2015 Volume 21 Issue 6 Pages 813-819
2015 Volume 21 Issue 6 Pages 813-819
Effects of the types of sugars and buffers, and pH on the formation of Maillard reaction pigments were investigated using the xylose-lysine and glucose-lysine model systems. Sugars (13.3 mM) and lysine (34 mM) were dissolved in acetate, phosphate, and tris(hydroxymethyl)aminomethane (Tris) buffers and heated at 100°C for 60 min. The profiles of browning, reversed-phase HPLC, sugar-decrease, and gel permeation chromatography of each sample were examined. Browning was stimulated by phosphate and inhibited by Tris. With the rise in pH value, browning occurred more intensively, more sugars decreased, and more melanoidins were formed. Xylose samples turned darker brown than glucose ones. The ratio of absorbance at 400 nm to that at 450 or 500 nm increased in the xylose system with the rise in pH value, while that in the glucose system decreased. Low-molecular-weight pigments, dilysyldipyrrolones, were formed only in the xylose system, while melanoidins were formed in both systems. Lysine stimulated the degradation of sugars, polymerization, and browning in both systems, although more intensively in the xylose system than in the glucose one.
The Maillard reaction between sugars and amino acids occurs ubiquitously during food processing and storage, and is critical in the formation of both desirable and undesirable pigments and flavors. The reduction of nutritional quality such as decrease in lysine and the formation of carcinogenic compounds by the reaction are also cause for concern.
One of the most characteristic contributions of the Maillard reaction to food is the change in color, i.e., browning. The formation of brown pigments, melanoidins, was first observed in 1912 by Louis Maillard following the heating of a solution of glucose and lysine; the reaction was thereafter referred to as the Maillard reaction (Eskin, 1990). Although intensive studies have subsequently been performed, the chemical structure of melanoidins remains unclear, because a wide variety of decomposition and polymerization of sugars and amino acids occurs during the Maillard reaction. Melanoidins formed by the reaction are heterogeneous polymers containing nitrogen (Wang et al., 2011). On the other hand, the regulation of the reaction is essential for food processing and storage. Factors or conditions, such as pH, buffers, temperature, moisture content, types of sugars and amino acids, and metals, involved in browning have been examined (Eskin, 1990).
Recently, our group observed that low-molecular-weight Maillard pigments, named dilysyldipyrrolones (DLDPs; Fig. 1), significantly contributed to the browning or color of a heated solution of xylose and lysine under weakly acidic conditions (Sakamoto et al., 2009; Nomi et al., 2011; Nomi et al., 2013). This result suggests that low-molecular-weight pigments in addition to melanoidins, high-molecular-weight brown compounds, should be considered in the color regulation of solutions containing sugars and amino acids. In general, increases in pH ensure that more sugars are in the open chain or reducing form, resulting in the promotion of the reaction between carbonyl and amino groups and pigment formation. Similarly, the amount of open chain or oxo form is greater in pentoses such as xylose and ribose than in hexoses such as glucose and fructose. Thus, the pentose system of the Maillard reaction turns brown more easily than the hexose system. It is well-known that buffers change the ionic environment in which the reaction takes place and influence browning (Eskin, 1990). However, this knowledge is derived from separate experiments and involved various experimental conditions. Thus, it is necessary to compare the factors affecting browning under the same conditions. Further, how the reaction conditions affect the formation of colored compounds including these derivatives in a solution containing sugars and lysine remains to be elucidated. In this study, we employed reversed-phase HPLC and gel permeation chromatography (GPC) equipped with a diode-array detector to evaluate the colored compounds in the model system. We compared xylose and glucose as a representative pentose and hexose, respectively, and described the effects of xylose and glucose, buffer type, and pH on the formation of Maillard pigments in the lysine model system.
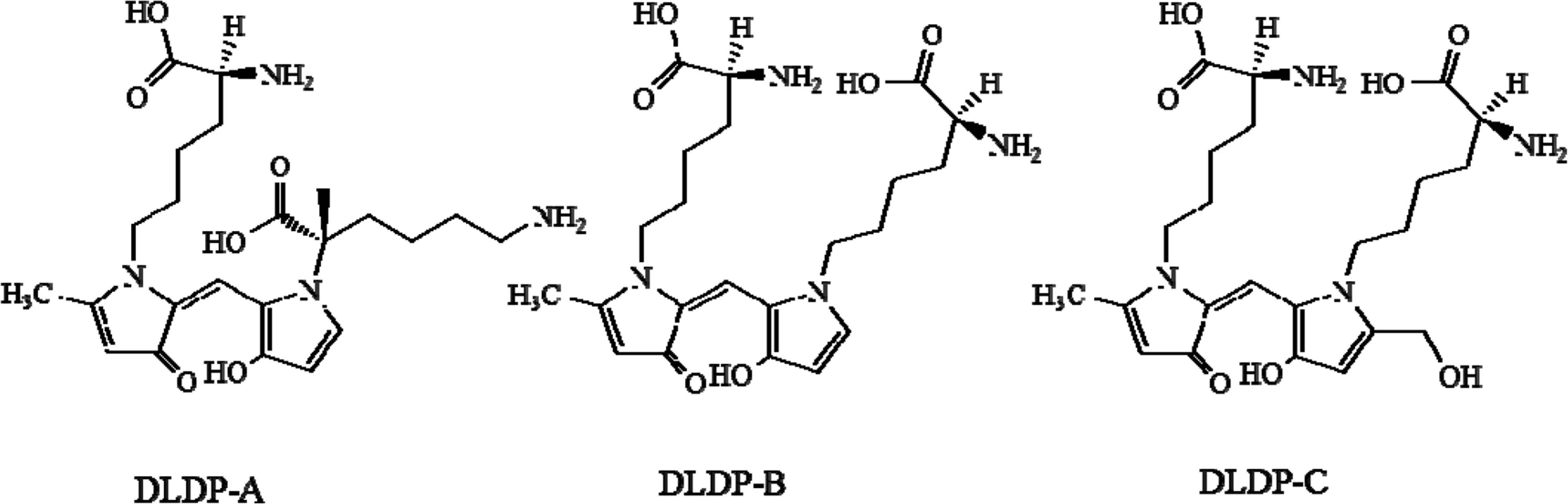
Structures of dilysyldipyrrolones (DLDPs) A, B, and C.
