2015 Volume 21 Issue 6 Pages 757-764
2015 Volume 21 Issue 6 Pages 757-764
Angiotensin II and aldosterone are key hormones regulating sodium and water balance. Investigations of the taste system in rat have shown that the amiloride-sensitivity of the taste nerve responses to NaCl is enhanced by aldosterone treatment over a time course of several hours. A recent study further revealed that angiotensin II suppresses the amiloride-sensitivity of the mouse gustatory NaCl responses and enhances sweet taste sensitivity within one hour, without affecting umami, sour and bitter responses. These results suggest the presence of a reciprocal regulatory mechanism of salty taste sensitivity by angiotensin II as an acute suppressor and aldosterone as a slow enhancer in peripheral taste organs, which may play an important role in maintaining sodium homeostasis. Moreover, the possible cross-talk between salty and sweet taste modulation by angiotensin II signaling may optimize sodium and calorie intake.
The sense of taste conveys critical information about the quality and nutritional value of food before it is ingested (Lindemann, 2001, Chandrashekar et al., 2006) and thus taste information is essential for maintaining nutritive, energy and electrolyte balance. Sweet, bitter, salty, sour and umami (a Japanese word that translates into ‘delicious’ and corresponds in many ways to ‘savory’ in English) tastes are generally accepted as the five basic taste modalities in humans and rodents. Of these, salty taste is indispensable for detecting and ingesting electrolytes, especially sodium. The sodium ion is a particularly important mineral in the regulation of nerve conductance, water homeostasis, and the regulation of pH and osmotic pressure. Thus, a disturbance in sodium balance affects many aspects of health. For example, hyponatraemia is related to a fall in the plasma sodium concentration, which creates an osmotic gradient between the extra- and intra-cellular fluid. The resulting movement of water into the cells results in tissue edema and neurological symptoms such as headache, nausea, confusion or vomiting (Madias and Adrogue, 2005). Patients with Addison's disease, caused by inadequate production of mineralocorticoid aldosterone (Aldo) which mediates the reabsorption of sodium in the kidney, show symptoms of fatigue, anorexia, weight loss, low blood pressure, and a specific sodium craving (Ten et al, 2001). Furthermore, the condition of adrenalectomized animals rapidly worsens and they die about one week after the procedure unless sodium is continually supplemented in their food (Richter, 1936). In contrast, excessive sodium intake is associated with various diseases such as hypertension, stroke, heart failure, and heart attack (He et al., 2011, Zhao et al., 2011). Hypernatraemia is defined as a rise in serum sodium concentration due to excessive water loss or hypertonic sodium gain: patients show symptoms such as anorexia, restlessness, nausea or vomiting (Madias and Adrogue, 2005). There is therefore an urgent need to clarify the regulatory mechanisms of salt taste sensitivity directly influencing sodium intake and to find novel ways utilizing salty taste modulation to reduce the salt content of food products. This review focuses on the neural and molecular mechanisms of salt taste perception, and the newly revealed roles of angiotensin II (AngII), which stimulates the release of Aldo from the adrenal gland, on salty and sweet taste sensitivities in the peripheral taste organs.
The diuretic agent amiloride, a sodium channel blocker, selectively suppresses taste responses to NaCl, but not to sweet, sour, and bitter substances in many mammals (Schiffman et al., 1983, Heck et al., 1984, Jakinovich 1985, Simon et al., 1986, Herness 1987, Ninomiya et al., 1989, Hellekant and Ninomiya, 1991). Subsequent studies using amiloride in rodents suggested that two distinct components underlie cellular sensitivity to NaCl: one that is amiloride-sensitive and another that is amiloride-insensitive. Both amiloride-sensitive and -insensitive components are observed in taste buds in fungiform papillae located on the anterior two-thirds of the tongue; these papillae are innervated by the chorda tympani (CT) nerve (Ninomiya and Funakoshi, 1988, Ninomiya, 1998, Yasumatsu, 2003). On the other hand, the taste buds in circumvallate and foliate papillae located on the posterior one-third of the tongue are innervated by the glossopharyngeal (IXth) nerve and have amiloride-insensitive components (almost no amiloride-sensitive response) (Ninomiya et al., 1991, Formaker and Hill, 1991). Furthermore, the amiloride-sensitive taste cells in the taste buds respond only to Na+, while the amiloride-insensitive taste cells respond not only to Na+ but also to other electrolytes such as K+ and/or H+ (Yoshida et al., 2009).
