2015 Volume 21 Issue 6 Pages 857-862
2015 Volume 21 Issue 6 Pages 857-862
In this study, probiotic ice cream are inoculated with Lactobacillus acidophilus (La-05; L), made with either refined (cane sugar; R) or unrefined sugar (coconut palm sugar; U) at concentrations of 15%, 18% and 21% (w/w). The changes in ice cream eating qualities, antioxidant capacity and the survival of La-05 were evaluated. Probiotic ice cream made with unrefined sugar had better microbial survival than when refined sugar was used. Ice cream made with 18% unrefined sugar showed the highest La-05 survivability. There were no significant effects on the sensory score among the probiotic ice cream with different sugar levels. However probiotic ice cream made with unrefined sugar was most preferred by panelists. The antioxidant capacity of the ice cream showed those made with unrefined sugar tend to have a higher antioxidant activity compared to those made with refined sugar. The replacement of refined with unrefined coconut palm sugar developed the viability of La-05, antioxidant capacity and total acceptability of probiotic ice creams.
In recent years the composition of foods containing added probiotic bacteria are increasingly popular because of the benefits to intestinal microbial and human health. Probiotic benefits include improvement in intestinal microbiota, activation of the immune system, reduction in serum cholesterol and inhibition of the growth of potential pathogens (Grajek et al., 2005). To take advantage of this development, many dairy products containing probiotics such as Lactobacilli and Bifidobacteria cultures have been formulated to enhance the nutritional and therapeutic of values of these products. It is important to sustain a high survival rate of the bacteria during the product shelf life in order to render them being effective. The minimum ingestion of 106–109 viable cells per day for humans can be achieved (Granato et al., 2010). Commercial dairy products such as yoghurts can not sustain adequate populations of viable probiotic bacteria during their shelf-life. However, probiotic microorganisms incorporated into ice creams have shown better viability during shelf-life (Heenan et al., 2004). One of main factors that affects the probiotic survivability in ice cream is sugar concentration. Sugars can reduce probiotic viability by osmotic stress and also increase their survivability by cryoprotective and prebiotics properties of sugars. These sugars effects on probiotics depend on the type and concentration of sugars, type of probiotic organism, freezing temperature and rate, freezing technology and storage time (Mohammadi et al., 2011). Refined sugar (e.g., lactose and particularly sucrose) is widely used in ice cream making. But the use of unrefined sugar containing various minerals, fatty acids, vitamins and botanical impurities may increase both the probiotic survivability and health properties of probiotic ice cream. Coconut unrefined sugar contains minerals such as of iron, zinc, calcium, phosphorus, magnesium and potassium, along with several short chain fatty acids, various vitamins such as vitamin C and vitamin B complex, polyphenols, antioxidants and inulin (Secretaria et al., 2007; Saxelby, 2014). Inulin, a non-digestible carbohydrate containing naturally- occurring fructooligosaccharides, which considered to have prebiotic and cryoprotective properties (Akin et al., 2007; Desmond et al., 2006). Vitamin C and phenolic compounds are important antioxidant agents that protect biomacromolecule from the damage induced by free radical. They also possess anti-aging, anti-tumor and antimutagen functions (Xia et al., 2011). In addition it also contains 16 kinds of amino acids (Xia et al., 2011). Coconut sugar has a low glycemic index (GI) and thus it is good for diabetics and suitable for weight maintenance (Kusumawaty et al., 2012).
The health benefits of antioxidants which include reduction of risks developing cancer, high blood pressure, diabetes and other diseases, caused the increase of their usage in functional foods with specific health properties (Oroian and Escriche, 2015). Hence, the objectives of this study were to evaluate the effects of different levels of refined and unrefined sugar on the nutritional (antioxidant capacities), physical and sensory characteristics of probiotic ice cream and the survivability of Lactobacillus acidophilus during frozen storage.
Materials Homogenized and pasteurized fresh milk (Dutch Lady, Malaysia) and UHT whipping cream (Arla Brand, Germany), refined cane sugar (Malayan Sugar Mfg Co. Bhd, Malaysia), coconut palm sugar (Gula Melaka, Malaysia), skim milk powder (Dutch lady, Malaysia), and vanilla were purchased from local grocery. Vanilla was added to aroma development. Cremodan SE 734 veg (Danisco AS, Copenhagen, Denmark; containing mono-and diacyl-glycerols of fatty acid, cellulose gum, guar gum and carrageenan) was used as stabilizer. Lactobacillus acidophilus (La-05) was obtained as pure freeze-dried probiotic culture from CHR-Hansen (Horsholm, Denmark).
