2015 Volume 21 Issue 6 Pages 821-826
2015 Volume 21 Issue 6 Pages 821-826
The aim of this study was to visualize the sorption of tocopherol acetate as a penetrant into polymer films. A matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS) technique was used to visualize the distribution of tocopherol acetate in films during the migration process. A phytic acid-aided MALDI-IMS using 2′,4′,6′-trihydroxyacetophenone as a matrix allowed us to successfully visualize the absorbed tocopherol acetate in both ethylene-vinyl acetate copolymer (EVA) and low-density polyethylene (LDPE) films after 1 week-storage of tocopherol acetate at 40°C. The IMS technique allowed us to also visualize the actual distribution of tocopherol acetate inside the EVA/barrier/EVA laminated films, in which tocopherol acetate sorption was blocked at the barrier layer.
The use of plastic polymer films is now in trend for drug-, food-, and cosmetic-packaging materials, because of their high processing properties and cost efficiency. However, their poor gas-barrier properties, in particular, flavor deterioration or loss of drug content by sorption, still limits their extensive use (Leufvén and Hermansson, 1994; Shimoda et al., 1984). Fayoux et al. (Fayoux et al., 1997) showed that the amount of d-limonene in orange juice was dramatically decreased with storage by sorption inside films. Sorption degradation was also issued in cosmetic fields, in which tocopherol acetate that is a typical cosmetic chemical was dramatically absorbed into polyethylene packaging film during 20 day-storage at 60°C (Fukahori et al., 1996). In a series of studies on sorbed penetrants into films, we demonstrated that factors affecting sorption were film properties such as glass transition temperature and density as well as the film affinity for penetrant (Matsui et al., 1992, 1994; Fukamachi et al., 1994). In this regard, we proposed a thermodynamic sorption equation that could predict the magnitude of sorption using a solubility parameter (SP) value (Matsui et al., 1992; Fukamachi et al., 1994). However, while the sorption equation was useful for homogenous films, it was restrictive when applied to laminated films. In addition, no direct information on the distribution of penetrants inside the films was available using conventional sorption experiments.
In a previous report, we visualized a fluorescent perylene distributed in cellulose acetate films using fluorescence laser spectroscopy. However, its application to visualize sorption phenomena was still restrictive to penetrants with self-fluorescence properties (Hasegawa, et al., 2010a, 2010b). In contrast, a recently developed matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS) is applicable to target compounds without any consideration of specific molecular properties. To date, in the biochemical omics field, MALDI-IMS application has increased, since it can provide hundreds of images of ionizable compounds in a single measurement. Thus, in this study, we attempted to apply the IMS technique for visualization of tocopherol acetate sorbed inside films. To the best of our knowledge, this is the first study demonstrating the visualization of sorbed penetrants in polymers using the MALDI-IMS technique.
Materials dl-α-Tocopherol acetate, nitrilotris [methylenephosphonic acid] (NTMP), and phytic acid were from Tokyo Chemical Ind. (Tokyo, Japan). α-Cyano-4-hydroxycinnamic acid (CHCA), 2,5-dihydroxybenzoic acid (DHB), sinapinic acid (SA), and 2′,4′,6′-trihydroxyacetophenone (THAP) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Citric acid, di-ammonium hydrogen citrate (di-AC) and phosphoric acid were purchased from Nacalai Tesque (Kyoto, Japan). Tartaric acid was purchased from Wako Pure Chemical Ind. (Osaka, Japan). All other chemicals were of analytical reagent grade and were used without further purification.
Preparation of film samples for IMS analysis Ethylene-vinyl acetate copolymer (EVA; vinyl acetate content: 32%) and low-density polyethylene (LDPE, 0.918 g/cm3) film samples with 500 µm thickness were manufactured by resin extrusion at 100°C and 200°C, respectively, by Toppan Printing Co. Ltd. For the preparation of laminated EVA film (EVA/barrier/EVA, thickness: 250/2/250 µm), an isocyanate-cured acrylic (IA) polymer (at a density of 1 g/m2) was primarily coated on the surface of 250 µm EVA film, followed by the sandwiching lamination of the barrier layer-coated EVA film with another 250 µm EVA film.