Amiloride is a blocker of the epithelial sodium channel (ENaC). Therefore, ENaC was predicted to be a potential amiloride-sensitive salt taste receptor based on the results of multiple studies (Schiffman et al., 1983, Heck et al., 1984, Jakinovich 1985, Simon et al., 1986, Herness 1987, Ninomiya et al., 1989, Hellekant and Ninomiya, 1991). It was demonstrated several years ago that the ENaC α-subunit (αENaC) plays an essential role as a receptor for amiloride-sensitive sodium taste in mice, since taste-cell-specific αENaC knockout (KO) mice exhibited a complete loss of amiloride-sensitive sodium taste response without any effect on responses to other salts or sweet, umami, bitter and sour substances (Chandrashekar et al., 2010). ENaC is a non-voltage-gated, sodium permeable, heteromeric ion channel composed of α-, β- and γ-subunits, of which the α subunit is essential and forms part of the pore. All ENaC subunits are present in the anterior fungiform papillae in rodents, whereas only αENaC can be easily detected in circumvallate and foliate papillae (Kretz et al., 1999, Lin et al., 1999, Shigemura et al., 2005, 2008). An analysis of the co-expression of αENaC with carbonic anhydrase4 (Car4) (a marker for type III taste cell responding to sour stimuli) or transient receptor potential cation channel subfamily M member 5 (Trpm5) (a marker for type II taste cell responding to sweet, bitter or umami stimuli) in taste buds showed that αENaC was observed both in some Car4 positive cells and in taste cells lacking both Car4 and Trpm5 signals (αENaC alone cells), suggesting that the αENaC alone cells are amiloride-sensitive salty taste cells (Chandrashekar et al., 2010). Furthermore, mouse inbred strains differ in the amiloride sensitivity of their CT nerve responses to NaCl. For example, in C57BL/6 (B6) and C3H/He mice, amiloride suppresses the CT response to NaCl to ∼50% of control (Ninomiya et al., 1989), whereas amiloride produces very small (∼20% of control) or no significant inhibition of the NaCl response in BALB/c (BALB), DBA/2 (DBA) and 129P3/J (129) mice (Ninomiya et al., 1989, 1996, Gannon and Contreras, 1995, Ohkuri et al., 2006). Genetic variation analysis between the amiloride-sensitive B6 and -insensitive 129 strains suggests that the substitution of arginine 616 in αENaC in the B6 strain to tryptophan in the 129 strain (R616W) reduced amiloride sensitivity (Shigemura et al., 2008). It was also reported that NaCl taste thresholds in the 129 and A/J strains are higher than those in B6, DBA, BALB, CE/J, NZB/BINJ and SJL/J strains; however, there were no significant correlations between the NaCl taste thresholds, the NaCl preferences, or the amiloride sensitivity of the CT nerve response to NaCl (Ishiwatari and Bachmanov, 2012). Psychophysical studies in humans have shown that taste intensity for Na+ and Li+ salts is also suppressed by amiloride treatment (Schiffman et al., 1983). Genetic variants in ENaC (βENaC (G442V) or αENaC (T663A)) relate to aldosterone and potassium excretion and risk for hypertension (Ambrosius et al., 1999). Salty taste acuity is affected by the joint action of αENaC (A663T) single nucleotide polymorphism and available zinc intake in women (Noh et al, 2013). The δENaC subunit is also predicted to be involved in the NaCl or sour responses, in addition to the α-, β- and γ-subunits (Huque et al., 2009). These results suggest that ENaC also works as an amiloride-sensitive salty taste receptor in human, although the species-specific composition of each ENaC subunit and their contribution to salt perception remain unknown.
