2014 Volume 20 Issue 3 Pages 621-628
2014 Volume 20 Issue 3 Pages 621-628
This study investigated functional dipeptide (anserine and carnosine) concentrations and antioxidant activities of Silky Fowl. Fresh meat from Silky Fowl contained 1.6- to 2.3-fold higher carnosine content compared with other chickens (p < 0.05). The carnosine content of fresh meat from Silky Fowl was higher than the anserine content, in contrast to that of common chicken. Our study showed that the meat extract of Silky Fowl is a good scavenger of the hydroxyl radical. Significant correlations between the total dipeptide content of meat extracts and hydroxyl radical scavenging (IC50 values) activity were additionally revealed by multiple regression analysis (breast: R = 0.684, p < 0.001; thigh: R = 0.729, p < 0.001). Since Silky Fowl is especially rich in carnosine, these findings make this breed potentially useful as a rich dietary source of antioxidant dipeptides.
Silky Fowl (Gallus gallus domesticus), originating from China, is a very unusual chicken with distinct characteristics: silky feathers, black-colored bones, and dark bluish skin. Silky fowl is characterized by melanin deposits on the surface of many organs, such as the skin, comb, bone, internal digestive organs, lung, brain and skeletal muscle, and has pigment granules in practically all of its tissues (Nozaki and Makita, 1998).
According to traditional Chinese medicine, Silky Fowl meat is beneficial for women’s diseases, while the eggs are beneficial for lung diseases (Akishinonomiya et al., 1994). It is likely that these traditions are one of the reasons people, even those outside China, expect pharmacological effects from Silky Fowl meat or eggs. Tian et al. (2007) reported that the carnosine content in Silky Fowl meat was 1.8-fold higher than that in White Plymouth Rock. However, the content of anserine, a dipeptide with antioxidant properties related to carnosine, in Silky Fowl meat has not yet been investigated.
The involvement of oxygen radicals in accelerated aging and the incidence of cancer is known, and dietary prevention of these effects is considered important. Carnosine (β-alanyl-L-histidine) and its metabolic derivative anserine (β-alanyl-1-methyl-L-histidine) are histidine-containing dipeptides, and are present in high concentrations in chicken breast muscle. Both dipeptides play beneficial roles in a number of physiological functions, as antioxidants (Yanai et al., 2004; Koide et al., 2007), and as anti-fatigue (Harada et al., 2002a) and anti-glycation (Hipkiss and Brownson, 2000) agents.
Silky Fowl meat is used in traditional Chinese medicine; however, there remains insufficient scientific evidence as to its properties and effects. Its black appearance might deter ordinary consumers from using it as a food. Revealing the functionality and antioxidant dipeptide contents of Silky Fowl meat is thus important for the potential health benefits of this natural food to be realized. Further, both dipeptides are water-soluble components, and are therefore easily extracted (Takai et al., 1993). Thus, it is expected that Silky Fowl could contribute to prepared foods in a form more acceptable to consumers, such as soup.
In this paper, we describe the content of histidine-containing dipeptides in the skeletal muscles and meat extracts of Silky Fowl, and evaluate the antioxidant activity in the meat extracts. We compare the values in breast and thigh muscles and their extracts with those of four other species of chicken.
Raw materials White-feathered Silky Fowls were bred and reared by the Tokyo Metropolitan Agriculture and Forestry Research Center (Tokyo, Japan). Five females (79 weeks of age) were placed in individual wire-floored cages, and fed a commercial formula feed (JA Higashinihon Kumiai Shiryou, Ltd., Gumma, Japan; crude protein (CP) 17%, metabolizable energy (ME) 3.18 Mcal/kg) for the last stage of broiler production. The birds had free access to water and feed. Two weeks later, the birds were killed by exsanguination from the cervical arteries, skinned, immersed in cold water with ice for 20 to 30 min. Next, skeletal muscles were dissected, and tendons and adipose tissue were removed. Breast and thigh muscles were immediately removed and stored at 4°C overnight. The two muscles studied were M. pectoralis superficialis and M. biceps femoris, with the adipose tissue removed. In addition, four types of chicken meat (Japanese Game Cross, Hinai- jidori, Nagoya Breed and broilers; five feathers from each chicken species were used) were purchased from a poultry wholesale store (Kagaya, Ltd., Tokyo, Japan). Broilers were about 8 weeks of age. Additionally, three other chicken species, 20 22 weeks of age, were used in this study. Deboned meats were stored at 4°C until use. Breast and thigh muscles were used for the experiments. M. pectoralis superficialis and M. biceps femoris were taken from both sides and peripheral adipose tissue was removed. Muscle samples were collected within 36 hours after slaughter. Females of the specific breeds were investigated; however, the broilers’ gender was unknown. The experimental procedure was in accordance with the guidelines for animal experiments of the Tokyo Metropolitan Agriculture and Forestry Research Center.