Sorption experiments of tocopherol acetate for films Tocopherol acetate sorption experiments in prepared film samples were performed as follows: Film sample cut into a circle disc with i.d. 2 cm was set on the inner side of the center of the perforated lid of a 50 mL-volume glass bottle as an inner seal of the cap (surface area: 3.14 cm2, Fig. 1). Twenty milliliters of tocopherol acetate solution (10 mg/mL ethanol) was then filled in the bottle and capped by the lid with the inner film. The bottle was then set upside down to immerse the inner film sample with the tocopherol acetate solution for 1 or 4 week-storage at 40°C. After storage, the film sample was detached from the lid and immediately frozen in liquid nitrogen.

Schematic work-flow for MALDI-IMS analysis of tocopherol acetate sorbed in films.
Overall work-flow for MALDI-IMS analysis of sorbed tocopherol acetate (upper), and the preparation scheme for sectioning sorbed polymer films (lower).
Cryosectioning of films for MALDI-IMS The prepared film sample was embedded in OCT compound (Tissue-Tek, Sakura Finetek, Tokyo, Japan) to prepare a frozen block. After setting the film-embedded OCT block on a cryomicrotome (CM1100 Leica, Wetzler, Germany), the embedded film was sliced cross-sectionally into a 12-µm-thick section. Each film section was collected by micro forceps to avoid contaminations and placed on a double-faced adhesive aluminum tape (TERAOKA SEISAKUSHO Co., Tokyo, Japan) on an indium-tin oxide (ITO)-coated conductive glass slide (Bruker Daltonics, Bremen, Germany).
Matrix spraying The matrix reagent (CHCA, DHB, SA, or THAP) was dissolved in acetonitrile/water (1:1, v/v) containing 0.1% trifluoroacetic acid (TFA) at a concentration of 5.0 mg/mL. Phosphoric acid, tartaric acid, citric acid, di-AC, NTMP, and phytic acid (each at 5.0 mM in matrix solution) were also used as matrix additives. To optimize the enhanced IMS visualization conditions of tocopherol acetate, an aliquot (20 nmol/0.2 µL) of 100 mM tocopherol acetate solution was manually spotted on the ITO glass slide or on EVA film section. The matrix solution was then sprayed uniformly over the ITO glass slide with an ImagePrep automatic matrix sprayer (Bruker Daltonics). Spraying conditions were as follows: Spray power, 20%; modulation, 20%; spray time, 1.3 s; incubation time, 10 s; and dry time, 55 s. One hundred and fifty spraying cycles were performed. The amount of matrix sprayed onto the slide was controlled within 10% variation by an automated spray-generator in ImagePrep (e.g., THAP: 3.14 ± 0.26 mg/slide).
MALDI-IMS analysis MALDI-IMS measurements were performed using an Autoflex III mass spectrometer equipped with a SmartBeam III (Bruker Daltonics) in both positive and negative ion linear modes. MS data were acquired in the range of 100 – 1000 m/z by averaging signals from 300 consecutive laser pulses. The MS parameters were as follows: Ion source 1, 20.00 kV; ion source 2, 18.80 kV; lens voltage, 7.50 kV; gain, 2.45; laser frequency, 200 Hz; laser power, 90%. IMS analysis was performed with a spatial resolution of 20 – 100 µm and the acquired MS spectra were analyzed by using Bruker FlexAnalysis software (ver. 3.3). The image data were reconstructed for visualization with a mass filter with a width of ± 0.2 m/z using Bruker FlexImaging software (ver. 2.1).
Statistical analyses Data for signal-to-noise (S/N) ratio were expressed as mean ± SEM. The statistical difference between two groups and more than two groups were analyzed using unpaired Student's t-test and one-way analysis of variance (ANOVA), respectively, followed by Tukey-Kramer's t-test for post-hoc analysis. P values <0.05 were considered statistically significant. All analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
Flavor deterioration by sorption into films during storage has been a serious issue when foods with high quality are in demand. Sorption is caused by the thermodynamic condensation of flavors on the surface of the film with high affinity (Matsui et al., 1992; Fukamachi et al., 1993). However, sorption dynamics inside films still remained unclear. Thus, in this study, we attempted to apply MALDI-IMS for direct visualization of penetrants located inside films to get insights regarding the sorption or distribution of penetrants in films. Since, tocopherol acetate, which presents phenyl ester bonds and is non-volatile, is suitable for MALDI ionization, we used it as the penetrant compound in this study.