With respect to amiloride-insensitive components, a variant of transient receptor potential vanilloid-1 (TRPV1t) has been proposed as a putative amiloride-insensitive salt taste sensor. Based on taste nerve recordings, amiloride-insensitive responses to NaCl is activated by vanilloids (resinferatoxin (RTX) and capsaicin, agonists of TRPV1) and temperature (>38°C), while antagonists of TRPV1 (capsazepine and SB-366791) inhibit amiloride-insensitive responses in wild-type mice and rats, but not in TRPV1 KO mice (Lyall et al., 2004). It has also been proposed that amiloride-insensitive component is composed of at least two populations of taste cells sensing sour and bitter tastants. Double-mutant Trpm5-knockout/polycystic kidney disease 2-like 1 (Pkd2L1) (a sour receptor candidate)-TeNT (synaptic machinery in sour taste cells silenced by tetanus toxin) mice showed a near complete loss of taste responses to a variety of salts without any effect on amiloride-sensitive sodium responses (Oka et al., 2013). These results suggest that amiloride-insensitive component is mediated by orchestration of various molecules and cellular pathways (Trpv1, bitter, and sour cells).
Aldo is biosynthesized in the adrenal gland and released into the blood through the renin-angiotesin system in response to decreases in water or sodium levels and helps increase the sodium permeability of the apical membrane of the kidney, lung, colon, and sweat and salivary glands. Aldo, like other steroid hormones, exerts its major biological effects by up-regulating target gene expression; consequently, the induction of channel proteins and changes in their activity requires several hours or days. For example, in the kidney, Aldo binds to the cytosolic mineralocorticoid receptor, then the hormone/receptor complex is translocated to the cell nucleus and enhances mRNA transcription of the αENaC target gene and following protein synthesis and protein trafficking into the cell surface from internal membranes is proceeded (protein synthesis-dependent mechanism over a time course of several hours) (Garty and Benos, 1988, Lingueglia et al., 1993, 1994, Loffing et al., 2001). The percentage inhibition of the CT nerve responses to 0.3 M NaCl by amiloride in taste organs was significantly greater in both normal and adrenalectomized rats pretreated with Aldo for 4 – 6 hours compared to control, non-amiloride-treated animals (Herness, 1992). Immunohistochemical analysis suggested that Aldo pretreatment (for 24 – 48 hours), a low sodium diet (for two weeks), or both together enhanced the intensity of apical immunoreactivity for β- and γENaC subunits in the fungiform, foliate and circumvallate taste buds. In addition, whole cell recording from isolated fungiform taste cells showed that the number of amiloride-sensitive cells and the current amplitude were increased by Aldo treatment (Lin et al., 1999). These results suggest that Aldo enhances amiloride-sensitive NaCl responses by the synthesis and translocation of ENaC protein from intracellular locations to the apical membrane in taste cells over the course of several hours.