Skeletal muscle preparation for anserine and carnosine content determinations Five grams of minced meat was homogenized with 50 mL of distilled water in an AM-8 homogenizer (Nihonseiki-seisakusyo, Ltd., Tokyo, Japan) at 10,000 rpm for 5 min in an ice-cold chamber. The homogenate was diluted in a measuring cylinder to 250 mL total with distilled water, and filtrated through No. 5A filter paper (Advantec Toyo Kaisha, Ltd., Tokyo, Japan). Then, 1.0 mL of filtrate was mixed with an equal volume of 10% (W/V) trichloroacetic acid. The mixture was left for 1 hour at room temperature, and centrifuged at 3,500 rpm for 20 min. The supernatant was filtrated through a 0.45μm membrane filter (Millipore, Bedford, USA). Then, 20 μL of each filtrate was applied to an Amino Acid Analyzer L-8900 (Hitachi, High- Technologies Corp., Tokyo, Japan). A standard L-amino acid solution was prepared by diluting a commercially available L-amino acid solution (type ANII and type B; Wako, Osaka, Japan) with water. Anserine and carnosine contents were analyzed.
Extraction of skeletal muscle for anserine and carnosine content determinations Heat extraction of fresh breast and thigh meat samples was performed as follows:
The meat samples (M. pectoralis superficialis and M. biceps femoris) were cut into rectangular prisms, and placed into discrete sealable polyethylene bag, and degassed. The bag was immersed in a thermostatic water bath at 70°C for 60 min, and cooled with tap water for 30 min. The exudate (as extract) was collected, and evaluated for dipeptide content. The extract was diluted 20-fold with lithium hydroxide buffer (pH 2.2); then, 1 mL of diluted extract was mixed with an equal volume of 10% (W/V) trichloroacetic acid for deproteinization. The analytical procedures (centrifugation, filtration, and measurement) were the same as above. The dipeptide (anserine and carnosine) contents of the meat extract were determined.
Evaluation of antioxidant activity Antioxidant activity against hydroxyl radicals in the solution was determined using a Radical Catch kit (ALOKA, Tokyo, Japan). The kit measured the amount of hydroxyl radicals generated by the Fenton reaction with luminescence produced by cobalt catalyzed hydrogen peroxide oxidation of luminol. The reaction mixture for measuring hydroxyl scavenging activity was as follows: 50 μL of cobalt chloride reagent, 50 μL of luminol reagent, and 20 μL of sample solution were mixed, and after preincubation for 5 min at 37°C, 50μL of hydrogen peroxide reagent was added. The amount of luminescence generated from 80 seconds to 120 seconds after the addition of hydrogen peroxide reagent was detected at 430 nm using a luminescence reader (Accu FLEX Lumi 400; ALOKA) according to the manufacturer’s instructions. Meat extracts were prepared at 100, 101, 102, and 103 dilutions using distilled water. The chemiluminescence intensity is given as the relative light unit (RLU). Hydroxyl radical scavenging activity was calculated as follows:
 |
IC 50 values represented the concentration of sample that showed a 50% rate of inhibition. In this experiment, the antioxidant activity of each extract was expressed in terms of the concentration of extract (no-diluted extract = 100%) that could inhibit 50% oxidation. Therefore, extracts having higher antioxidant activity have lower IC50 values.
Statistical analysis All data were analyzed for statistic significance using one-way ANOVA. When significant effects were detected (p < 0.05), comparisons between means were carried out using Tukey’s multiple range test. Regression analysis for the histidine-containing dipeptide concentration and antioxidant effects of skeletal muscle extracts was carried out. Statistical analysis was conducted using R software (http://www.R-project. org; Ihaka and Gentleman, 1996).
Anserine and carnosine contents of skeletal muscles Anserine and carnosine contents of skeletal muscles are shown in Tables 1 and 2, respectively. Breast meat from Silky Fowl (SF) had significantly ( p < 0.05) lower anserine content than Japanese Game Cross (JG), Nagoya Breed (NB), and Hinai-jidori (HJ). However, SF had the highest (789.3 mg/100 g meat) carnosine content, 1.7- to 1.9-fold higher those that of the other four chicken varieties ( p < 0.05). In breast meat, total functional dipeptide (anserine + carnosine) contents were in the order of SF ≥ JG ≥ NB ≥ HJ ≥ broilers (BR). The differences in dipeptide content between SF and both HJ and BR were significant (p < 0.05).