Preparation of starter culture Each strain (1 g) was cultured in 100 mL of sterilized skimmed milk (10 w/v), amplified by the addition of 0.05% (w/v) L-Cysteine hydrochloride, 1% (w/v) yeast extract and 2% (w/v) glucose. The incubation was carried out under aerobic condition in a water bath (Julabo, Haake Model SWD 20, Germany) at 42°C until a pH of 5.0 was reached (Magarinos et al., 2007).
Preparation of intermediate culture Inoculation culture for each strain was prepared fresh by adding 4 mL of starter culture into 100 mL of sterilized skimmed milk. Incubation was carried out under anaerobic condition. Anaerobic conditions were created using anaerocult A sachets, anaerobic jar and anaerotest® strip (Merck) prior to incubation in a still water bath (Julabo, Haake Model SWD 20, Germany) at 42°C until pH has reduced to 5.0 (Magarinos et al., 2007).
Preparation of ice cream Ice cream was prepared using formula as shown in Table 1.
| SampleA | Ingredient | ||||||
|---|---|---|---|---|---|---|---|
| Milk (% w/w) | Cream (%) (Fat = 35% w/w) | Skim milk powder (% w/w) | Refined Sugar (% w/w) | Unrefined sugar (% w/w) | Stabilizer-Emulsifier (% w/w) | Vanillin (% w/w) | |
| U1 | 55.4 | 20 | 8 | 0 | 15 | 0.5 | 0.1 |
| U2 | 55.4 | 20 | 8 | 0 | 18 | 0.5 | 0.1 |
| U3 | 55.4 | 20 | 8 | 0 | 21 | 0.5 | 0.1 |
| R1 | 55.4 | 20 | 8 | 15 | 0 | 0.5 | 0.1 |
| R2 | 55.4 | 20 | 8 | 18 | 0 | 0.5 | 0.1 |
| R3 | 55.4 | 20 | 8 | 21 | 0 | 0.5 | 0.1 |
The milk was heated to 40 – 45°C before a thorough mixing with cream (35% w/w fat) followed by the addition of skim dry milk powder, vanilla, stabilizer and sugar. The mixtures were subjected to two homogenization stages (16000 rpm, 70°C, 5 min; Ika Homogenizer T-25 basic Ultra Turrax, Germany). The mixtures were pasteurized at 80°C for 10 min in a water bath and then cooled to 4°C prior to overnight aging at 4°C. The mixture was then inoculated with 4% (w/w) fermented milk (intermediate culture) followed by thorough gentle mixing. The inoculated ice cream mix was subjected to freezing in a 1.5 L batch ice cream maker (Baumatic gelato1ss, UK; rotor speed 50 round/min, 40 min, −30°C) followed by packing in 100 mL plastic cups. The cups were firmly covered using the lids prior to storage at _20°C in a freezer (Aboulfazli et al., 2014).
Chemical analysis The pH of melted ice creams were measured using digital pH-meter whereas titratable acid (TA) was determined by slowly titrating 10 g samples with 0.1 N NaOH, using several drops of phenolphthalein (1% w/v) as color indicator. The total solids were measured by drying samples at 100 ± 1°C for 3.5 h using an air oven (Akin et al., 2007). All measurements were performed three times.
DPPH Antioxidant Analysis Extraction of Ice cream sample Ice cream sample was extracted by using water according to Amirdivani and Baba (2011). Briefly, probiotic ice cream sample (10 g) was homogenized with 2.5 mL of sterile distilled water and this was subsequently acidified to pH 4.0 with HCl (0.1 M). The acidified homogenized ice cream was then heated in water bath (45°C) for 10 min followed by centrifugation (5000 g, 10 min, 4°C). NaOH (0.1 M) was added to adjust the pH of supernatant to 7.0. The neutralized supernatants were re-centrifuged (5000 g, 10 min, 4°C) and the supernatants were stored in a −19°C freezer until required for further analysis.