AngII is a principal mediator of the renin-angiotensin system and plays important roles in the regulation of cardiac function and sodium re-absorption. AngII is also a potent mediator for sodium appetite (a strong motivation to seek, obtain and ingest sodium) and preference (a desire for sodium in the absence of need). For example, injections of AngII (500 ng/µL every 8 min for 8 hours) into the lateral cerebral ventricle in rats causes increased intake of hypertonic 1.8% or 2.7% NaCl solution (animals usually reject hypertonic NaCl) during a 1 hour test period, whereas intracranial carbachol injection or intracellular dehydration by subcutaneous injection of 1.5 M (8.8 %) NaCl induces intake of water but has no effect on intake of concentrated NaCl solution in rats (Buggy and Fisher, 1974). A single injection of AngII into the preoptic area also produces an initial increased intake of 2.7% NaCl during the first hour. AngII treated rats preferred NaCl to KCl solutions (Avrith and Fitzsimons, 1980). Smaller doses of AngII (5 – 50 ng) administered as a single injection into the third ventricle restore the intake of 1% NaCl in rats in which induced sodium appetite had been abolished by bilateral nephrectomy (Chiaraviglio, 1976). The enhanced sodium intake following intracranial AngII administration is observed even in bilaterally adrenalectomized or hypophysectomized rats (Avrith, 1980). These results suggest that sodium appetite and intake are induced by intracranial AngII within an hour, are not secondary effects due to stimulation of hormones released from the adrenal cortex or pituitary gland, and that the induced appetite is specific for sodium.
Circulating AngII also produces dose-dependent salt appetite and stimulates sodium intake. For example, bilateral nephrectomy in rats greatly reduces sodium appetite (Fitzsimons and Stricker, 1971). Intravenous chronic AngII administration induces salt appetite, which is similar to that observed after an overnight period of sodium deprivation and an injection of furosemide (Fitts et al., 2007). Salt appetite induced by intravenous AngII infusion is observed even in adrenalectomized rats, suggesting this induction may be independent of the effects of Aldo induced by AngII (Dalhouse et al., 1986). Captopril is an inhibitor of angiotensin converting enzyme1 (Ace1) which cleaves the C-terminal histidine-leucine of AngI to generate AngII. Intravenous infusion of captopril reduces or abolishes salt appetite rapidly without affecting the conversion of AngII inside the blood-brain barrier in sodium-depleted rats (Thunhorst and Fitts, 1994). These results suggest that induced sodium appetite can be explained by the action of peripheral circulating AngII.
Salty taste is crucial for evaluating the sodium content of food. Thus, sodium taste sensitivity might be related to sodium appetite induced by AngII, as described above. However, the contribution of AngII to salty taste sensitivity remains unclear.
AngII interacts with at least two AngII receptors, designated type1 (AT1) and type2 (AT2), and belonging to the G-protein coupled receptor (GPCR) family. The predominant biological effects of AngII, such as vasoconstriction and sodium re-absorption, are believed to be mediated through the AT1 receptors. AT1 receptors are widely distributed throughout the body, including vascular smooth muscle, adrenal gland, kidney, heart, lung, liver and brain. In contrast, AT2 receptors are thought to oppose the actions mediated by AT1 (de Gasparo et al., 2000). Aldo induced by circulating AngII elevates the amiloride-sensitive sodium responses in the taste nerves and cells, as described above. Such enhanced salty taste sensitivity due to Aldo, however, would increase aversive responses to a hypertonic NaCl solution. Increased taste sensitivity due to Aldo might be mediated through the synthesis and translocation of ENaC protein from intracellular locations to the apical membrane in taste cells. Such multi-step enhancement of taste sensitivity would require many hours. Thus, these Aldo effects on salt taste sensitivity would not account for the acute facilitation of sodium intake by AngII administration. We therefore hypothesized that if receptors for AngII, but not for Aldo, were expressed in peripheral taste cells, circulating AngII may act as an acute suppressive mediator for amiloride-sensitive salt responses. The sequential and opposing regulation by an acute suppressor (AngII) and a slow enhancer (Aldo) on peripheral salt taste sensitivity would contribute to and regulate sodium intake, and play an important role in sodium homeostasis. To explore this hypothesis, we examined gustatory nerve and behavioral responses to taste stimuli after an intraperitoneal (i.p.) injection of AngII and the expression of AngII receptors in mouse taste organs by RT-PCR, in situ hybridization, and double immunohistochemistry (Shigemura et al., 2013).