Sample preparation and estimation of browning Buffer solutions (0.2 M acetate-Na buffer (pH 3.5–6.0), 0.2 M phosphate-Na/K buffer (pH 5.5–8.0) and 0.2 M tris (hydroxymethyl) aminomethane (Tris)-HCl buffer (pH 7.0–9.0)) containing 13.3 mM of sugar (xylose or glucose) and 34 mM of l-lysine monohydrochloride were heated in boiling water for 60 min. Absorbance at 400, 450, and 500 nm were measured by photodiode array spectrophotometry (Multi Spec-1500; Shimadzu, Kyoto, Japan). Each sample was prepared at least three times for analyses.
HPLC analysis Samples were analyzed using a reversed phase HPLC system equipped with photodiode array detection under the following conditions: pump, HITACHI L-6320 Intelligent Pump (Hitachi, Tokyo, Japan); column, COSMOSIL 5C18-MS-II (i.d. 4.6 × 250 mm; Nacalai Tesque, Kyoto); eluent, solution A (0.1% trifluoroacetic acid (TFA) − water/MeOH = 98/2, V/V) and solution B (0.1% TFA − water/MeOH = 50/50, V/V), 0% B for 0 – 2 min, 0 to 5 0% B for 2 – 12 min and 50% B for 12 to 30 min; flow rate, 1 mL/min; column temperature, 40°C; detector, HITACHI L-4500 Diode Array Detector (Hitachi); detection wavelength, 250 – 500 nm (280 nm and 400 nm for quantification). DLDPs A, B, and C on the chromatograms were identified by the retention times and spectra using standard samples.
GPC analysis Sample solutions were analyzed using an HPLC system equipped with GPC column and a photodiode array detector under the following conditions: pre-column, TSK guard column PWXL (i.d. 6.0×40 mm; Tosoh, Tokyo,); column, TSK-gel G2500PWXL (i.d. 7.8×300 mm; Tosoh); eluent, Milli-Q water; flow rate, 0.5 mL/min; column temperature, 40°C; detector, HITACHI L-4500 Diode Array Detector (Hitachi); detection wavelength, 250 – 500 nm (400 nm for quantification).
Sugar analysis Sugars in sample solutions were analyzed using an HPLC system equipped with an evaporative light scattering detector by the external standard method under the following conditions: column, COSMOSIL SUGAR-D (i.d. 4.6 × 250 mm; Nacalai Tesque) for xylose and Shodex Asahipak NH2P — 50 4E (i.d. 4.6 mm × 250 mm; Asahikasei, Tokyo) for glucose; eluent, acetonitrile/water = 75:25 (V/V); flow rate, 1 mL/min; column temperature, 40°C; pump, JASCO PU-980 Intelligent Pump (JASCO, Tokyo); detector, Alltech 3300 ELSD (Alltech, Grace, IL, U.S.A.).
Fluorescence analysis Fluorescence intensities of sample solutions were measured with a spectrofluorophotometer (RF-540; Shimadzu) at 370 nm for excitation and 450 nm for emission. Sample solutions were further analyzed using an HPLC system with fluorescence detection under the following conditions: column, YMC-Pack R&D ODS-A (i.d. 4.6 × 250 mm; YMC, Kyoto); eluent, solution A (0.1% TFA − water/MeOH = 98/2, V/V) and solution B (0.1% TFA − water/MeOH = 50/50, V/V), 0% B for 0 to 2 min, and 0 to 50% B for 2 to 12 min, and 50% B for 12 to 30 min; flow rate, 1 mL/min; column temperature, 40°C; detector, HITACHI FL Detector L-2485 (Hitachi); detection wavelength, 370 nm for excitation and 450 nm for emission.
Effects of pH and buffer type on the Maillard reaction in the xylose-lysine system At first, the effects of pH and buffer type on the browning of a solution containing xylose and lysine were examined (Fig. 2). Acetate, phosphate, and Tris were used as the buffers. Maillard browning was estimated by measuring the absorbance at 400, 450 and 500 nm. Among samples of the same buffer type, browning occurred more intensively with the rise in pH value. However, buffer type had a larger effect on browning than pH. Browning was most intensively observed in the phosphate buffer among the three buffer types.
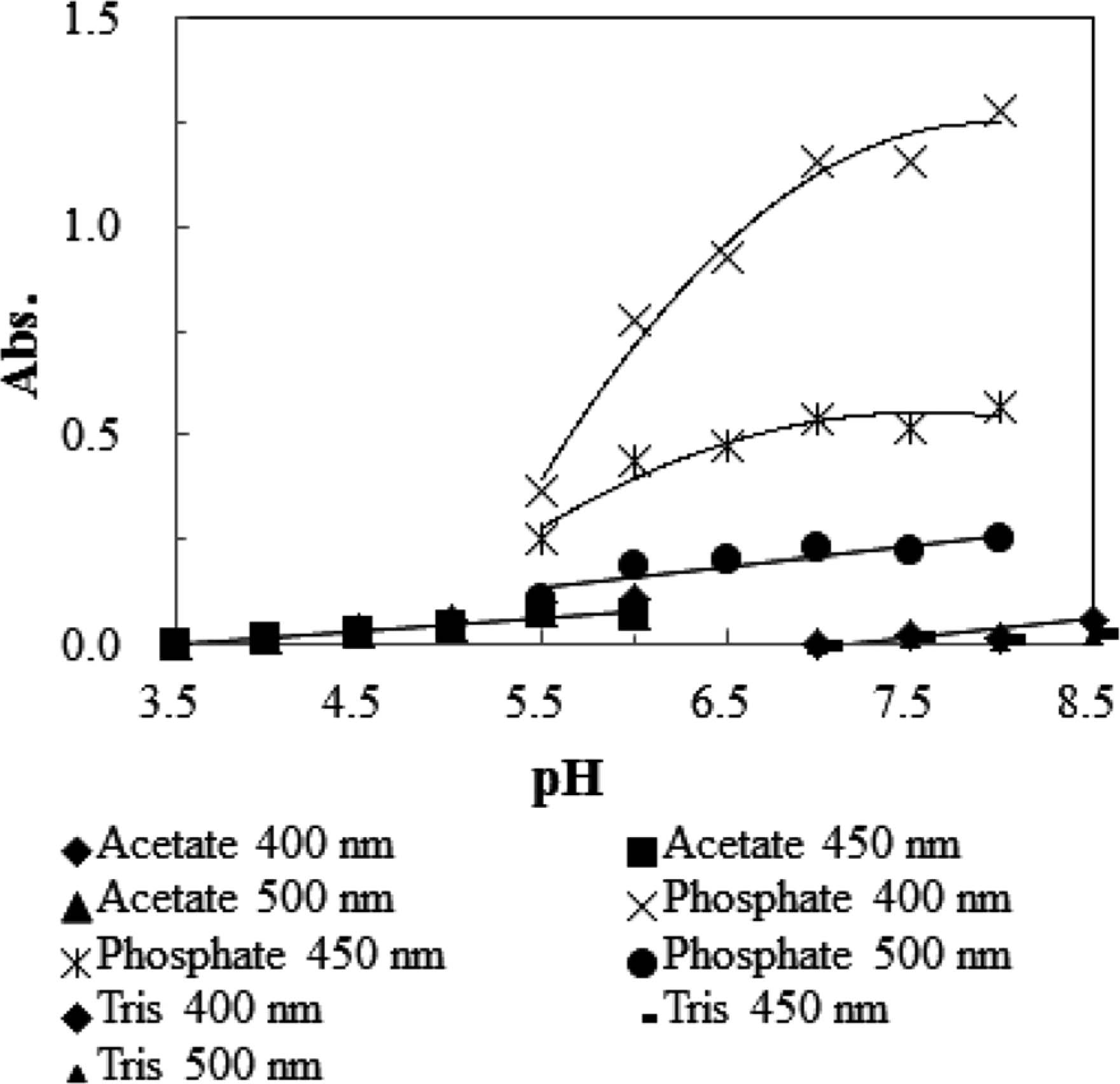