In thigh meat, HJ had the highest anserine content of the five breeds. The anserine contents of thigh meats were in the order of HJ ≥ JG ≥ NB ≥ SF ≥ BR. There was a significant difference in anserine content between SF and HJ (261.9 and 326.0 mg/100 g, p < 0.05), but there was no significant difference among SF, JG, NB, and BR (p > 0.05). However, the highest carnosine content for thigh meat was observed in SF (288.2 mg/100 g), which contained 1.6- to 2.3-fold higher carnosine compared with the other four breeds (p < 0.05). The total contents of carnosine and anserine of thigh meats were in the order of SF > HJ ≥ JG ≥ BR ≥ NB. There was a significant difference in total dipeptide content between SF and three of the breeds (JG, BR or NB:p < 0.05).
| Breed\Item | Aneserine | Carnosine | Total |
|---|---|---|---|
| Silky Fowl | 631.8 ± 12.4b | 798.3 ± 85.9a | 1430.1 ± 87.3>a |
| Japanese Game Cross | 809.5 ± 15.6a | 455.5 ± 36.8b | 1265.0 ± 25.1ab |
| Hinai Jidori | 762.6 ± 33.1a | 441.3 ±49.9b | 1203.9 ± 39.9b |
| Nagoy a Breed | 780.7 ± 23.7a | 478.9 ± 23.8b | 1259.6 ± 37.4ab |
| Broiler | 647.9 ± 12.6b | 417.2 ± 14.8b | 1065.1 ± 14.6b |
| Breed\Item | Aneserine | Carnosine | Total |
|---|---|---|---|
| Silky Fowl | 261.9 ± 9.4b | 288.2 ± 20.4a | 550.1 ± 27.7a |
| Japanese Game Cross | 291.8 ± 7.7abc | 161.0 ± 8.6b | 452.8 ± 12.4bc |
| Hinai Jidori | 326.0 ± 9.6a | 164.5 ± 16.4b | 490.4 ± 12.5ab |
| Nagoy a Breed | 277.2 ± 8.8bc | 126.7 ± 13.2b | 403.9 ± 13.4c> |
| Broiler | 254.9 ± 7.6bc | 183.4 ± 14.7b | 438.3 ± 15.8bc |
Anserine and carnosine contents of heat extracts from skeletal muscles Anserine and carnosine content of heat extracts from skeletal muscles are shown in Tables 3 and 4, respectively. Among the breast extracts, there was a significant difference in anserine content between JG and BR (71.5 and 58.1 μmol/mL ext., respectively: p < 0.05), but there was no significant difference between SF (63.9 μmol/mL) and the other four breeds (p > 0.05). However, the highest carnosine content was observed in the SF breast extract (48.8 μmol/mL), which contained 2-fold more carnosine compared with the other four breeds (p < 0.05). Total contents of dipeptides (anserine + carnosine) for breast extracts were in the order of SF > JG ≥ HJ ≥ NB ≥ BR.
Among thigh extracts, HJ (28.0 μmol/mL) had significantly higher anserine content than the other four breeds (p < 0.05), but there was no significant difference among SF, JG, NB, and BR (p > 0.05). However, the highest carnosine content was observed in the SF thigh extract (12.1 μmol/mL), which contained 2-fold more carnosine as compared with NB (5.9 μmol/mL:p < 0.05). Total dipeptide (anserine + carnosine) contents of thigh extracts were in the order of HJ > SF = JG ≥ BR ≥ NB.