Salty taste transduction occurs through amiloride-sensitive and -insensitive sodium transport pathways (Ninomiya and Funakoshi, 1988, Ninomiya, 1998, Yasumatsu, 2003). Thus, the CT nerve responses involving both amiloride-sensitive and -insensitive components were investigated in B6 mice. After an i.p. injection of AngII, the CT nerve responses to NaCl started to decrease and reached near maximum suppression (∼70% of control) at 30 min, then recovered to near control levels one hour after AngII administration. On the other hand, the CT nerve responses to NaCl in the presence of 30 µM amiloride did not change after an i.p. injection of AngII. These results suggest that AngII affects amiloride-sensitive salt responses selectively, but not amiloride-insensitive salt responses. Surprisingly, a significant enhancing effect of AngII on sweet taste responses (to the natural sugars glucose and sucrose, and to the artificial sweeteners saccharin and SC45647) was also observed at 10 – 30 min post-injection. AngII did not influence CT responses to KCl, sour (HCl), bitter (quinine hydrochloride: QHCl) or umami (monosodium glutamate: MSG) tastants. The effects of Ang II on nerve responses were inhibited by pretreatment with a specific antagonist of AT1, CV11974 (Kinugawa et al., 1993). These results suggest that AngII acts on the peripheral taste organs of mice via AT1, suppresses CT nerve responses to NaCl through amiloride-sensitive receptors, and enhances CT nerve responses to sweeteners selectively (Shigemura et al., 2013).
A time course study of the effects of AngII on the CT nerve responses showed significant increases of amiloride-sensitive sodium responses at 90 – 120 min after the AngII injection. Such slow enhancing effects on sodium responses may be due to the action of Aldo because Aldo is stimulated by AngII and is thought to be a slow enhancing mediator of amiloride-sensitive salt taste responses in taste cells (Lin et al., 1999, Garty and Benos, 