Optimization of MALDI-IMS measurement For enhanced MALDI-IMS detection of tocopherol acetate spotted on ITO glass slide (20 nmol/0.2 µL spot), four matrix reagents (i.e., CHCA, DHB, SA, and THAP) were examined. As shown in Fig. 2, the highest MS detection of tocopherol acetate by IMS was observed in THAP matrix in positive mode with a high S/N ratio of 17.7 among the four common matrices. The lower tocopherol acetate detection as [M+H]+ in other matrices would be due to the production of other molecular ion species such as [M]+ and [M+Na]+ and/or their low ionization ability for tocopherol acetate. In addition, a 6.3-fold higher IMS detection of tocopherol acetate was observed when 5.0 mM phytic acid in THAP matrix solution was sprayed on EVA film (20 nmol/0.2 µL spot), compared to THAP alone (Fig. 3). The phytic acid-induced high MS detection of tocopherol acetate on EVA film (Fig. 3) is most likely due to the reduction of MS-interfering complex formation with salts (e.g. Na+) from ubiquitous impurities in film, glassware, solvent, and reagents (Chen et al., 2003). As reported previously, phytic acid in matrix solution also allowed a homogenous coating (or spraying) of THAP matrix onto the target section (Hong et al., 2013). In the present study, a marked MS signal of tocopherol acetate with Na+ ([M+Na]+) in THAP alone (Fig. 4A) was strongly attenuated by the presence of phytic acid in THAP (Fig. 4B), indicating the efficacy of phytic acid as a matrix additive on enhanced visualization of sorbed penetrants in films (Figs. 4C and D).

Mass spectra of tocopherol acetate (20 nmol/0.2 µL spot) spotted on ITO-coated glass slide with various matrix reagents (CHCA, DHB, SA, and THAP, at each concentration of 5.0 mg/mL). Arrow shows the m/z value of tocopherol acetate in positive or negative mode ([M+H]+ (473 m/z) or [M-H]− (471 m/z)). Visualized images of tocopherol acetate were inserted into each spectrum with a width of ± 0.2 m/z at raster spot of 100 µm. N.D., not detected.

Effect of matrix additives on MALDI-IMS detection of tocopherol acetate (20 nmol/0.2 µL spot) spotted on EVA film section in positive mode. Concentration of each additive in 5.0 mg/mL THAP solution was 5.0 mM. Results are expressed as the mean of S/N ratio ± SEM. Data without common letters are significantly different (P < 0.05) according to Tukey-Kramer's t-test.

Mass spectra of tocopherol acetate (20 nmol/0.2 µL spot) spotted on EVA film in positive mode in the absence (A) and presence (B) of 5.0 mM phytic acid in THAP matrix solution. Observed product ions regarding matrix and tocopherol acetate (TocAc) were [THAP+Na]+ (191 m/z), [TocAc+Na]+ (495 m/z), together with [THAP+H]+ (169 m/z) and [TocAc+H]+ (473 m/z). S/N ratios of each target product ion for THAP (C) and tocopherol acetate (D) in the presence (■) and absence (□) of 5.0 mM phytic acid are expressed as mean ± SEM. Significant difference between groups was analyzed by Student's t-test.
Visualization of tocopherol acetate in EVA and LDPE films by MALDI-IMS Under the optimal MALDI-MS conditions, sections of EVA and LDPE films immersed in tocopherol acetate (10 mg/mL) at 40°C for 1 week were subjected to an IMS analysis. As shown in Fig. 5, tocopherol acetate was sorbed into the inner EVA and LDPE films with storage. Although the MS detection intensity by MALDI-MS measurement was semi-quantitative (Albrethsen et al., 2011), a ca. 8.0-fold higher intensity of tocopherol acetate in EVA film, compared with that in LDPE film (Fig. 5), suggested that tocopherol acetate was favorably sorbed into EVA film rather than in LDPE film. The favorable sorption in EVA film may be explained by the high affinity or small distance in SP values (δc) between tocopherol acetate and EVA (δc EVA = 4.4 MPa1/2, δc LDPE = 9.0 MPa1/2) (Matsui et al., 1992). So far, few investigators attempted to use MALDI-IMS to evaluate polymer characteristics such as cross-linking (Crecelius et al., 2011) and surface analysis (Krueger et al., 2013). However, to the best of our knowledge, this is the first application of the technique for dynamic evaluation of sorption behavior in films.