| Breed\Item | Aneserine | Carnosine | Total |
|---|---|---|---|
| Silky Fowl | 63.9 ± 2.2ab | 48.8 ± 3.7a | 112.7 ± 5.0a |
| Japanese Game Cross | 71.5 ± 0.6a | 23.6 ± 1.7b | 95.1 ± 1.2b |
| Hinai Jidori | 65.5 ± 3.7ab | 20.1 ± 2.7b | 85.6 ± 3.3bc |
| Nagoy a Breed | 62.6 ± 0.9ab | 22.2 ± 1.3b | 84.8 ± 1.9bc |
| Broiler | 58.1 ± 2.0b | 22.0 ± 1.4b | 80.1 ± 2.8c |
| Breed\Item | Aneserine | Carnosine | Total |
|---|---|---|---|
| Silky Fowl | 17.8 ± 0.7b | 12.1 ± 1.0a | 29.9 ± 1.6a |
| Japanese Game Cross | 21.2 ± 1.5b | 8.7 ± 0.8ab | 29.9 ± 2.0b |
| Hinai Jidori | 28.0 ± 1.7a | 9.0 ± 1.1ab | 37.0 ± 1.1a |
| Nagoy a Breed | 18.7 ± 1.3b | 5.9 ± 0.6b | 24.6 ± 1.6b |
| Broiler | 18.1 ± 0.9b | 8.0 ± 0.6b | 26.0 ± 1.2b |
Antioxidant activity of heat extracts from skeletal muscles Hydroxyl radical scavenging activities (IC50 values) of heat extracts from skeletal muscles are shown in Fig. 1. In breast extracts, antioxidant activities were greater in the order of SF ≥ JG ≥ HJ ≥ NB ≥ BR. There was a significantly greater antioxidant activity in SF than in three of the other breeds (HJ, NB and BR).
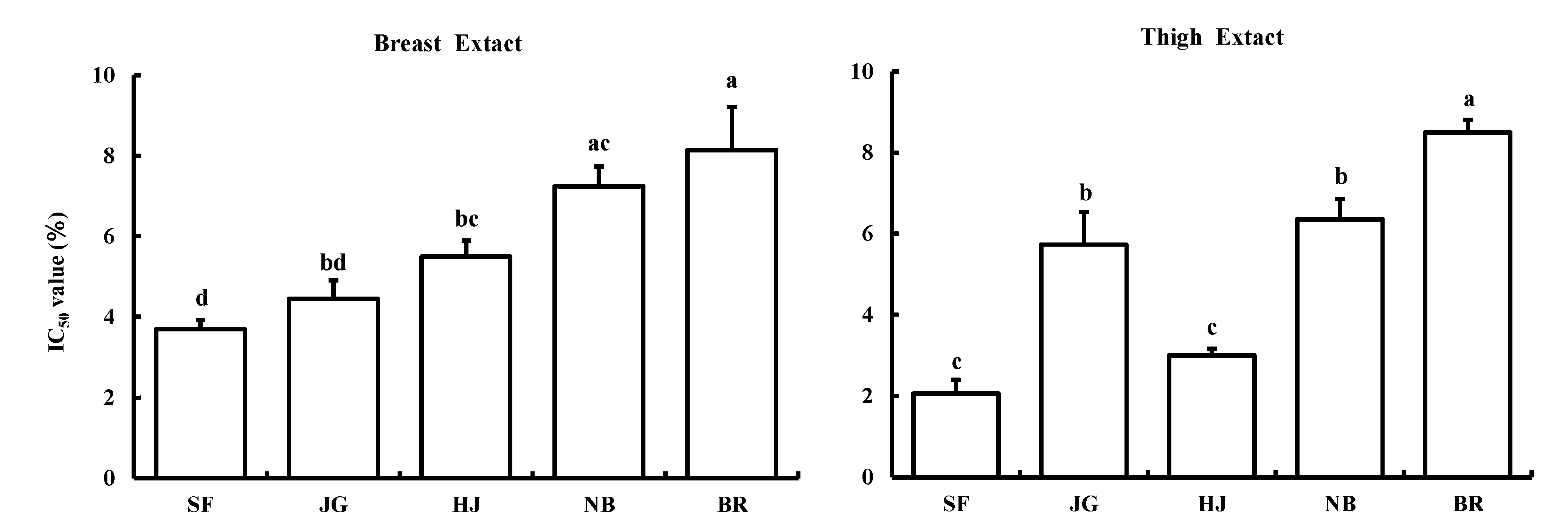
Comparisons of IC50 values among meat extracts measured using the chemiluminescence method. SF: Silky Fowl, JG: Japanese Game Cross, HJ: Hinai Jidori, NB: Nagoya Breed, and BR: Broiler. Breast: M. pectoralis superficialis; thigh: M. biceps femoris. Values are expressed as means ± standard errors (n = 5). Means with different letters are significantly different (p < 0.05).
In thigh extracts, antioxidant activities were greater in the order of SF ≥ HJ > JG ≥ NB > BR. There were significantly greater antioxidant activities in SF and HJ than in the other three breeds (JG, NB and BR).