1988, Lingueglia et al., 1993, 1994, Loffing et al., 2001). Taken together, AngII may play a role in increasing sodium intake by reducing amiloride-sensitive sodium taste responses acutely, and subsequently stimulated Aldo may act to stop excessive sodium intake by enhancing amiloride-sensitive responses slowly (Shigemura et al., 2013) (Fig1).
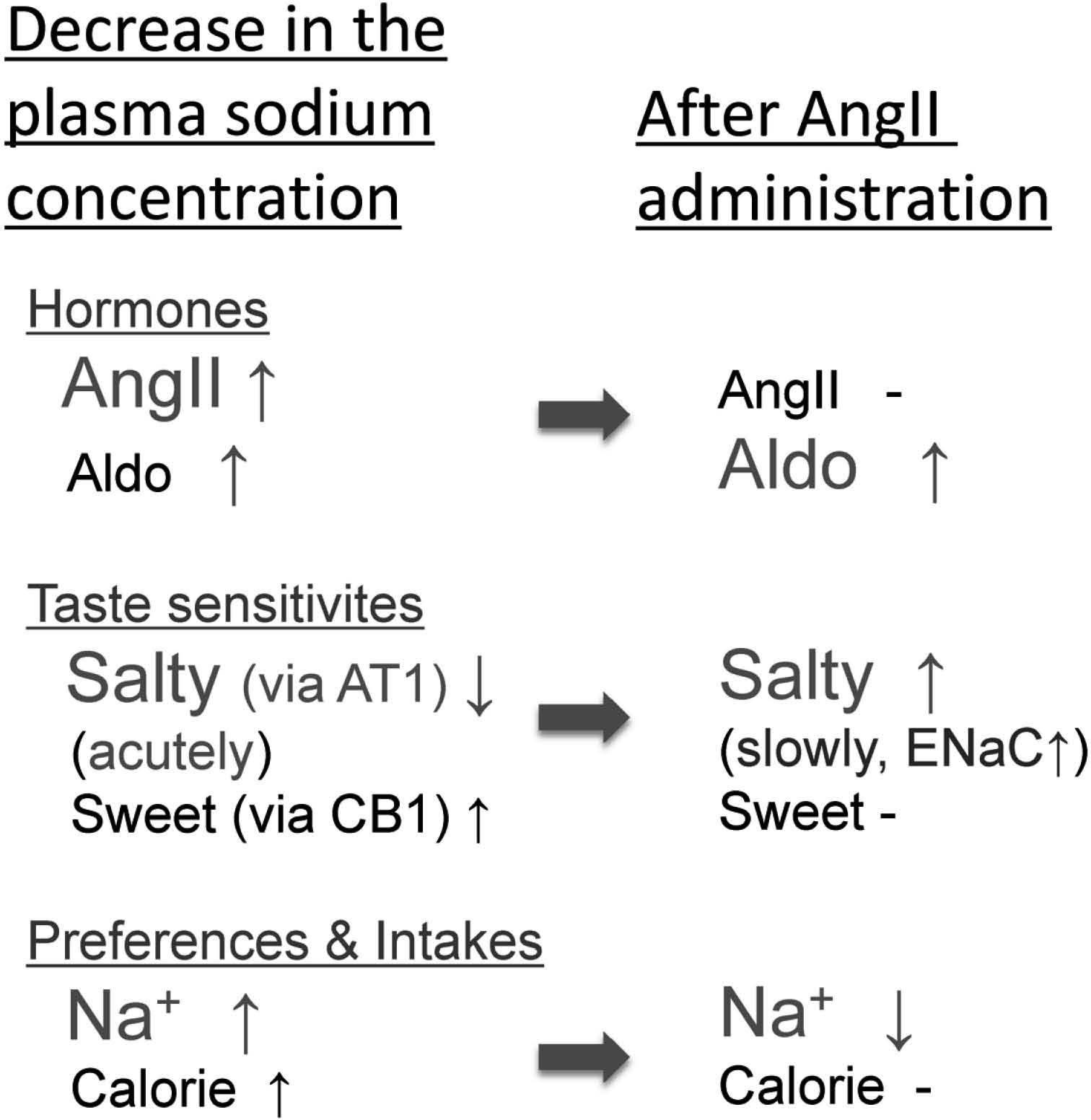
Possible regulatory mechanism of salty taste sensitivity by angiotensin II (AngII) and aldosterone (Aldo). AngII plays a role in increasing sodium preference and intake by reducing amiloride-sensitive sodium taste responses acutely via AngII type I receptors (AT1) expressed in amilorode-sensitive salt taste cells; subsequently stimulated Aldo acts to stop excessive sodium intake by enhancing amiloride-sensitive responses slowly. Concurrently, AngII enhances sweet taste sensitivity via endocannabinoid receptor1 (CB1) expressed in sweet taste cells, thus possibly contributing to increase calorie intake. The cross-talk between salty and sweet taste modulation by AngII signaling may optimize sodium and calorie intakes.
Behavioral responses after AngII injection were also examined by using a brief-access test (per 10 sec) which can measure the number of licks for taste substances without post-ingestive effects (Murata et al., 2003, Shigemura et al., 2004). The number of licks for preferred distilled water (DW) and sweeteners were in the range 65 – 80, while the number of licks for aversive tasting solutions such as 0.3 – 1.0 mM QHCl was around 10 – 15. The behavioral tests showed that lick rates for NaCl (amiloride-sensitive) and sweeteners, but not for KCl, sour, bitter or umami substances, were significantly reduced by pretreatment with the AT1 antagonist, CV11974. These results suggest that salty taste inhibition and sweet taste enhancement by AngII on the CT nerve responses critically influence ingestive behaviors for sodium and sweet taste compounds (Shigemura et al., 2013).