Distribution of tocopherol acetate sorbed into EVA and LDPE films for 1 week-storage at 40°C by MALDI-IMS. The visualized image of tocopherol acetate ([M+H]+, m/z = 473) was obtained by MALDI-IMS with a width of 0.2 m/z at raster spot of 20 µm. White dotted lines in pictures represent the edges of cross sectional film segment on ITO-coated slide glass. Scale bar: 100 µm.
Evaluation of sorption barrier property of laminated EVA/barrier/EVA film by MALDI-IMS Since no direct observation of sorption in films permitted to clarify the distribution of the penetrant inside the sorbed films, barrier characteristics of laminated films remained in question so far. Thus, we first attempted to visualize the absorbed tocopherol acetate in laminated EVA film with isocyanate-cured acrylic layer. As shown in Fig. 6, the inside of the laminated EVA film at 1 and 4 week-storage was successfully visualized by MALDI-IMS. At 1 week-storage, tocopherol acetate absorption was only observed in the upstream side of the laminated EVA film, indicating that tocopherol acetate sorption was completely blocked at the barrier layer. The IMS technique also provided useful information on the evaluation of the barrier property in laminated films, since after a longer (4 week-) storage period, tocopherol acetate sorption was observed beyond the barrier layer (Fig. 6).
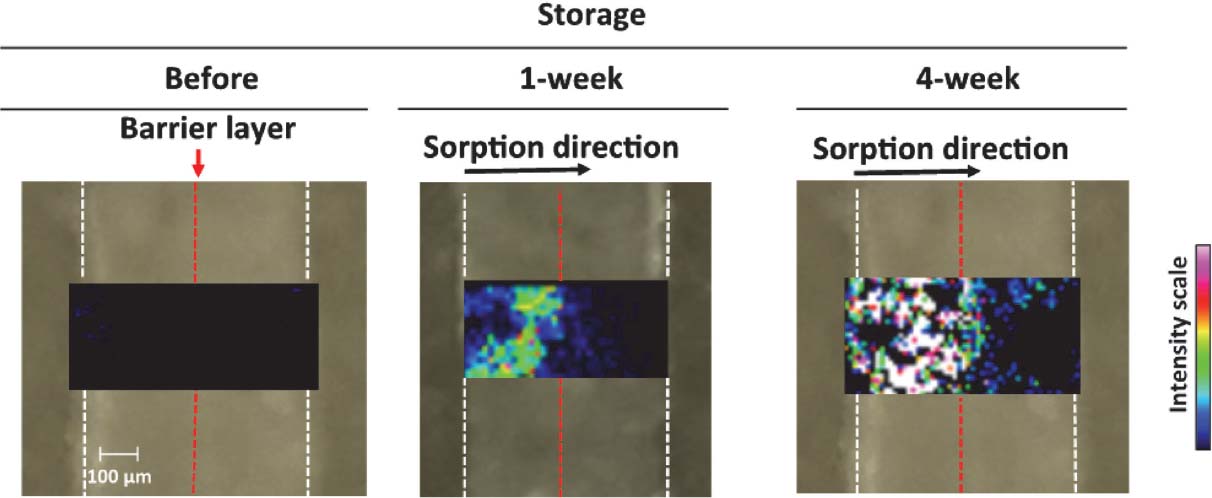
Distribution of tocopherol acetate sorbed into laminated EVA film for 1 and 4 week-storage at 40°C. Laminated EVA/barrier/EVA (250/2/250 µm) films composed of isocyanate-cured acrylic polymer as the barrier layer were used for sorption experiments. The image of tocopherol acetate ([M+H]+, m/z = 473) was obtained by MALDI-IMS with a width of 0.2 m/z at raster spot of 20 µm. White and red dotted lines in pictures represent the edges of the cross-sectional film segment and barrier layer, respectively. Scale bar: 100 µm.
The present study demonstrated for the first time that a phytic-acid-aided MALDI-IMS technique was applicable for the visualization of sorption dynamics in laminated films. As reported by Palmgrén et al. (2006), the reduction of packed drugs with polymer films by sorption was also issued in the pharmaceutical field. Thus, the advantage of direct IMS visualization of penetrants distributed across cross-sectional directions in films will provide new insights regarding evaluation and protection on sorption in any packaging fields.
Acknowledgements The cost of publication was supported in part by the Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University. The authors thank Ms. Kaori Miyazaki for her technical assistance.