Relationship between antioxidant activity (IC50 values) and dipeptide contents of meat extracts The relationship between IC50 values and dipeptide contents of heat extracts from skeletal muscles are shown in Figs. 2 (breast) and 3 (thigh), respectively. In breast extracts, negative correlations were observed between IC50 values and dipeptide contents. There was a significant negative correlation between IC50 values and carnosine contents (r = -0.575, p < 0.01), but there was no significant relationship between IC50 values and anserine contents (r = -0.359, p = 0.078). In thigh extracts, significant negative correlations were observed between IC50 values and dipeptide contents (anserine: r = -0.414, p < 0.05; carnosine: r = -0.603, p < 0.01). We also used multiple regression analysis to determine the relationship between dipeptide contents and antioxidant activities of meat extracts (Table 5). Anserine and carnosine contents were employed as predictor variables, and IC50 values as a response variable. Significant correlations between the dipeptide contents of meat extracts and IC50 values were revealed by multiple regression analysis (breast: R = 0.684, p < 0.001; thigh: R = 0.729, p < 0.001), and adjusted R2 values were 0.420 and 0.484, respectively. The adjusted R2 = 0.420 in breast muscle was increased from the single regression analysis in which anserine or carnosine content was employed as a predictor variable (anserine: r2 = 0.129; carnosine: r2 = 0.331, respectively). Similarly, the adjusted R2 = 0.484 in thigh muscle was increased from the single regression analysis (anserine: r2 = 0.183; carnosine: r2 = 0.364, respectively). Multiple regression analysis indicated that both anserine and carnosine content significantly contributed to explaining the radical scavenging capacity of the extracts. The standard partial regression coefficients (SPRCs) of anserine and carnosine in breast muscle were -0.371 and -0.583, respectively. Similarly, SPRC of thigh muscle was -0.409 and -0.600, respectively.
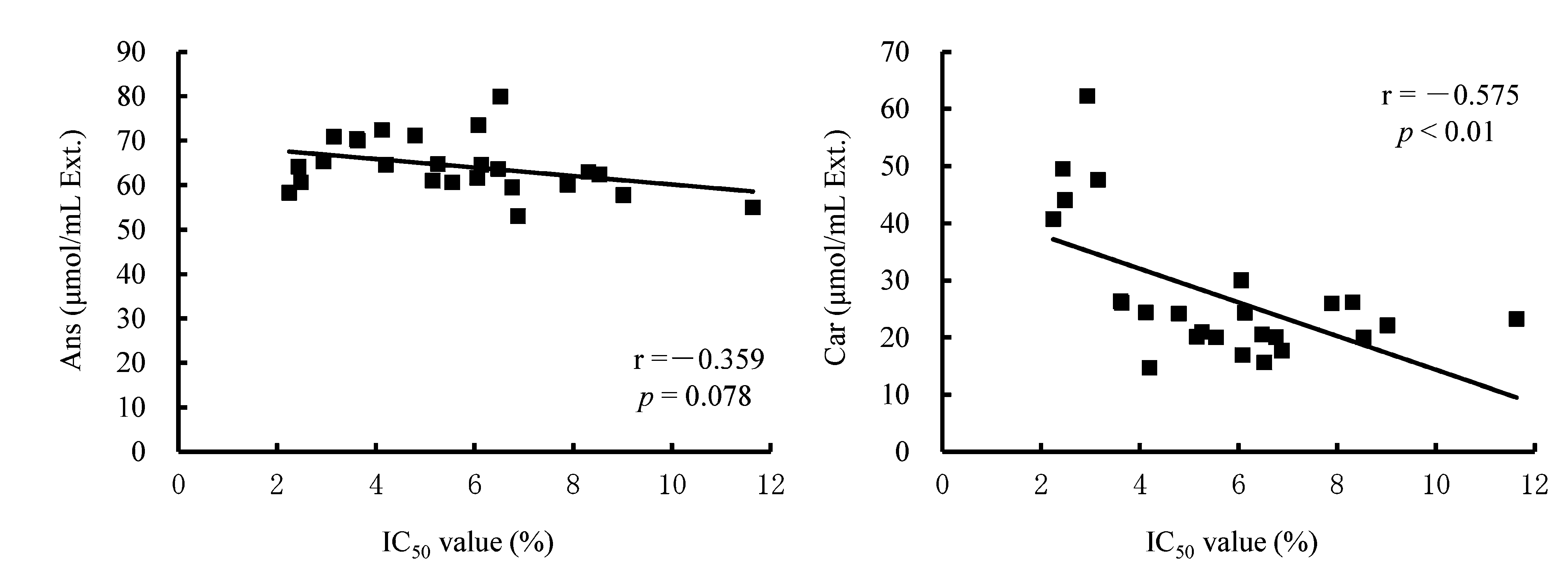
Relationship between the IC50 values measured using the chemiluminescence method and dipeptide concentrations of breast meat extracts. IC50 value: concentration of extract producing 50% inhibition of oxidation. Ans: anserine; Car: carnosine. Breast: M. pectoralis superficialis.
