2015 Volume 21 Issue 5 Pages 659-664
2015 Volume 21 Issue 5 Pages 659-664
A convenient and environmentally friendly method was developed for extraction and enrich of Sudan I from tomato sauce and chilli products. The method is based on an ultrasound-assisted dispersive liquid-liquid microextraction with solidification of floating organic drop (UADLLME-SFO) after a preliminary QuEChERS procedure, which was followed by high performance liquid chromatography with photodiode array detection (HPLC-PAD). The several parameters involved in the UADLLME-SFO step was optimized. The optimal variables obtained were 30 µL of 1-dodecanol as the extraction solvent, 1.0 mL of acetone as disperse solvent, ultrasonic irritation for 15 min, and no salt addition. Under the optimum conditions, the limit of detection for Sudan I was as low as 1.5 µg kg−1. The recoveries obtained was between 79% and 92% (RSD, 4.8% – 7.1%, n = 7). The proposed method was successfully applied to the determination of Sudan I in four kinds of real samples.
Food safety is a serious problem for public health in the world. The harmful substances in food may be from environmental pollution, food processing, and illegal additives (Bidari et al., 2012; Nascimento et al., 2015; Sivaperumal et al., 2015). Sudan I has been detected in many food products, such as spices, sausage and egg, which was maliciously added to boost up the typical reddish color of products (Oplatowska et al., 2011; Enríquez-Gabeiras et al., 2012; Hu et al., 2012; Elyasi et al., 2013). It is reported that Sudan I is a possible carcinogen and mutagen to human (Moller and Wallin, 2002; Stiborova et al., 2002). Therefore, it has been forbidden as an additive in food by both the FSA (Food Standards Agency) and the EU (European Union). With the increasing of various food products, it is imperative and significative to establish a sensitive, rapid and convenient method for the determination of Sudan I in food.
Currently, the methods involving in detecting Sudan dyes in food samples are mainly high-performance liquid chromatography (HPLC) coupled with UV/vis or MS detection (Enríquez-Gabeiras et al., 2012; He et al., 2007; Qi et al., 2011; Yu et al., 2008; Zhu et al., 2014). To avoid the expensive MS dectector, some sample pretreatment procedures are necessary for complex food matrices. Solid-phase extraction (SPE), solid-phase microextraction (SPME), molecularly imprinted matrix solid-phase dispersion, molecularly imprinted solid-phase extraction combined with ultrasound-assisted dispersive liquid-liquid microextraction and gel permeation chromatography, have been applied to clean-up procedure the food samples before Sudan I detection (Qi et al., 2011; Enríquez-Gabeiras et al., 2012; Xin et al., 2011; Yan et al., 2011; Hu et al., 2012). But there are disadvantages involving in these methods such as time-consuming, expensive, costing large amounts of organic solvents and producing large amounts of organic waste.
At present, “quick, easy, cheap, effective, rugged and safe” (QuEChERS) sample preparation is the common extraction technique for pesticides analysis in complex food matrices (Wilkowska and Biziuk, 2011; Al-Rahman et al., 2012; Delgado-Zamarreño et al., 2012). However, compared with other techniques, the technique gets the poor enrichment factor. Recently, dispersive liquid-liquid microextraction based on solidification of floating organic droplet (DLLME-SFO) as a simple, convenient and environmental-friendly sample pretreatment method has been developed for isolating and preconcentrating compounds from water samples with excellent enrich effect (Rezaee et al., 2010). The combining sample treatment of QuEChERS procedure and DLLME-SFO method may produce good extraction efficiency for Sudan I in complex food matrices. In addition, as a freshly extraction technique, ultrasound irradiation may keep the cloudy state of DLLME-SFO procedure, which may improve the dispersion of extraction solvent and reduce dispersive solvent consumption (Capelo et al., 2006; Xia et al., 2012; Xu and Pan, 2013). The UADLLME-SFO method may be successfully used to separation and concentration of Sudan I in food samples by combining with QuEChERS procedures. However, no related report about this can be found in the literature.