Determination of Antioxidant Activity The scavenging rate of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical inhibition (DRI) was evaluated according to the procedure reported by Skrede et al. (2004).The extracted ice cream sample (100 µL) or methanol (blank) was mixed with 3.9 mL of DPPH (Sigma-Aldrich, Germany) reagent (0.0277 g DPPH L−1 methanol).The mixtures were held at ambient temperature for 2 hours in the dark. The absorbance (Abs) of the supernatant-DPPH mixture was then measured at 515 nm. The amount of sample required to decrease the DPPH concentration by 50% (EC50) was calculated. The absorbance measured was used in calculating the % inhibition of DPPH oxidation (Apostolidis et al., 2007) as follows:
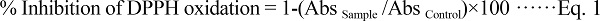 |
Microbial viable cell count (VCC) in ice cream The VCC was measured immediately after inoculating the probiotic culture and again after 1, 30, 60 and 90 days of frozen storage. The samples (10 g) were decimally diluted with sterile peptone water (1 g L−1; Merck). One milliliter aliquot dilutions were pour plated in triplicate on MRS agar. The plates were incubated in an incubator (Revco Ultima, USA) at 38 ± 1°C for 72 h under aerobic condition in the presence of 5% CO2 (v/v). The bacterial viability was represented as survival rate (Magarinos et al., 2007).
Sensory analysis The ice creams were organoleptically evaluated by thirty four consumer panelists (25 – 30 year; 19 males, 15 females), using a sensory rating scale of 1 – 10 for taste and flavor, and 1 – 5 for consistency and 1 – 5 for appearance and colour (Akin et al., 2007). The properties evaluated contained (a) three characteristics for appearance and colour (no criticism: 5, dull colour: 4 – 1, unnatural colour: 3 – 1), (b) seven properties for taste and flavor (no criticism: 10, cooked flavor: 9 – 7, lack of sweetness and too sweet: 9 – 7, lack of flavor: 8 – 6, rancid and oxidized:6 – 1, and other: 5 – 1) and (c) seven terms describing texture and body (no criticism: 5, coarse: 4 – 1, crumbly: 4 – 2, weak: 4 – 1, fluffy: 3 – 1, gummy: 4 – 1, sandy: 2 – 1).
Statistics The statistical analysis was performed using SAS statistical software, Version 6.12 edition followed by Duncan's multiple range method for mean comparison. The criterion for statistical significance was p < 0.05 (Homayouni et al., 2008). The experiments were assayed in triplicates.
Physicochemical analysis The chemical compositions of the ice creams are presented in Table 2. The pH and titratable acidity (TA) were unchanged by making probiotic ice cream with different levels of refined and unrefined sugar and also during the 90 days frozen storage (pH = 6.60 ± 0.01 until 6.63 ± 0.02; TA = 0.070 ± 0.002 until 0.073 ± 0.002 % v/v). No significant change in pH and titrable acidity (p > 0.05) during storage of ice cream with different sugar levels was also reported by Akin et al., 2007. Total solids were higher in ice creams with higher sugar amount.
| SamplesA | Total solids (g/100g)B | Overrun (% w/w)B |
|---|---|---|
| U1 | 38.15 ± 0.15c | 34.06 ± 0.61c |
| U2 | 41.20 ± 0.17b | 35.50 ± 0.50b |
| U3 | 44.12 ± 0.15a | 37.30 ± 0.59a |
| R1 | 38.10 ± 0.08c | 33.10 ± 0.66d |
| R2 | 41.10 ± 0.07b | 35.20 ± 0.76b |
| R3 | 44.10 ± 0.08a | 36.30 ± 0.40b |
Ice creams made with unrefined sugar had the tendency to have a higher overrun than those made with refined sugar (Table 2). Among ice creams with same sugar level, the overrun was higher in ice creams containing unrefined sugar than those containing refined sugar (U1 > R1 and U3 > R3). The increase in sugar levels also caused increase in overrun. Akin et al (2007) reported that an increase in inulin and sugar contents increased overrun values of probiotic ice creams because of stabilizing properties of both ingredients. Akalin et al. (2008) found the addition of inulin significantly increased the viscosity, overrun, and improved the melting properties and texture of ice cream. Since, unrefined sugar contains inulin (Xia et al., 2011), the use of this type sugar may be contribute to increase of stabilizing properties to ice cream.