Effect of pH and buffer on the browning in the xylose-lysine system of the Maillard reaction. A buffer solution (0.2 M acetate, 0.2 M phosphate, and 0.2 M Tris) containing 13.3 mM xylose and 34 mM lysine was heated at 100°C for 60 min.
Next, the effect of buffer on the formation of low-molecular-weight Maillard pigments was examined. DLDPs A, B, and C (Fig. 1) are reported as the major low-molecular-weight Maillard pigments in the xylose-lysine system under weakly acidic conditions (Sakamoto et al., 2011; Nomi et al., 2013). Figure 3 shows the reversed-phase HPLC profiles of the reaction solutions consisting of different buffers. Low-molecular-weight Maillard pigments were apparent as peaks having absorption maxima at or near a visible region, while melanoidins were broad convex or trapezoidal peaks showing no specific absorption maxima in the UV-Vis region. DLDPs A, B, and C were clearly detected in the reaction solution containing the acetate buffer (Fig. 3-A), while they were not detected in the reaction solution containing the Tris buffer (Fig. 3-C). DLDP C was the major low-molecular-weight pigment in the phosphate buffer, while DLDPs A and B were those in the acetate buffer. While the reason is unclear, this result suggests that buffer type has an effect on not only the promotion of browning but also the pathway of the Maillard reaction. Since definite differences in the browning and HPLC profiles were observed between the phosphate and Tris-HCl buffer samples, further experiments were conducted. Buffer solutions (pH 7.5) of Tris-HCl, Tris-phosphoric acid (Tris-Pi), NaOH-phosphoric acid (NaOH-Pi), and Na/K is and phosphate on browning. Figure 4 shows the browning and sugar decomposition of the reaction solutions containing different buffers. Browning was estimated by the absorbance at 400 nm. The absorbance at 400 nm of the reaction solution of Tris-Pi was lower than those of NaOH-Pi and NaKPi, while that of Tris-Pi was higher than that of Tris-HCl. This result clearly showed that Tris inhibited Maillard browning while phosphate facilitated it. Similarly, phosphate promoted the decomposition of sugars while Tris inhibited it. Many previous studies have shown phosphate's ability to facilitate the Maillard reaction. For example, Sumaya-Martinez et al. (2005) showed that phosphate had a stronger effect on browning and the antiradical activity of Maillard reaction products than citrate or Tris-HCl buffers, and that a positive correlation was observed among browning, antiradical activity, and phosphate concentration. Bell (1997) reported that the browning and glycine loss increased with the increase in phosphate buffer concentration and that it was due to the bifunctional catalytic ability of phosphate anions. Rizzi (2004) suggested that phosphate acted as a polyatomic anion to facilitate the aldose/ketose equilibration of reducing sugars and aldose sugar dehydration and provide reactive intermediates, α-dicarbonyls, leading to browning. It is reported that sodium or potassium phosphate contains small amounts of transition metals that promote the Maillard reaction and browning (Hayase et al., 1996), thus contaminating metals in the system might stimulate browning. However, browning in the Tris-Pi buffer was more intensive that that in the Na/Pi buffer. It was also reported that Tris inhibited the Maillard reaction (Andreassen and Oxlund, 1985). Tris competes with the deprotonation of amino groups of lysine and reduces the deprotonated amino groups of lysine. As a result, the Maillard reaction or browning was considered to be repressed in the Tris buffer (Rizzi, 2008). Except for the Tris-HCl buffer samples, the formation of DLDPs was observed using HPLC analyses (data not shown). Since xylose decomposition was only minimally observed in Tris-HCL buffer, few pigments were considered to be formed.