In the present study, the UADLLME-SFO method was investigated to isolate and enrich Sudan I in tomato sauce and chilli products after a pretreatment step of QuEChERS. This investigation aims to verify if the proposed method was successfully applied to determination of Sudan I in solid and semi-solid complex food matrices.
Chemicals and reagents Sudan I, 1-dodecanol, 1-undecanol, hexadecane, 1,10-dichlorodecane were bought from Sigma (MO, USA). Acetonitrile, acetone, methanol and ethanol were of HPLC grade and purchased from Merck (Darmstadt, German). Ultrapure water (18.2 mΩ•cm) obtained from a Millipore purification system (MA, USA) was used throughout the experiment. A stock solution of Sudan I (1000 µg mL−1) was prepared in methanol and stored at 4°C. The calibration standard solutions (20 – 5000 µg L−1) were prepared from the stock solution by serial dilutions with methanol.
Apparatus The HPLC system used throughout this study was a Waters 1525 Binary HPLC Pump separations module with a Waters 2996 photodiode array detector (MA, USA). The system also included an auto-injector, and an RP C18 analytical column (4.6 mm x 250 mm, 5 um, Waters, MA, U.S.A.). Evaluation and quantification were performed with an Empower chromatography system (MA, USA).
The ultrasound-assisted procedure was carried out in a KQ-600E ultrasonic device (Changzhou, China) with an ultrasound power of 600 W, heating power of 800 W, and frequencies of 40 kHz, equipped with digital timer and temperature controller. In addition, a digitally regulated centrifuge, universal 32R from Hettich (Kirchlengen, German), was used for sample preparation.
Sample preparation and QuEChERS procedure Four food samples, including chili sauce, chili oil, chili powder and tomato sauce, were purchased from local supermarkets. A portion of solid or semisolid homogeneous sample or liquid oil (about 1 g) was introduced into a 25 mL glass centrifuge tube, and suspended in 10.0 mL of the mixture of acetonitrile and water (80/20, v/v). After standard solution additions, the mixture was irradiated with ultrasound for 20 min at room temperature, and 4 g of anhydrous MgSO4 and 1 g NaCl were added (Wilkowska and Biziuk, 2011). After shaking during 2 min on the vortex, the mixture was centrifuged at 3500 × g for 5 min. The acetonitrile supernatant was collected, and dried by rotary vaporization. The residue was redissolved in 1 mL of acetone for the further assays.
UADLLME-SFO procedure Five milliliters of water was transferred into a 10 mL vial (screw-cap glass test tube), and a combination mixture of 1 mL of the acetone extracts and 30 µL of 1-dodecanol as extraction solvent was rapidly injected into water using a 2 mL syringe, where acetone was dispersive solvent. The vial was placed in the ultrasonic bath, and irritated for 15 min. The capped vial was centrifuged at 3500 × g for 10 min. Then, the vial was moved into a beaker containing water and ice for 5 min, and the solidified extraction solvent was collected and transferred into a conical vial by a little special spoon, which were almostly 17 µL after melting at room temperature. Finally, 10 µL of this extraction solution was injected into the HPLC column for subsequent analysis.
HPLC analysis The mobile phase used for the chromatographic separation of Sudan I consisted of water and acetonitrile (20:80, v/v) at a flow-rate of 1 mL min−1. The column temperature was kept at 27°C. The photodiode array detector was set at 3D mode, and the spectra were recorded between 400 and 550 nm for peak characterization. The retention time of Sudan I was about 11.8 min. Quantification of Sudan I was carried out by peak area at 474 nm. All the experiments were conducted in triplicate, and the average values and standard deviation were reported. The chromatograms of standard Sudan I and the spiked sample are shown in Fig. 1.

Chromatograms of Sudan I standard solution (a) and spiked sample (b).