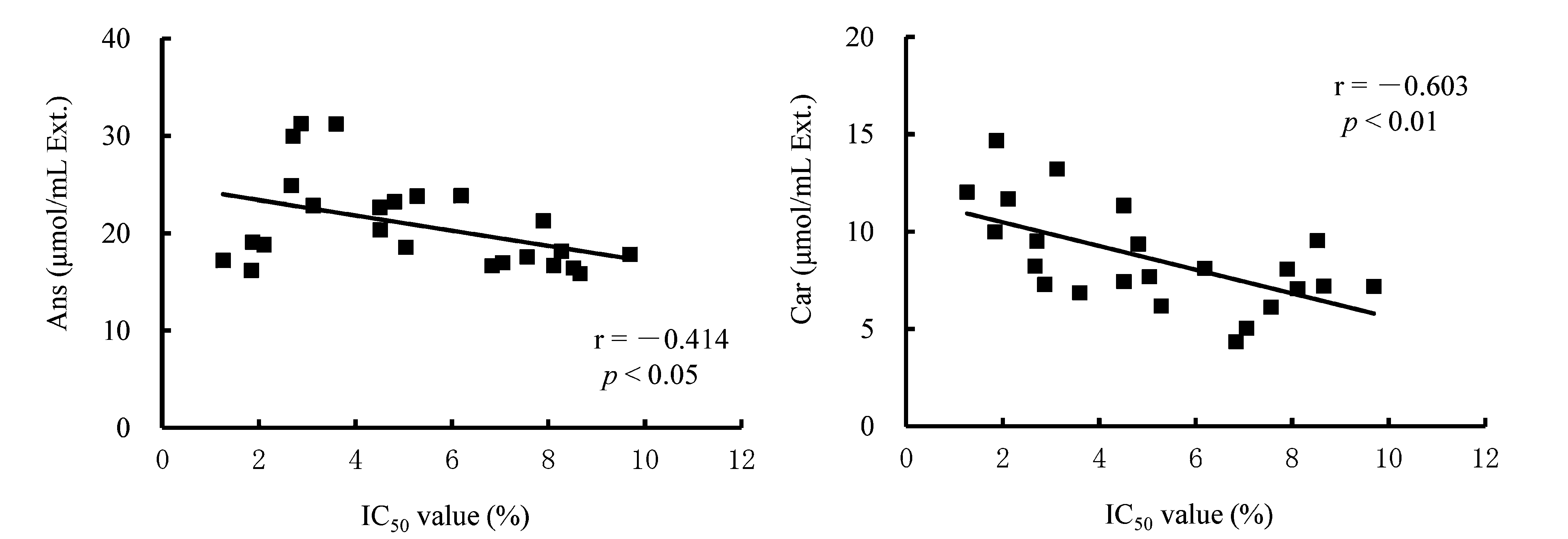
Relationship between the IC50 values measured using the chemiluminescence method and dipeptide concentrations of thigh meat extracts. IC50 value: concentration of extract producing 50% inhibition of oxidation. Ans: anserine; Car: carnosine. Thigh: M. biceps femoris.
| Y variable | Equation (SPRC) | R | R2 | F value |
|---|---|---|---|---|
| IC50 value (Breast Ext) | Y=17.634-0.139X1-0.114X2 (-0.371, -0.583) | 0.684 | 0.420 | 9.684*** |
| IC50 value (Breast Ext) | Y=14.785-0.218X1-0.594X2 (-0.409, -0.600) | 0.729 | 0.484 | 11.320*** |
SPRC: standardized partial regression coefficient. R2: adjusted coefficient of determination. IC50 value: concentration of extract producing 50% inhibition of oxidation. Breast: M. pectoralis superficialis, and thigh: M. biceps femoris. *** p < 0.001.
Anserine is also known as methyl carnosine. Anserine is expected to exert similar functions as carnosine; supported by the fact that previous studies revealed that these dipeptides exhibited antioxidant effects (Kohen, 1988; Aruoma, 1989). In general, carnosine and anserine contents of chicken breast muscle are higher than those of thigh muscle (Saegusa et al., 1987). Anserine is contained at higher levels than carnosine in both muscles, at a ratio of approximately 3:1 (Abe and Okumura, 1995). On the other hand, the content of carnosine is higher than anserine in pork and beef. Also, the content ratio differs depending on the type of muscle or animal species (Watanabe et al., 1989; Abe and Okuma, 1995). In this study, a comparison of M. pectoralis superficialis and M. biceps femoris dipeptide contents revealed that breast muscles were higher than thigh muscles in both anserine and carnosine, as previously reported. Moreover, the breast muscle contained both dipeptides at more than double the thigh muscle level in the five chicken species investigated. Additionally, in the four chicken species except for Silky Fowl, anserine contents exceeded carnosine contents, as in the common chicken. In contrast, both muscles of Silky Fowl contained higher carnosine contents compared with anserine contents. This was a remarkable characteristic not observed in other chickens.