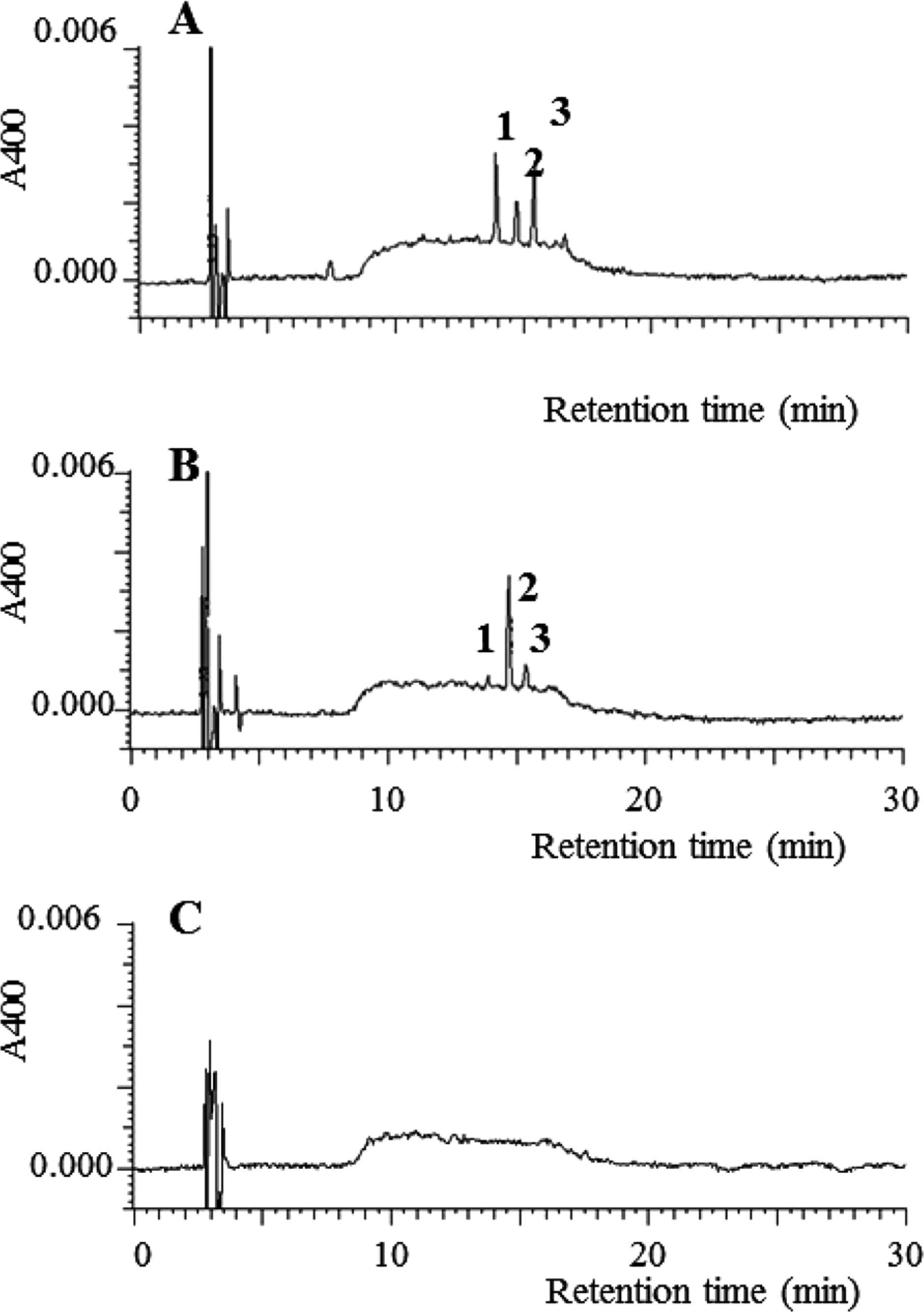
Effect of buffer on reversed-phase HPLC profiles in the xylose-lysine system of the Maillard reaction. A, acetate buffer (pH 6.0); B, phosphate buffeer (pH 6.5); C, Tris buffer (pH 9.0). 1, DLDP A; 2, DLDP C, 3, DLDP B.
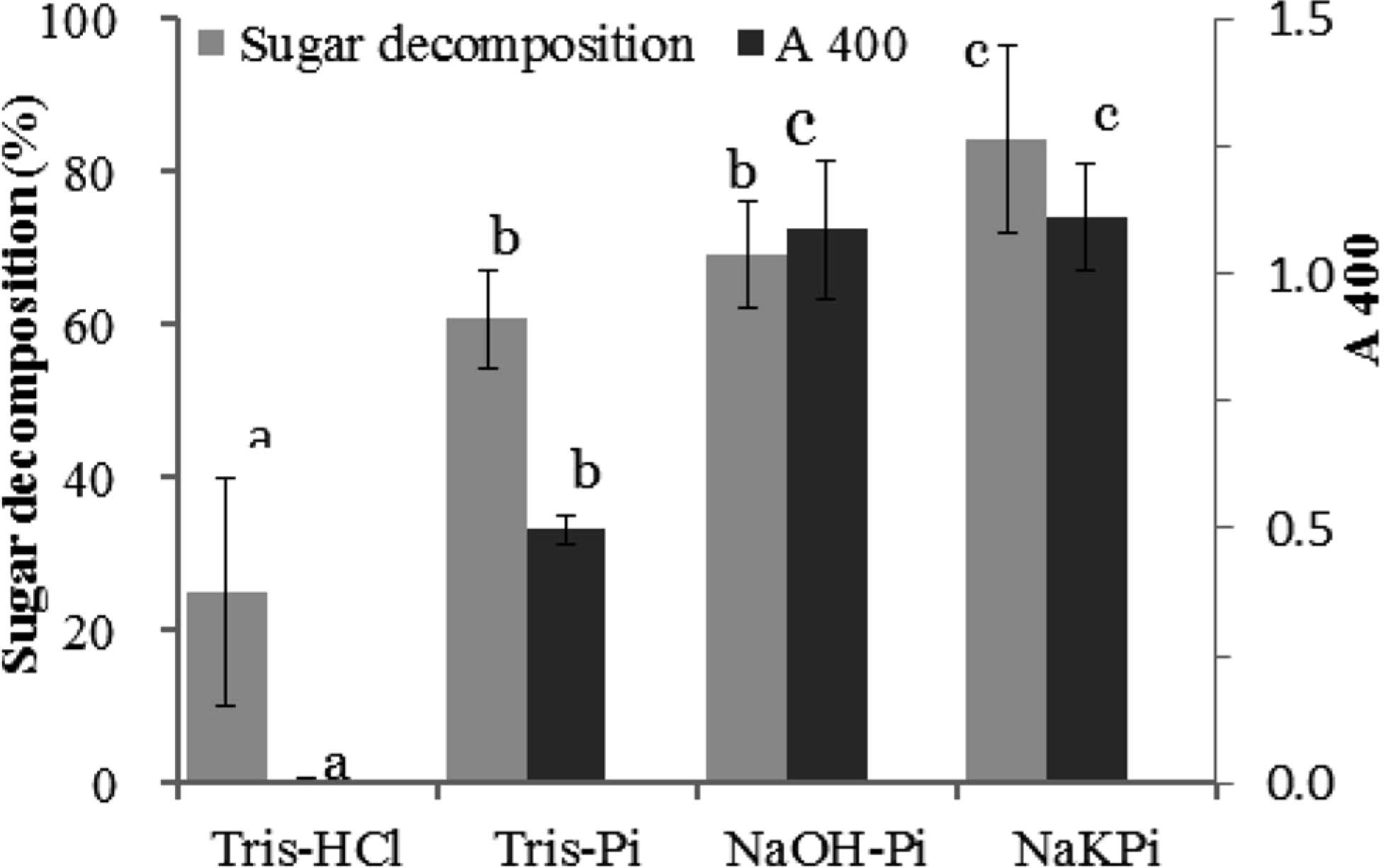
Effect of phpsphate and Tris on browning and sugar decompsition in the xylose-lysine system of the Maillard reaction. Tris-HCI, Tris-phosphate (Tris-Pi), Na phosphate (NaOH-Pi), and Na/K phosphate (NaKPi) ware used as buffers (n = 3). The concentrations of Tris and phosphate were 0.2 M. In Tris-phosphate buffer, the concentration of Tris was 0.2 M.