Antioxidant activities Probiotic ice cream made with unrefined sugar (antioxidant activity = 30%) showed a higher antioxidant activity than refined sugar (antioxidant activity = 0%) (Table 3). Unrefined sugar contains higher phenolic compounds than refined sugar (Xia et al., 2011). Other impurities unrefined coconut palm sugar such as manganese is known to exhibit antioxidant and free-radical fighting properties (Secretaria et al., 2007). Increased antioxidant activities were found in the probiotic ice cream with increasing amount of added refined and unrefined sugar (Table 3). Probiotic ice creams containing refined sugar also had antioxidant activity because of the presence of probiotic bacteria. Moneim et al (2000) investigated on antioxidant activity and biological evaluations of probiotic bacteria strains and found probiotic cell free extract had antioxidant activity. The probiotic count was higher in ice creams with higher amount of sugars. However, the antioxidant activities of the probiotic ice creams (11.07% – 45.3%; Table 3) were relatively low, because milk used in the ice cream making was not fermented. Thus fermentation of the milk by L. acidophilus increases free radical scavenging-linked antioxidant activity which can potentially enhance functional properties of probiotic ice cream (Donkor et al., 2007).
| Samples | Antioxidant activity (%)A |
|---|---|
| U1 | 31.90 ± 1.34b |
| U2 | 33.30 ± 2.62b |
| U3 | 45.30 ± 0.45a |
| R1 | 11.07 ± 1.39d |
| R2 | 17.73 ± 3.24c |
| R3 | 24.30 ± 4.65c |
Effects of sugar on the L. acidophilus viability during frozen storage The mechanical stresses associated with the mixing which incorporates oxygen into the mixture and freezing are known to be during viable cell counts (VCC) (Haynes and Playne, 2002). Fig. 1 shows the changes in VCC counts in probiotic ice creams during 3 months frozen storage. The reduction in VCC during freezing is associated with the freeze injury of cells (Haynes and Playne, 2002). Table 4 shows the survival rate of La-05 in samples during 90 days of frozen storage. Among ice creams with same sugar level, the survival rate of La-05 was higher in ice creams containing unrefined sugar than those containing refined sugar after 60 (U1 > R1, U2 > R2 and U3 > R3) and 90 (U1 = R1, U2 > R2 and U3 > R3) days of frozen storage. Although, no significant difference was observed in survivability of La-05 between U1 and R1 (sugar level 15%) after 90 days of frozen storage. La-05 showed higher survivability in ice creams containing unrefined sugar than those containing refined sugar except in sugar level 18% (U2 < R2) after 30 days of frozen storage. La-05 had a higher survival in ice creams containing refined sugar than those containing unrefined sugar except in sugar level 21% (U3 > R3) after 1 day of frozen storage. The survival rate of La-05 in samples after 90 days freezing tended to be higher in ice creams containing unrefined (85.04 – 90.60%) than in those containing refined sugar (82.65 – 87.27%) (Table 4) (p < 0.05). Unrefined sugars are known to contain components of nutritional importance such as manganese, magnesium and amino acids (it contains 16 amino acids which glutamic acid has the highest content among them (Purnomo, 2007) which act as growth promoters for L. acidophilus (Ahmed et al., 1990)). Unrefined coconut palm sugar also contains in addition the presence of inulin may serve as prebiotic to further enhance (Saxelby, 2014) and support the growth and survival of La-05 (Akin et al., 2007). Prebiotics are mostly oligosaccharides which non digestible but probiotics are able to metabolize them and enhance their growth and/or activity. Palframan et al. (2003), Akin et al. (2007), and Akalin and Erisir (2008) reported the addition of inulin to ice cream improved the survivability of probiotics during frozen storage. And also inulin has cryoprotective properties, hence it can improve the survival of probiotic in ice creams during frozen storage (Mohammadi et al., 2011).