It is thought that the myofiber composition is affected by environmental factors, such as advancing age, genetic factors, and rearing conditions. Myofibers in the M. pectoralis superficialis are mainly type IIB. On the other hand, muscle fiber type distribution in the thigh muscle differs among muscles. The M. biceps femoris of intact male chickens are composed of type II fibers (Ono et al., 1983). In the middle gluteal muscle, the carnosine contents are 3.8- to 4.2-fold greater in type II than in type I fibers (Dunnett and Harris, 1995). Type II fibers are classified as type IIB and type IIA; the carnosine content of type IIB fibers is higher than that of type I and type IIA fibers (Swell et al., 1992; Dunnett and Harris, 1995). Chicken Iliotibialis lateralis, composed of type IIA and IIB fibers, has been observed to have a progressive increase of type IIA fibers and decrease of type IIB fibers with advancing age (Ono et al., 1993). The same report showed that the allocation of type IIB fibers in the I. lateralis of New Hampshire chickens at 1 to 35 weeks of age was reduced from 78.6% to 46.1% by advancing age. On the other hand, Nakamura et al. (2003) reported that the percentage distribution of type IIB fibers in the I. lateralis of male Silky Fowl at 50 weeks of age was 58.3%. One reason the carnosine content of M. biceps femoris of Silky Fowl is higher may be related to the proportion of myofiber allocation. In general, broilers are about 8 weeks of age, and JG, HJ, and NB are consumed at about 20 weeks of age. Thus, the Silky Fowl (81 weeks of age) used in this study was aged compared to the other chickens. Therefore, the carnosine content might be higher in the M. biceps femoris of Silky Fowl specimens younger than 81 weeks of age.
M. pectoralis of Silky Fowl is composed only of IIB fibers (Nakamura et al., 2003). The fiber diameter of M. pectoralis increases with advancing age, but configuration of the fiber type is unchanged (Ono et al., 1993). Therefore, it might be difficult to explain Silky Fowl breast carnosine content by muscle fiber type. M. pectoralis of broilers was composed of two types of fibers, IIR and IIW (Nikki et al., 2012). However, males were used in many of these reports. It is necessary to further examine the histochemical properties of female Silky Fowl.
After force feeding of chicken breast extracts (containing 3.89% anserine and 1.17% carnosine) to mice, both dipeptides were found in the blood, and significant increases in their concentrations were evident in M. quadriceps femoris (Harada et al., 2002b). Suzuki et al. (2004) showed that anserine in human plasma was slightly detectable 30 min after chicken breast extract supplementation, but carnosine was not detected. However, the related amino acid (β-alanine, histidine, and 1-methylhistidine) concentrations in human plasma was increased. Suzuki et al. (2004) considered that ingested carnosine and anserine were broken down into constituent amino acids by serum carnosinase. Constituent amino acids of carnosine and anserine are resynthesized to carnosine by cellular components of skeletal muscle (Bauer and Schulz, 1994). Therefore, the intake of chicken breast extracts (containing 0.6% anserine and 0.2% carnosine) for 30 days increases carnosine concentration in human muscle significantly (Sato et al., 2003). Another study showed that the increase of 8-OHdG and 8-isoprostane was suppressed by oral ingestion of histidine-containing dipeptide (Tanaka et al., 2008). A mixture of anserine and carnosine, isolated from chicken extract, can prevent DNA degradation by four major reactive oxygen species that occur in vivo (Takahashi et al., 2011). Human serum TAC (Total Antioxidant Capacity) rises when carnosine is ingested (Antonini et al., 2002). These results show that oral administration of anserine and carnosine is effective for suppression of oxidative damage by reactive oxygen species.