Comparison of browning between xylose and glucose systems The browning estimated by absorbance at 400, 450, and 500 nm was compared between the glucose-lysine and xylose-lysine systems at pH from 5.5 to 8.0 (Fig. 5-A). Xylose samples turned brown more intensively than glucose ones at every pH value. With the rise in pH value, the absorbance values at all wavelengths were increased in both xylose and glucose systems, showing that browning occurred more strongly under high pH conditions. These observations corresponded to that previously reported (Eskin, 1990). The ratio of absorbance at 400 nm to that at 450 or 500 nm was then calculated to indicate color tone. As a result, the ratio increased in the xylose system with the rise in pH value, while that in the glucose system decreased (Fig. 5-B). The ratio in the glucose-lysine system under weakly acidic conditions was smaller than that in the xylose-lysine system, while under weakly basic conditions there was little difference apparent between the two systems. This result shows that sugar type has an effect on the color tone of Maillard pigments in addition to the browning intensity. Although the higher values often suggest brighter or clearer color tone, in this case the lower value means more reddish yellow or orange, as DLDPs formed in the xylose-lysine system have an absorption maximum at 440 nm. Sugar decomposition was compared between the glucose-lysine and xylose-lysine systems (Fig. 6-A). In the absence of lysine, glucose was stable at pH 5.5 to 7.0 and xylose was partly decomposed. Decomposition occurred more rapidly at higher pH than at lower pH. As a greater amount of the open chain form is present in xylose solution than that in glucose one, xylose is more rapidly decomposed and reacted with lysine than glucose. The relationship between residual sugar and browning was then examined (Fig. 6-B). The more sugar was decomposed, the greater the browning. The browning of the solution showing the same degree of sugar decomposition was compared to discriminate the effect of sugar decomposition and browning in the xylose-lysine and glucose-lysine systems. At the same level of sugar decomposition, for example 70% (Fig. 6-B), the xylose-lysine system showed the most intensive color followed by the glucose-lysine system. There was no apparent difference between glucose and xylose solutions. This result shows that lysine definitely contributes to browning or pigment formation in addition to sugar decomposition, and that decomposition products of xylose more strongly contribute to the browning than those of glucose in the presence of lysine.
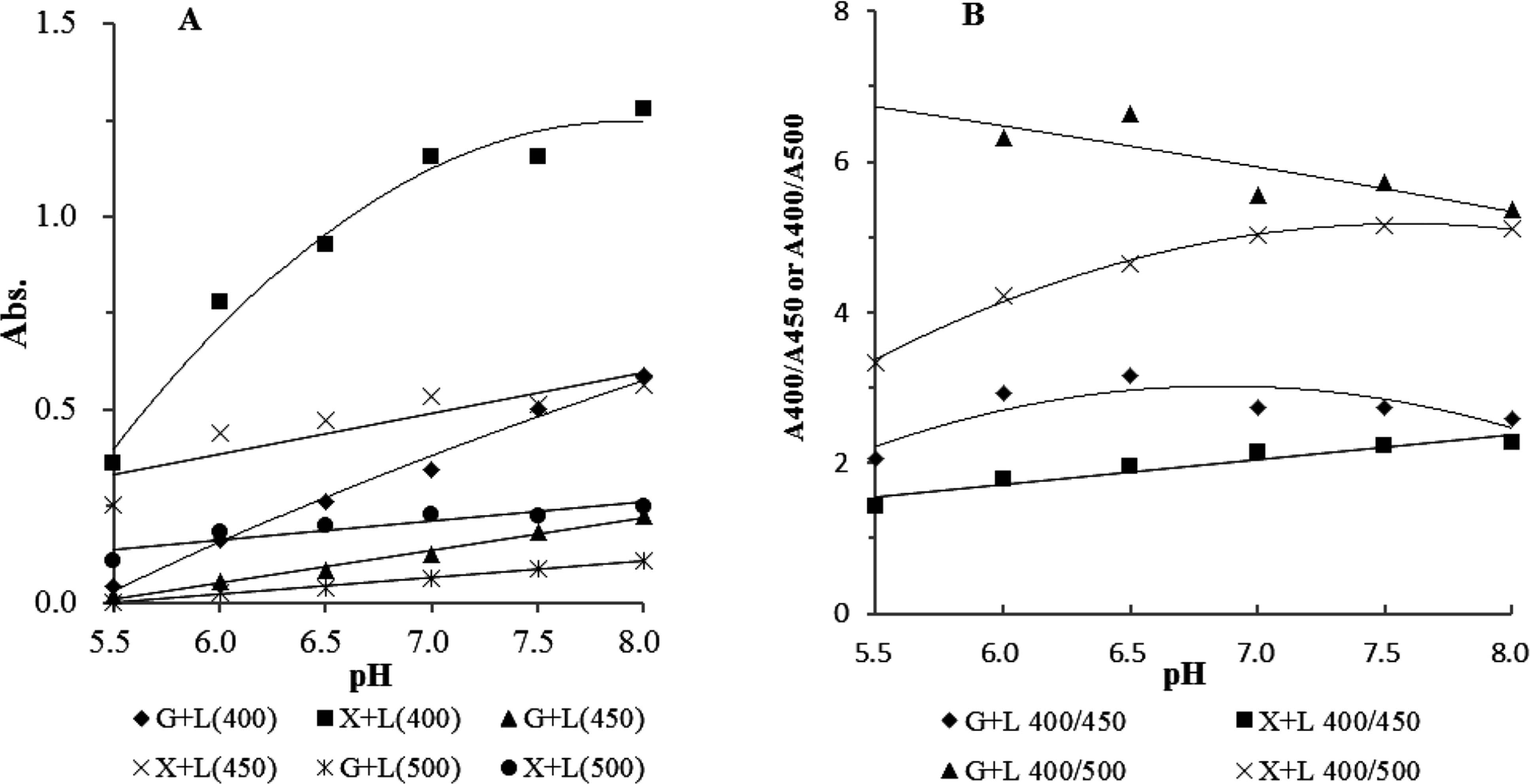
Comparison of xylose and glucose on the browing. A buffer solution (0.2 M phosphate, pH 5.5–8.0) containing 13.3 mM xylose (X) or glucose (G) and 34 mM lysine (L) was hated at 100°C for 60 min, and then the absorbance at 400, 450, and 500 nm was measured (A). A400/A450 ro A400/A500 of each sample was plotted (B).