Taste buds are onion-shaped structures consisting of 50 – 100 taste cells that interact with gustatory nerves. In the human oral cavity, taste buds are distributed on the tongue, palate, epiglottis, larynx and pharynx. The taste buds on the tongue are embedded in fungiform papillae in the anterior part, in circumvallate papillae in the posterior part, and in foliate papillae in the lateral sides of the tongue (Roper, 2013). The taste bud contains at least three morphologically distinct cell types, type I, II and III (Murray, 1973). Type I cells are the most abundant cells in taste buds and are defined ultrastructurally as showing electron-dense cytoplasm, elongated and pleomorphic nuclei, extended lamellate processes around other types of taste cells, and express a glial glutamate/aspartate transporter (GLAST) (Lawton et al., 2000) and an ecto-ATPase2 (Entpd2) (Bartel et al., 2006). These characteristics suggest that Type I cells act to isolate individual taste cells functionally, and to uptake and clear neurotransmitters following their release at synapses likes as glial cells within the taste buds. Type II cells consist of at least three subsets of cells that respond to sweet, umami and bitter tastants and express GPCR taste receptor type 1 (T1r) or type 2 (T2r) families: T1r2+T1r3 heterodimer as a sweet taste receptor, T1r1+T1r3 heterodimer as an umami taste receptor, and T2r receptors as bitter taste receptors (Kitagawa et al., 2001, Montmayeur et al., 2001, Max et al., 2001, Sainz et al., 2001, Nelson et al., 2001, Bachmanov et al., 2001, Chandrashekar et al., 2000). Gα-gustducin (McLaughlin et al., 1992), phospholipase C β2 (PLCβ2) (Miyoshi et al., 2001), inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) (Clapp et al., 2001) and Trpm5 (Pérez et al., 2002, Talavera et al., 2005, Damak et al., 2006) are common transduction cascade molecules downstream of these T1rs and T2rs taste receptors. Based on ultrastructural criteria, type II cells do not have conventional synapses. Type III cells are defined as having identifiable synaptic contacts with the gustatory nerves and are believed to express sour taste receptor candidate Pkd2L1 (Lopez-Jimenez et al., 2006, Ishimaru et al., 2006, Horio et al., 2011) and synaptic membrane proteins such as synaptosomal-associated protein 25 (SNAP25) (Yang et al., 2000, Clapp et al., 2006), protein gene product 9.5 (PGP9.5) (Iwanaga et al., 1992), glutamic acid decarboxylase 67 (GAD67) (Tamamaki et al., 2003), and neural cell adhesion molecule (NCAM) (Nelson and Finger, 1993).
Based on these studies, expression analysis of the AngII receptors AT1 and AT2 in taste tissues were performed by RT-PCR, in situ hybridization, and double colored immunohistochemistry. AT1 mRNAs were expressed in fungiform and circumvallate papillae, but not in epithelial tissue lacking taste buds in B6 mice. The expression pattern was similar to that of RT-PCR bands for the type II taste cell marker Trpm5 and the type III taste cell marker Pkd2L1. AT2 mRNAs were not detected in any of these taste tissues. In situ hybridization analyses detected AT1 mRNAs in a subset of taste cells in both fungiform and circumvallate papillae but not in surrounding epithelial cells. Double labeled immunohistochemistry showed that AT1 proteins were co-expressed with an amiloride-sensitive salt taste receptor, αENaC, or a sweet taste receptor component, T1r3, but not with a sour receptor candidate, Pkd2L1, in a subset of taste cells in both fungiform and circumvallate papillae in B6 mice (Shigemura et al., 2013). It had been reported that amiloride-sensitive taste cells express ENaC subunits, but not the type II cell marker Gα-gustducin or the type III cell marker SNAP25 (Yoshida et al., 2009, Yang et al., 2000, Clapp et al., 2006). αENaC expressing cells do not express Trpm5 in fungiform or palate taste buds (Chandrashekar et al., 2003). These results suggest that AT1, but not AT2, is expressed in mouse taste bud cells in both the anterior and posterior tongue, and the amiloride-sensitive salt and sweet taste modulations by AngII occur in a distinct subset of taste cells expressing ENaC or T1r3 via AT1 (Shigemura et al., 2013).