Optimization of the UADLLME-SFO conditions Selection of extraction and disperser solvents. According to melting point and density, several solvents, including 1-undecanol, 1-dodecanol, 1,10-dicholorodecane and n-hexadecane, were tested as extraction solvents for UA-DLLME-SFO, and their characteristics are presented in Tab. 1 (Rezaee et al., 2010). At the same time, other several solvents, including acetone, acetonitrile, ethanol and methanol, were investigated as dispersive solvents for optimization of UA-DLLME-SFO procedure. The UA-DLLME-SFO was performed by 0.5 mL of dispersive solvent combining with 25 µL of extraction solvents at room temperature (25°C) for 20 min without salt addition.
| Extraction solvent | Melting point (°C) | Boiling point (°C) | Density (g mL−1) |
|---|---|---|---|
| 1-Dodecanol | 22 | 259 | 0.831 |
| 1, 10-dichlorodecane | 16 | 179 | 0.996 |
| n-Hexadecane | 18 | 287 | 0.887 |
| 1-undecanol | 11 | 248 | 0.830 |
The effects of extraction and disperser solvents on the extraction efficiency of Sudan I are illustrated in Fig. 2. Seen from Fig. 2, the recovery by 1-undecanol, n-hexadecane and 1-dodecanol was almost at same level (65 – 85%), but the recovery by 1,10-dichlorodecane was very low (about 40%). Furthermore, the highest recovery (85%) was obtained by a combination of 1-dodecanol with acetone. Therefore, 1-dodecanol and acetone were selected as optimal extraction and dispersive solvents in the further studies, respectively.

Effect of different combination of extraction solvents and dispersive solvents on Sudan I extraction efficiency. Other conditions: Dispersive solvent volume, 0.5 mL; Extraction solvent volume, 25 µL; Extraction time, 20 min.
Effect of extraction solvent volume. To examine the effect of extraction solvent volume on the extraction efficiency, different volumes of 1-dodecanol (15, 20, 25, 30 and 35 µL) was tested with 0.5 mL of acetone, and the results are shows in Fig. 3. The results revealed that the recovery increased with the increase of 1-dodecanol volume, and the highest recovery was obtained with 30.0 µL of 1-dodecanol. When volume of 1-dodecanol increased further (above 30 µL), the recovery kept almost change. The extraction behavior was accordant with the previous report (Asadollahi et al., 2010). Thus, 30.0 µL was selected as the optimum volume of extraction solvent in the following steps.

Effect of volume of extraction solvent on Sudan I extraction efficiency. Other conditions: Disperser solvent acetone volume, 0.5 mL; Extraction time, 20 min.
Effect of dispersive solvent volume. To obtain the optimal volume of dispersive solvent (acetone), different volumes of acetone (0.5, 1.0, 1.5, 2.0 mL) were investigated with 30 µL of 1-dodecanol. From Fig. 4, the recovery slightly increased with the increase of acetone volume in the range of 0.5 – 1.0 mL, but a further increase in the acetone volume from 1.0 to 2.0 mL resulted in a decrease in the recovery. This was because at low volumes of dispersive solvent, the extraction solvent 1-dodecanol was not dispersed well in the solution, and extraction was not complete. However, at too high volumes of dispersive solvent, the solubility of extraction solvent 1-dodecanol in the solution increased, which would result in a decrease in the recovery (Rezaee et al., 2010). In addition, smaller variability in the recovery was observed when using 1.0 mL of acetone rather than 0.5 mL. Therefore, 1.0 mL was selected as volume of dispersive solvent acetone in the next step experiment.

Effect of volume of dispersive solvent on Sudan I extraction efficiency. Other conditions: Extraction solvent 1-dodecanol volume, 30 µL; Extraction time, 20 min.
Effect of extraction time. Effect of different extraction time (0, 15, 30, 45, 60 and 90 min) on the recovery of Sudan I was investigated under ultrasound irradiation. The results are presented in Fig. 5. From Fig. 5, the recoveries increased from 0 to 15 min, but slightly decreased from 15 to 90 min. The highest recovery was obtained at 15 min. The results were different from that reported in the literature by DLLME-SFO procedure, where no significant change in recovery was observed with different extraction time (Chou et al., 2009). This was because of the difference between DLLME-SFO and UA-DLLME-SFO. Ultrasound irradiation might increase dispersion of extraction solvent 1-dodecanol in the solution which resulted in increase in the recovery, but ultrasound irradiation of long time could cause degradation of Sudan I which would result in decrease in the recovery. Thus, 15 min was chosen as the optimum time for the further experiments.