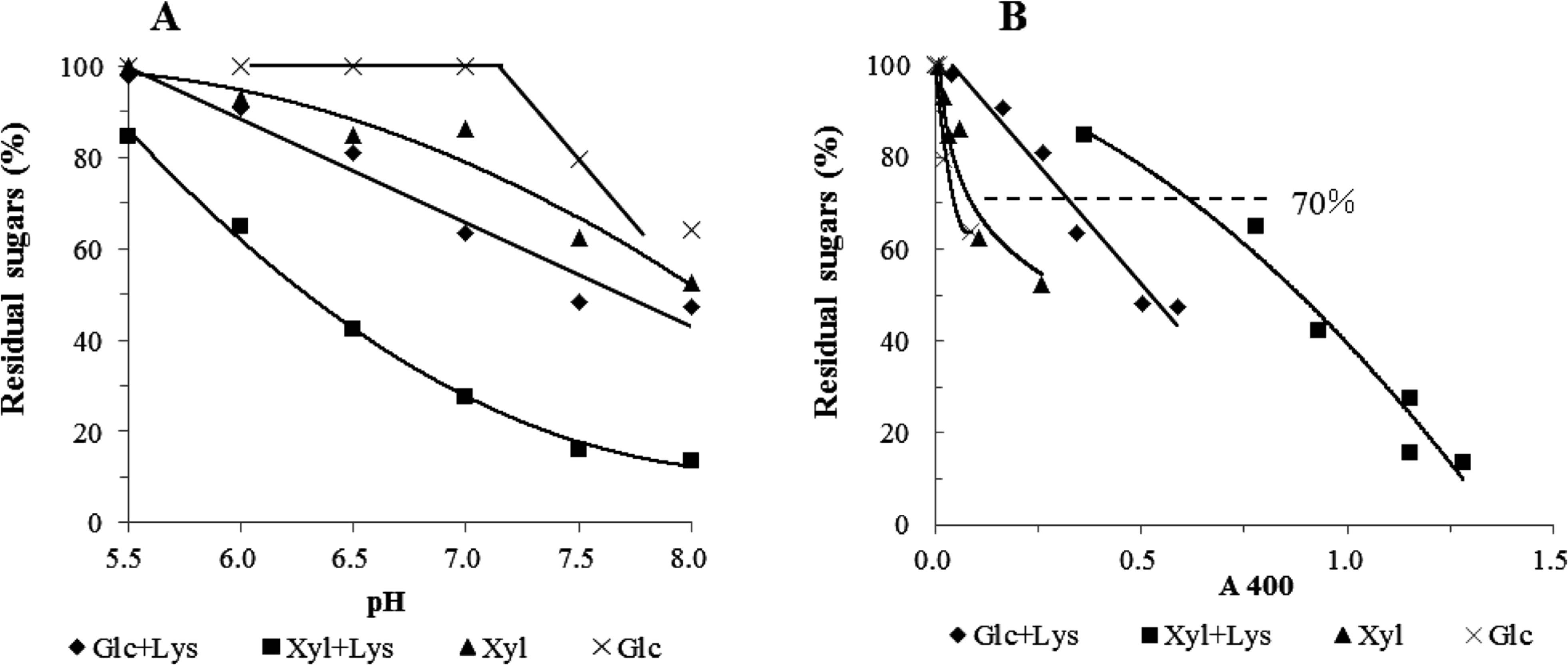
Sugar degradation in the xylose-lysine and glucose-lysine systems in the Maillard reaction. A buffer solution (0.2 M phosphate, pH 5.5–8.0) containing 13.3 mM xylose (Xyl) or glucose (Glc) in the absence or presence of 34 mM lysine (Lys) was heated at 100°C for 60 min, and then the residual sugars were measured (A). The residual sugar and A400 of each solution was plotted (B).
The glucose-lysine and xylose-lysine systems were compared using reversed-phase HPLC monitored with absorbance at 400 nm and fluorescence (Ex, 370 nm; Em, 450 nm). Low-molecular-pigments such as DLDPs were detected only in the xylose-lysine system, while melanoidins were detected in both systems (Figs. 7-A2 and -B2). The convex broad peaks are considered melanoidins (Bailey et al., 1996). The chromatograms detected by fluorescence (Fig. 7-A1 and -B1) were not consistent with those by browning or absorbance at 400 nm (Fig. 7-A2 and -B2), although correlations between browning (A400) and fluorescence were apparent in model solutions of sugar and lysine (r2 = 0.87 in Fig. 8-A and 0.92 in Fig. 8-B). In Fig. 7-1 several new peaks appeared in the area considered as melanoidins in Fig 7-2 and the shapes of both chromatograms differed. This suggests that melanoidins consist of various chemical structures or chromophores showing different color and florescence.