Endocannabinoids, such as 2-arachidonoyl glycerol (2AG) and anandamide (N-arachidonoylethanolamine, AEA), are orexigenic mediators acting through cannabinoid receptor 1 (CB1) in the hypothalamus and limbic forebrain and induce appetite and stimulate food intake (Jamshidi and Taylor, 2001, Cota et al., 2003). It was recently revealed that endocannabinoids enhance sweet taste sensitivity via CB1 in peripheral T1r3 expressing sweet taste cells (Yoshida et al., 2010). Moreover, AT1 and CB1 form receptor heteromers, resulting in the potentiation of AT1 signaling in hepatic stellate cells from ethanol-administered rats (Rozenfeld et al., 2011). CB1 is transactivated by AT1 stimulating 2AG production in Chinese hamster ovary cells (Turu et al., 2009). These results raised the possibility that the activation of AT1 by AngII administration stimulates CB1 signaling in sweet taste cells, thereby enhancing sweet taste sensitivity. To investigate this possibility, the effects of AngII on sweet taste responses were examined by using CB1-knockout (KO) mice (Ledent et al., 1999). The CT nerve responses to NaCl in CB1-KO mice were significantly decreased after AngII treatment as compared to B6 control mice, whereas the responses to sweeteners were not changed in CB1-KO mice. These results suggest that AngII acts to modulate sweet taste responses via CB1 receptors in sweet taste cells independently of αENaC expressing amiloride-sensitive salt taste cells (Shigemura et al., 2013). The enhancing effects of AngII on sweet taste sensitivity may contribute to increase calorie intake, which may play a role in regulating calorie homeostasis.
AngII modulates amiloride-sensitive salt and sweet taste responses via AT1 receptors expressed in peripheral taste cells (Fig. 1). These modulations by AngII occur in a distinct subset of αENaC-positive amiloride-sensitive salty taste cells or T1r3-positive sweet taste cells. These results suggest that the taste organ is a newly appreciated peripheral target of AngII. The specific suppression of amiloride-sensitive salt taste sensitivity by AngII may contribute to increase NaCl intake. The reciprocal and sequential regulation of salt taste sensitivity by AngII as an acute suppressor and Aldo as a slow enhancer may play an important role in sodium homeostasis in cooperation with the brain and other organs. Concurrently, AngII may contribute to increase calorie intake by enhancing sweet sensitivity via CB1 receptors. The cross-talk between salty and sweet taste modulations by AngII may optimize sodium and calorie intakes.
The author declares no competing financial interests.
Acknowledgements This research was supported in part by Grants-in-Aid 24659828 and 15K11044 (N.S.) for Scientific Research from the Ministry of Education, Culture, Sports and Science, Japan.
aldosterone
AngIIangiotensin II
AT1 (AT2)angiotensin II receptor type 1 (type 2)
Car4carbonic anhydrase 4
CB1cannabinoid receptor 1
CTchorda tympani
DWdistilled water
GAD67glutamic acid decarboxylase 67
GLASTglial glutamate/aspartate transporter
ENaCepithelial sodium channel
Entpd2ecto-ATPase2
GFPgreen fluorescent protein
HClhydrochloric acid
i.p.intraperitoneal
IP3R3inositol 1,4,5-trisphosphate receptor type 3
KClpotassium chloride
KOknockout
MSGmonosodium glutamate
NaClsodium chloride
NCAMneural cell adhesion molecule
PGP9.5protein gene product 9.5
Pkd2L1polycystic kidney disease 2-like 1
PLCβ2phospholipase C β2
QHClquinine hydrochloride
RT-PCRreverse transcriptase polymerase chain reaction
SNAP25synaptosomal-associated protein 25
T1r (1-3)taste receptor family 1 member (1-3)
Trpm5transient receptor potential cation channel subfamily M member 5
TRPV1transient receptor potential vanilloid 1