Effect of extraction time on Sudan I extraction efficiency. Other conditions: Disperser solvent acetone volume, 1.00 mL; Extraction solvent 1-dedocanol volume, 30 µL.
| Sample | Conc. (µg kg−1) | Spiked (µg kg−1) | Found (µg kg−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| chili oil | 2.56 | 2.5 | 4.53 | 79 | 4.8 |
| chili sauce | 2.71 | 2.5 | 5.01 | 92 | 5.9 |
| tomato sauce | not detected | 2.5 | 2.09 | 84 | 7.1 |
| chili powder | 0.30 | 2.5 | 2.45 | 86 | 5.6 |
| Method | LOD (µg kg−1) | Solvent volumes | Extraction time (min) | References |
|---|---|---|---|---|
| SPE-HPLC a | 4.6 | 100 mL | 35 | Qi et al., (2011) |
| MSPD-HPLC b | 50 – 90 | 8 mL | 25 | Enríquez-Gabeiras et al., (2011) |
| SM-HPLC c | 4.2 | 4 mL | 25 | Lopez-Jimenez et al., (2010) |
| GC-MS d | 2.0 – 4.2 | 50 mL | 35 | He et al., (2007) |
| UA-DLLME-SFO e | 1.5 | 30 µL | 15 | This study |
Notes:
Effect of salt. To assess effect of ionic strength of the sample solution on the recovery of Sudan I, different concentration of NaCl solution (0, 5, 10, 15, 20%, w/v) were investigated, which included the possible saline in food samples. The experimental results (Fig. 6) showed that the salinity had no significant impact on the recovery. Therefore, no salt need be added in the experiment.
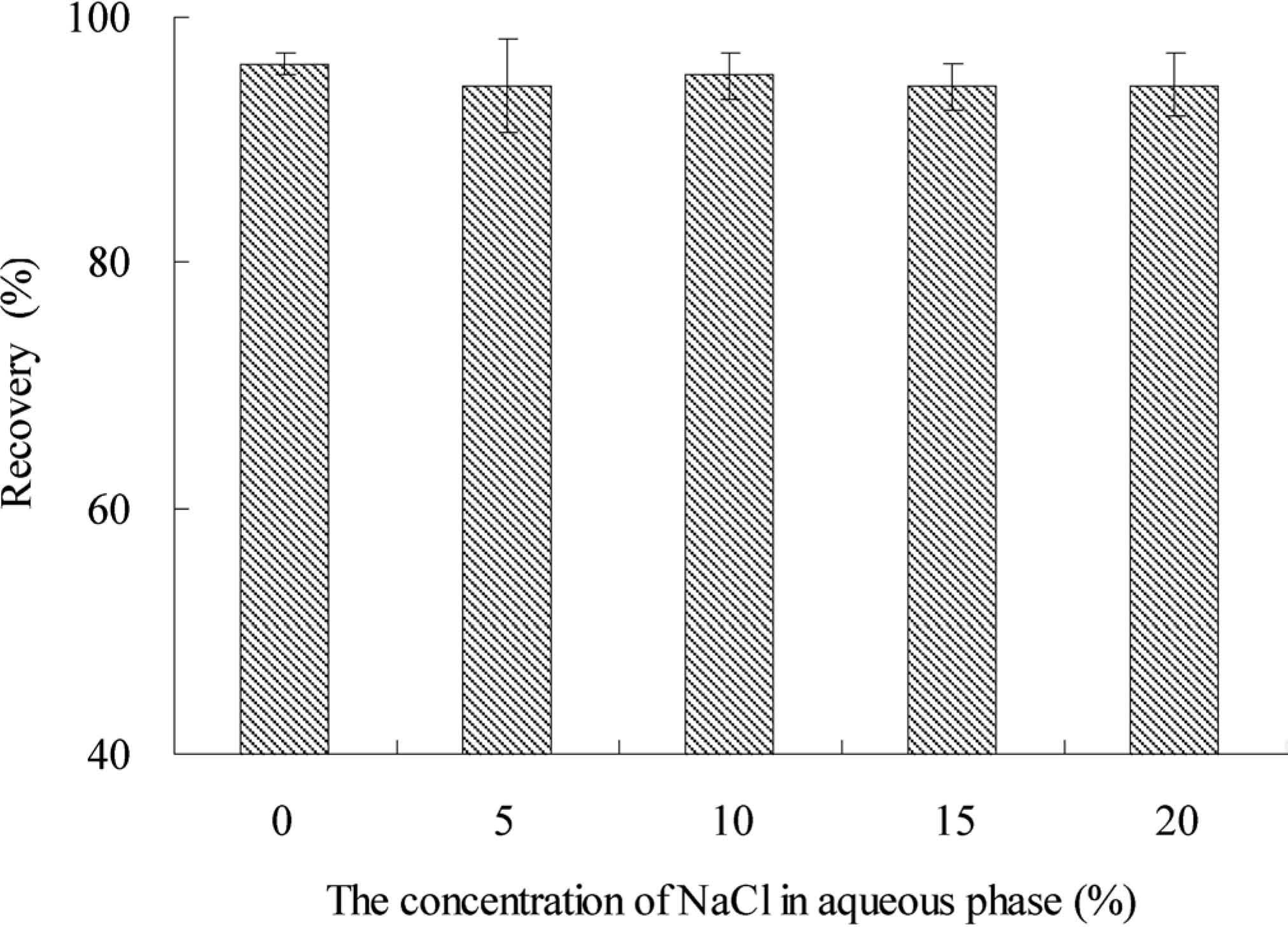
Effect of the concentration of NaCl on Sudan I extraction efficiency. Other conditions: Disperser solvent acetone volume, 1.00 mL; Extraction solvent 1-dedocanol volume, 30 µL; Extraction time, 15 min.
Validation and application of the proposed method Under the optimum conditions, the method was evaluated for linear range, limit of detection, and reproducibility. The linear range was 20 – 5000 µg L−1 with a correlation coefficient (R2) of 0.9997. The limit of detection (LOD) and limit of quantification (LOQ) were calculated as 1.5 and 3.1 µg kg−1 according to IUPAC. The relative standard deviations were 4.46 and 2.67% for 20 and 500 µg L−1 of Sudan I (n = 7), respectively.