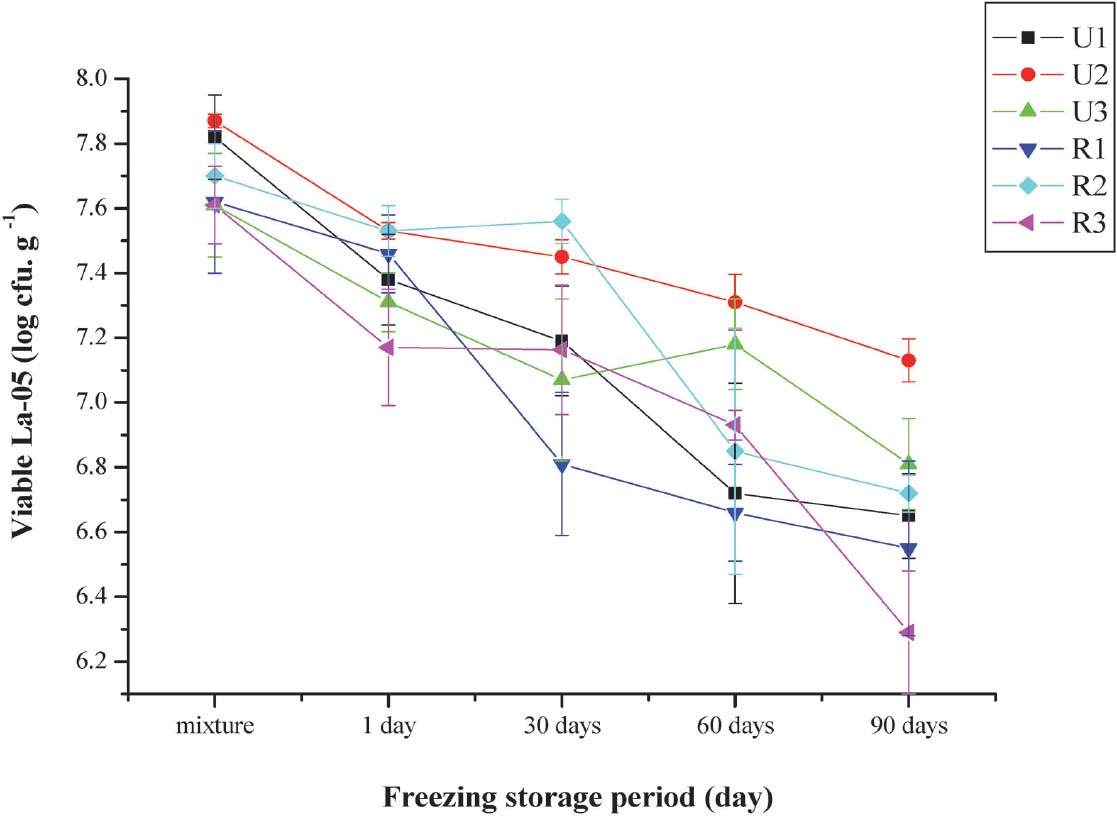
The L. acidophilus counts (log10 cfu/g) in ice cream at different levels of refined and unrefined sugar during 90 days storage at −20°C (p < 0.05).
| Survival rate (%)B | ||||
|---|---|---|---|---|
| SampleA | After 1 day | After 30 days | After 60 days | After 90 days |
| U1 | 94.37c | 1.94d | 85.93f | 85.04d |
| U2 | 95.68b | 94.66b | 92.88b | 90.60a |
| U3 | 97.37a | 92.50c | 93.56a | 89.49b |
| R1 | 97.77a | 89.25e | 86.89e | 85.84d |
| R2 | 97.66a | 98.18a | 88.96d | 87.27c |
| R3 | 94.22c | 94.08b | 91.06c | 82.65e |
Sugar is important in the formulation of ice cream. The maximal survival of probiotic strains at 18% w/w sugar may be explained by the optimal osmotic pressure at this concentration (Medici et al., 2004). The importance of optimal osmotic pressure was demonstrate in the highest survivability of La-05 during 3 months frozen storage for U2 (94.56%) which was even higher than R2 ice cream (89.24%). This result is similar to those reported by Akin et al. (2007) which showed viable bacteria counts were highest at 18% refined sugar compared to 15% and 21%.
Viable La-05 in all probiotic ice cream after 3 months of freeze storage ranged from 1.95 × 106 cfu/g to 1.25 × 107 cfu/g ice cream (Fig. 1). These VCC values indicate the probiotic ice creams produced such a high viability of probiotic is essential because a minimum count of 106 cfu/g is required at the moment of consumption (Granato et al, 2010) to have a beneficial result.
Sensory evaluation The probiotic ice creams prepared using both types of sugar can be regarded acceptable by the consumer panelists, because mean scores above 14 out of 20 point scale (Fig. 2). Probiotic ice cream made with unrefined palm sugar was found note to affect the structure, creaminess, and flavor of the products. None of the ice cream was judged to be sandy, fluffy, watery or weak. Hagen and Narvhus (1999) and Salem et al. (2005) investigated on the different properties of probiotic ice creams and mentioned all ice cream incubated with probiotics were acceptable and gave a good total impression with no marked off-flavor.

Sensory properties of probiotic ice cream made with different levels of refined cane and unrefined coconut palm sugar.
In conclusion, unrefined sugar improved the survivability of L. acidophilus in ice cream during 90 days storage at −20°C when compare to refined cane sugar. In addition, it also increased the antioxidant activity and total acceptability of probiotic ice creams. The replacement of refined cane sugar with unrefined coconut palm sugar in probiotic ice cream may be adopted to increase the functional properties of probiotic ice cream (increased L. acidophilus survivability and antioxidant activities).