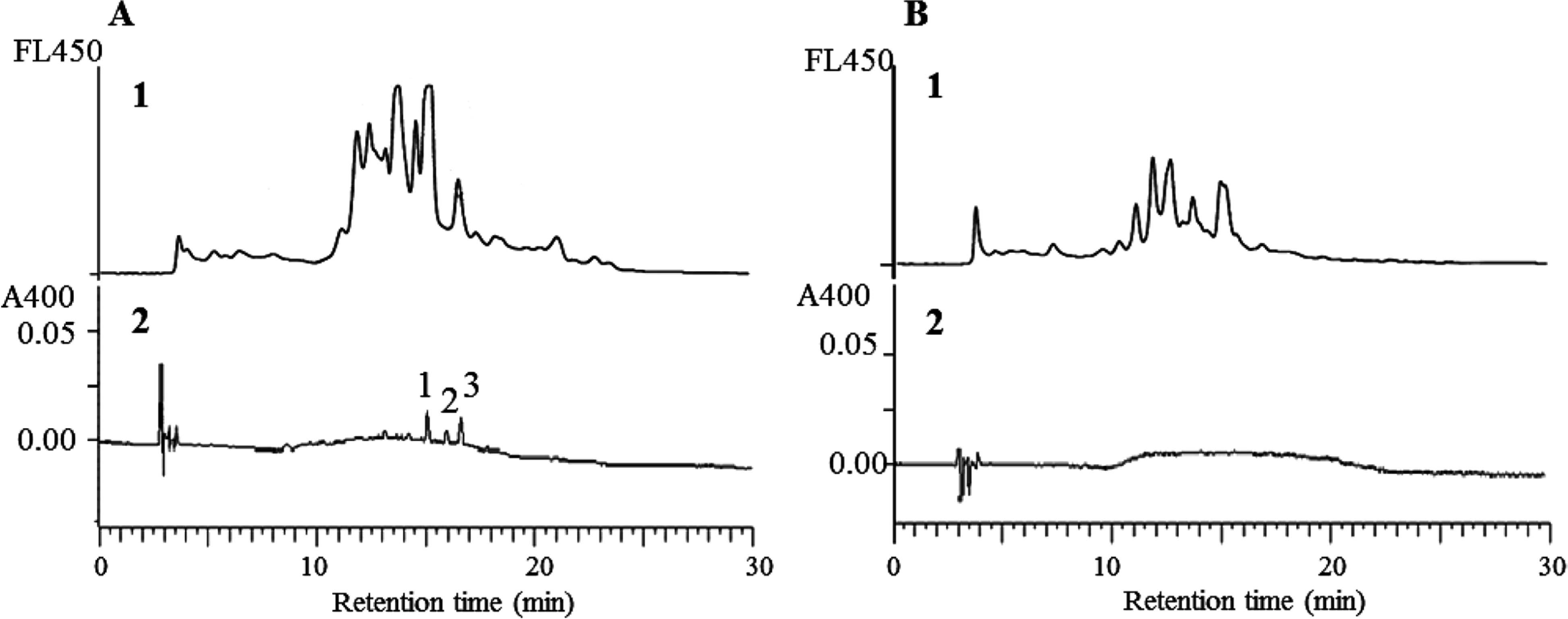
Profiles of reversed- phase HPLC of the xylose-lysine (A) and glucose-lysine (B) systems of the Maillard reaction. Samlres were prepared in 0.2 M Na/K phosphate buffer (pH 7.0). The HPLC was monitored by flurescence (1, FL450; Ex, 370 nm; Em, 450 nm) and 400(2). 1, DLDP A; 2, DLDP C, 3, DLDP B.
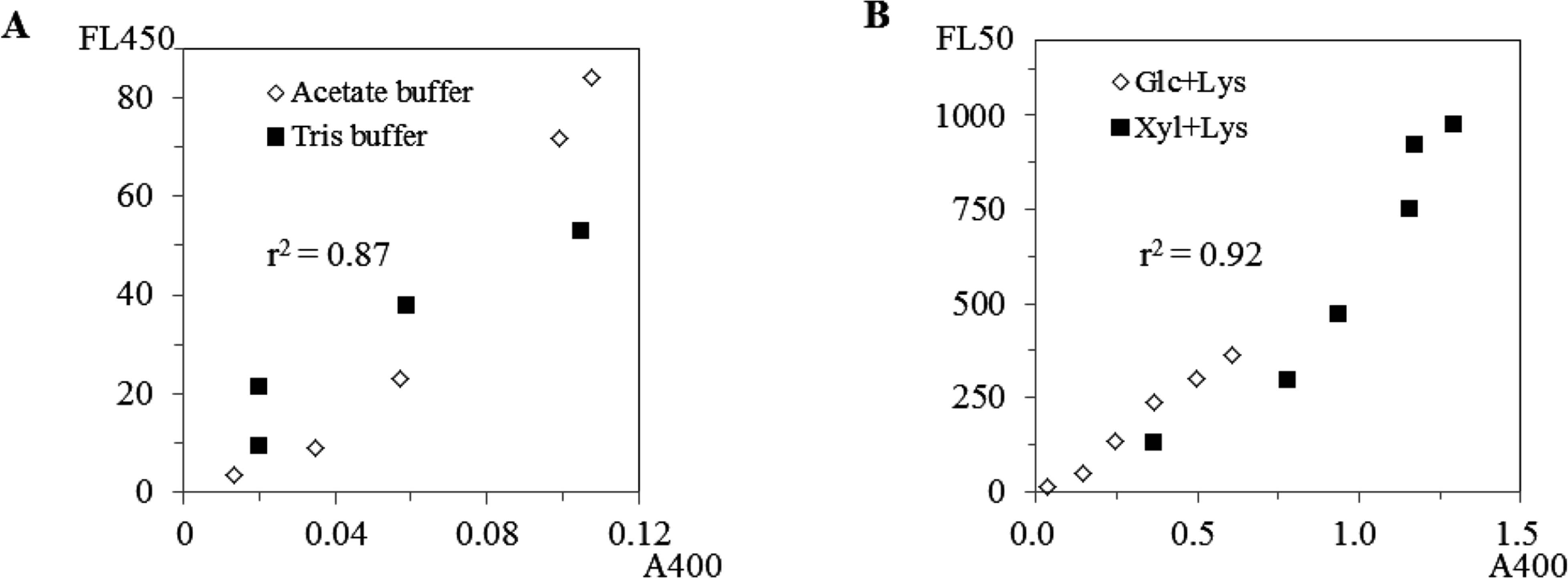
Relations between visible absorbance (A400) and fluorescence (FL450) of sugar lysine model solutions. Xylose and lysine were dissolved in acetate buffer (pH 4–6) or Tris buffer (pH 7.5–9) and heated at 100°C for 1 h (A). Xylose or glucose, and lysine were dissolved in phospate buffer (5.5–8) and heated at 100°C for 1 h (B).
