed by existing regulations. The target pesticides for the developed method are currently in use in cultivation of bamboo shoot, which could assure the high applicability and practicability of the new method. Thereby, the developed method improves the general extraction efficiency and decrease the matrix effect in comparison with the traditional standard method; and it allows completing sample treatment within 30 min. Moreover, there is the first report on the application of MSPD for pesticide residue analysis in bamboo shoot, and the good results represent MSPD might be a good tool for developing pesticide residue analysis for other plant food.
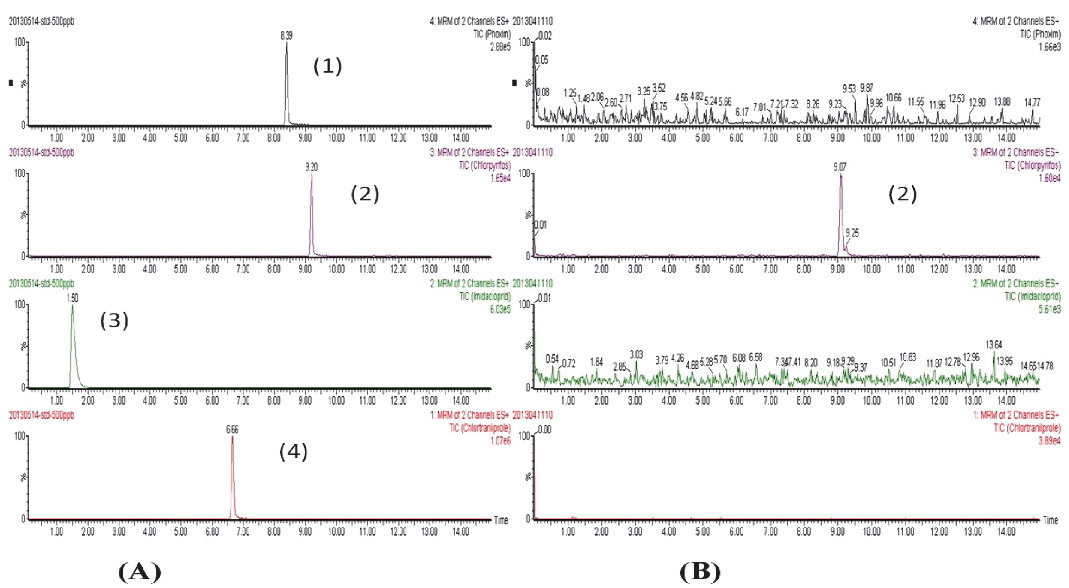
Typical chromatograms obtained by LC-MS/MS of: (A): standard mixture solution of the selected pesticides, (B): a real sample with chlorpyriofs detected. Peak identification: (1) phoxim, (2) chlorpyrifos, (3) imidacloprid, (4) chlorantraniliprole.
Acknowledgements This work was supported by the Special Funds from the Central Scientific Research Institute of Public Welfare (RISF61252), Special Fund for Forestry Scientific Research in the Public Interest (201304705) and the Applied Research Project in the Public Interest of Zhejiang Province (2013C32106). The authors state that there are no conflicts of interest.