Dietary histidine enhances breast muscle anserine and carnosine contents in broilers, whereas β-alanine content is reduced (Haug et al., 2008). Oral administration of β-alanine to male broiler chicks increased breast muscle carnosine, but did not affect the anserine content (Tomonaga et al., 2012). The carnosine content of breast muscle is increased in male White Leghorn chicks (Julia strain), whereas the anserine content is reduced (Tomonaga et al., 2005). In addition, β-alanine administration showed no effect on the contents of the two dipeptides in the breast muscle of 4-week- old male broilers (Tomonaga et al., 2006). Intarapichet and Maikhunthod (2005) showed that the carnosine content of female meat was higher than that of male meat at the same live weight. They also speculated that feed quality might have a greater effect on carnosine content than the age of chickens. In other words, the dipeptide content of skeletal muscle is affected by feed, chicken breed, age, and gender.
In this study, the rearing conditions and exact ages of the four chicken species that were used for comparison with Silky Fowl are unknown. It is necessary to align these conditions in order to more accurately compare the differences in dipeptide contents of different chicken species. However, Tian et al. (2007) reported that the muscle carnosine content of Silky Fowl was higher than that of White Plymouth Rock chickens under the same rearing conditions and age. Therefore, the carnosine content of Silky Fowl skeletal muscle observed in this study appears to be characteristic of Silky Fowl. The present study shows that the functional dipeptide content (anserine + carnosine) and the carnosine content of Silky Fowl skeletal muscle is the richest among the five chicken species. Thus, Silky Fowl can be considered a better chicken breed for carnosine and anserine supplementation.
Breast meats contained higher carnosine contents compared with thigh meats in both the extracts and raw meats. In contrast, the antioxidant activity of the thigh muscle was higher than that of breast muscle (Intarapichet and Maikhunthod, 2005). It is considered that other antioxidant compounds combined to give the thigh meat extracts higher inhibition efficiency despite their lower amounts of carnosine. In addition, an ultrafiltrate (using a 5,000 MW cut off membrane) from meat extracts had approximately 20% higher carnosine, but given equal amounts of carnosine, the unfiltered extract had greater antioxidant activity than the ultrafiltrate (Maikhunthod and Intarapichet, 2005). This suggests that the lower activity of the ultrafiltration permeate could be due to the loss of other antioxidant compounds in the retentate of the ultrafiltration membrane. In this study, the carnosine and anserine contents of M. pectoralis superficialis were higher than those of M. biceps femoris in both the meat extracts and raw meats. Moreover, when comparing the breast and thigh meat extract dipeptide contents of Silky Fowl, the breast extracts had 3.59-fold higher anserine, 4.03-fold higher carnosine, and 3.77-fold higher functional dipeptides (anserine + carnosine) than thigh extracts. However, the thigh meat extract concentration required for 50% inhibition of hydroxyl radical activity was lower than that of the breast meat extract. Similar results were also observed in the four other chicken species.
M. pectoralis superficialis, which is so-called white muscle, is composed of type IIB fibers. M. biceps femoris, which is so-called red muscle, is made up of type IIA and IIB fibers. Red muscle is richer in myoglobin, as oxygen-to-ATP synthesis is required for muscle contraction in thighs. The capillary density of red muscle is higher than that of white muscle; thus, red muscle has many blood pigments due to hemoglobin. Usually, the molecular weight of proteins in nature is 5,000 or more. Hemoglobin (64,500 MW) and myoglobin (17,800 MW) are both water-soluble, but cannot pass through an ultrafiltration membrane with a cutoff of 5,000 MW. Copper and iron are generally more prevalent in thigh meat than breast meat. In addition, iron- and copper-binding proteins are related to the elimination and suppression of free radicals. In the present study, the antioxidant activity of the thigh meat extract was higher than that of the breast meat extract. The difference between the content of water-soluble protein and metal of the breast and thigh meat might affect the antioxidant activity of skeletal muscle extracts. The carnosine content in the extract of Silky Fowl skeletal muscle was the highest among the five chicken species, with breast muscle containing 2.07- to 2.42-fold higher carnosine, and thigh muscle containing 1.34- to 2.05-fold higher carnosine, than the other chicken species. Antioxidant activity was also high among the five chicken species. SPRC indicates that carnosine’s effect on hydroxyl radical scavenging activity is greater than anserine’s. Both anserine and carnosine are good scavengers of the hydroxyl radical (Aruoma et al., 1989). Both anserine and carnosine showed antioxidant activity in a concentration-dependent manner. Additionally, no synergistic effects on antioxidation were found between anserine and carnosine (Wu et al., 2003).
The results obtained in this study clearly indicate that Silky Fowl skeletal muscle contained antioxidant dipeptides at high concentrations, and its heat extract had the greatest antioxidant activity. These findings show that Silky Fowl skeletal muscle would be a useful source of dietary anserine and carnosine.