Next, pigments formed in the glucose-lysine and xylose-lysine systems were compared using GPC equipped with a DAD detector. Figure 9 shows typical chromatograms and UV-Vis spectra of the xylose-lysine system. A small amount of high-molecular-weight pigments > 5 kDa were observed in the system. In the glucose-lysine system, high-molecular-weight pigments were similarly minimally detected (data not shown). Pigments are not necessarily eluted in order of molecular-weight and may interact with the column matrix. However, when these solutions were subjected to centrifugal ultra-filtration (cut-off, 3 kDa), most of the color was passed to the < 3 kDa fraction. This result corresponded with that reported by Hofmann (1998). Although melanoidins have higher molecular-weight than low-molecular-weight pigments such as DLDPs, the molecular weight of melanoidins formed in this system might not be so high.

Gel permeation chromatography (GPC) profile of the xlyose-lysine and UV-vis spectra. Pigments were categorized into melanoidins (Mel), intermediate pigments (IP), and low-molecular-weight pigments (LMP) according to UV-vis spectra.
Pigments appearing on the chromatogram of Fig. 9 were categorized into three groups according to UV-Vis spectra. Here, pigments are indicated as peaks detected by absorbance at 400 nm. Pigments that had negligible or minimal specific maximum absorption, those that had a specific maximum absorption under 350 nm, and those that had a specific maximum absorption over 350 nm were divided into melanoidins, intermediate pigments, and lower-molecular-weight pigments, respectively. All of the pigments contribute to the brownish color, but the contribution of each pigment to color tone differs as their spectra are different. We calculated the ratios of these pigments to the total area and examined the effect of sugar type and pH on the ratio of these pigment groups (Fig. 10). With the rise in pH value, the amount of total color compounds increased in both systems but the content differed. Melanoidins were the major pigment in both systems at the pH range of 5.5 to 8.0. Lower-molecular-weight pigments contributed to the color in the xylose system under acidic conditions but did not in the glucose system. In the xylose-lysine system, with the increase in pH, the ratio of both melanoidins and intermediate pigments increased, while the ratio of low-molecular-weight pigments, mainly DLDPs, decreased. In the glucose-lysine system, the ratio of melanoidins decreased with the increase in pH and that of intermediate pigments increased.
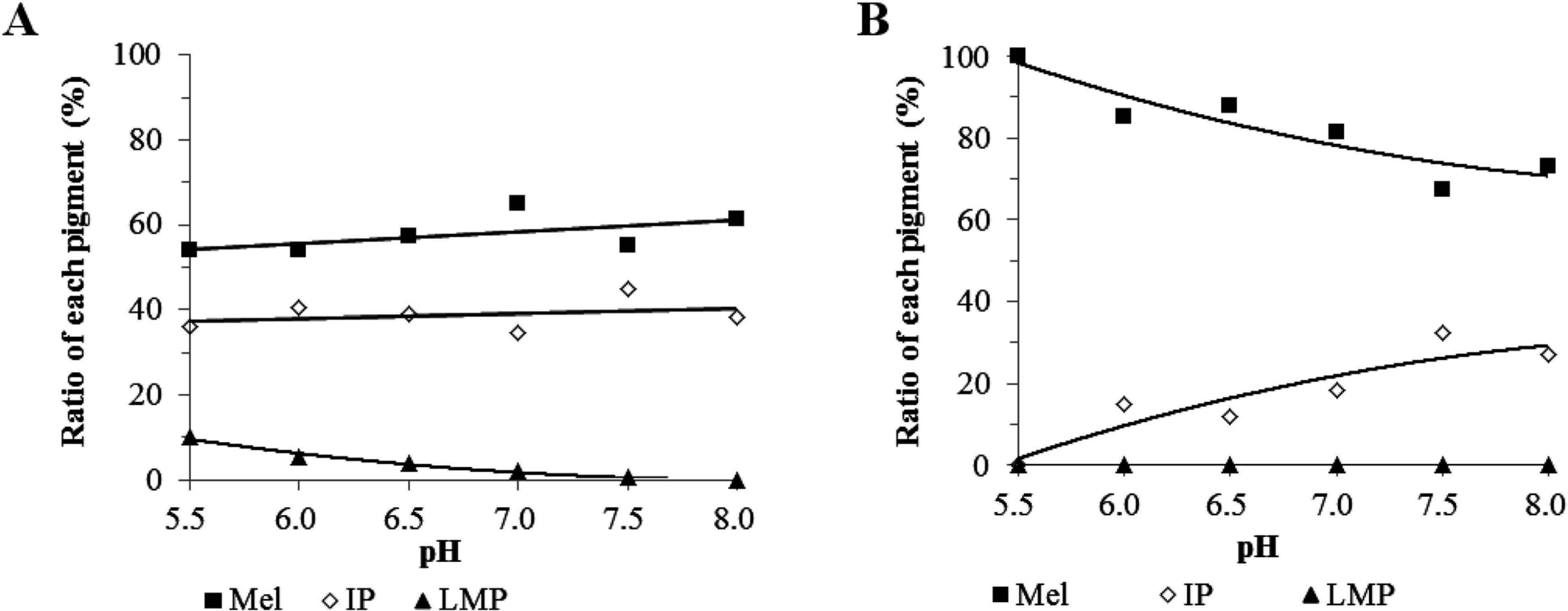
Comparsion of the xylose-lysine (A) and glucose (B) systems of the Maillard reaction on the category of pigment. GPC pigments were divvied into melanoidins (Mel), intermediate pigments (IP), and low-molecular-weight pigments (LMP) according to UV-Vis spectra.
Buffer type had a definite effect on Maillard browning, which was stronger than that of pH. Sugar type also influenced the formation of pigments. Xylose was more rapidly degraded than glucose; however, the browning after decomposition was similar between xylose and glucose in the absence of amino acids. Amino acids stimulated both steps of sugar decomposition and pigment formation during the Maillard reaction, and the stimulation was larger in the xylose system than in the glucose system. Low-molecular-pigments were formed only in the xylose-lysine system, contributing to the orange color of the system. Factors such as sugar and buffer types, and pH are critical to control the intensity and tone of color formation by the Maillard reaction.
Acknowledgements This study was supported by a Grant-in-Aid for Scientific Research (B) (no. 26282016) from the Japan Society for the Promotion of Science.