Analysis of Sudan I in tamato sauce and chili products The proposed method has been applied to determination of Sudan I in four kinds of real food samples, including chili oil, chili sauce, chili powder and tamato sauce. Seen from Tab. 2, Sudan I was found in all chilli-products samples and the maximum value was 2.71 µg kg−1. The recoveries at each spiking level were in the range of 79% – 92% with the RSDs in range of 4.8% – 7.1%.
Comparison with other methods The present method was compared with those methods reported in the literature for the determination of Sudan I (Enríquez-Gabeiras et al., 2012; He et al., 2007, Lopez-Jimenez et al., 2010; Qi et al., 2011). Seen from Tab. 3, the present method had the lowest detection limit (1.5 µg kg−1), which was even more sensitive than that of the GC-MS method. In addition, the present method consumed less organic solvent than other methods, which indicated that it was an environmental-friendly method.
In this work, an environmental-friendly, efficient and easy-to-operate method of UADLLME-SFO combined with QuEChERS was firstly and successfully applied for isolation and enrichment of the Sudan I in tamato sauce and chilli products. Under the optimal experiment parameters, the satisfactory extraction performance and analytical merits were achieved. The limit of detection of the proposed method was 1.5 µg kg−1, which was even lower than the result obtained by GC-MS. The proposed method was successfully applied to the determination of Sudan I in four kinds of real food samples, and Sudan I levels obtained in the four kinds of samples were from not detected to 2.71 µg kg−1.
Acknowledgements This research was supported by the Doctoral Scientific Research Foundation of Guangdong Medical College, and the open funding of Guangdong Provincial Key Laboratory of Food, Nutrition and Health, the Hundred-Talents Scheme of Sun Yat-Sen University, and the Science, Technology and Information Bureau of Tianhe District in Guangzhou City (Project No. XB1310, 109G004 and 118Z004).