2015 Volume 21 Issue 6 Pages 863-867
2015 Volume 21 Issue 6 Pages 863-867
In this study, we investigated the effects of freezing temperature (−10°C, −30°C, and −80°C) on the freeze-thaw fractionation of soymilk. The physicochemical properties of soymilk samples were analyzed after freeze-thawing and fractionation (upper and lower layers). The weight ratios, protein and lipid contents, and 11S/7S ratios of the upper layers were essentially equivalent to those of the lower layers, regardless of the freezing temperature employed. On the other hand, the particle size of the lower layer increased as the freezing temperature was elevated. However, when the lower layers were heated, the particle size distributions were again found to be equivalent across the various freezing temperatures tested. Furthermore, the heated lower layers exhibited similar soymilk calcium coagulability and texture of the produced tofu curd across the various freezing temperatures. These results suggested that the fractions obtained by the freeze-thaw fractionation of soymilk were unaffected by freezing temperature.
Soymilk is consumed as a healthful beverage, and is an important source of tofu curd (soybean curd). Therefore, the physical properties of tofu curds depend on the physicochemical properties of soymilk such as protein content (Toda et al., 2003; Toda et al., 2006) and the 11S/7S ratio (Saio et al., 1969; Kohyama et al., 1995; Yagasaki et al., 2000; Guo and Ono, 2005). However, the 11S/7S ratio in soymilk is dependent on the soybean variety (Toda et al., 2008). Thus, it is difficult to modify the 11S/7S ratio during preparation. Therefore, the utilization of individual 7S or 11S globulins obtained by suitable fractionation holds promise for improving the functional and textural properties of soybean products.
We previously reported (Morita and Yokoi, 2011) a simple freeze-thaw method for the fractionation of these proteins in soymilk, and obtained two kinds of soymilks that had different protein and lipid contents as well as 11S/7S ratios. The tofu curd made from the 7S globulin-rich fraction was soft and smooth, whereas that made from the 11S globulin-rich fraction was firm. However, the effects of freezing temperature on the freeze-thaw fractionation of soymilk have not been reported. The formation of ice crystals is well known to be modified by the freezing rate (Ueno et al., 2004), and the morphology, size, and distribution of ice crystals formed during the freezing process of foods influence the quality of the resultant food products (Rahelić et al., 1985; Jibu et al., 2009; Ottestad et al., 2011). In addition, the investigation of freezing temperature is important to facilitate the commercial utilization of freeze-thaw fractionation of soymilk.
In this study, we investigated the effects of freezing temperature on the freeze-thaw fractionation of soymilk, and evaluated the applicability of freeze-thaw fractionation of soymilk using a wide variety of freezing temperatures.
Preparation of soymilk Soymilk was manually prepared from commercially available soybeans (Glycine max (L.) Merrill, Enrei). Soymilk samples were prepared by the nama-shibori method with modifications (Toda et al., 2007). Soybeans were washed and soaked in distilled water at 20°C for 16 h. The swollen soybeans were then drained and ground into a homogenate with a volume of distilled water equivalent to six times their dry weight. The homogenate was filtered through an absorbent cotton sheet to remove the okara, and the filtrate was termed raw soymilk.
Freeze-thaw treatment of soymilk The raw soymilk was aliquoted into individual plastic tubes (40 mL each). These soymilk samples were frozen at −10°C, −30°C, and −80°C for 7 days, and then thawed at 10°C for 24 h. A temperature recorder (TR-52i; T and D Corp., Ltd., Matsumoto, Japan) was placed inside the sample to monitor the temperature during freezing.
Evaluation of the physicochemical properties of soymilk After freeze-thawing, the raw soymilk was separated into two layers; the supernatant (upper) layer was carefully collected by decantation as to not include the precipitate (lower) layer, and the wet weight of the upper layer was determined. The weight ratios of the upper and lower layers to the total weight were obtained. The protein content of the soymilk was determined by the Kjeldahl method, using a nitrogen-to-protein conversion factor of 6.25. The lipid content of the soymilk was determined by chloroform-methanol extraction.
Protein analysis The protein in the soymilk was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) and stained with Coomassie Brilliant Blue R-250. The intensities of the stained protein bands in the gel were determined using CS Analyser 3.0 image analysis software (ATTO Co., Ltd., Tokyo, Japan).
Particle size distribution The particle size distribution of the soymilk was analyzed with a laser diffraction particle size analyzer (SALD-2100; Shimadzu Co., Ltd., Kyoto, Japan).
Assessment of the coagulability of soymilk The coagulability of the soymilk samples was evaluated by the modified method of Ono et al. (1993). Accordingly, the lower layer was adjusted to 50 g kg−1 protein and heated in an oil bath at 95°C for 2 min, then cooled in ice-cold water. The heated lower layer was added to various concentrations of calcium chloride. After mixing, the mixture was kept at room temperature for 30 min. After centrifugation of the mixture at 3000 × g for 10 min, the precipitate was collected and its wet weight was determined. The precipitation value was obtained as the weight ratio of the precipitate to the total weight.
Preparation of tofu curds After cooling the heated lower layer in ice-cold water, 0.30% glucono-delta-lactone (GDL) was added and the components were mixed. Then, the mixture was poured into a stainless steel cylinder (23 mm diameter and 25 mm height) and heated at 80°C for 1 h, followed by cooling at 20°C. The generated tofu curd was carefully removed from the cylinders and cut into a columned sample 20 mm in height. The breaking stress of the tofu curd was measured with a rheometer (Rheoner RE-33005; Yamaden Co., Ltd., Tokyo, Japan) using a disk adapter 30 mm in diameter. The moving speed of the adapter was 1 mm/s.
Statistical analysis Data were subjected to statistical analysis using one-way analysis of variance (ANOVA) and multiple range comparison by Tukey's test. A p value of < 0.05 was considered statistically significant.
Effects of freezing temperature on the freeze-thaw fractionation of soymilk The raw soymilk samples were frozen at −10°C, −30°C, and −80°C, and passed through the zone of maximum ice crystal formation (0 to −5°C) for 222 min, 147 min, and 29 min, respectively (Fig. 1). After freeze-thawing, the soymilk samples were separated into two layers (upper and lower), with similar fractions for each of the freezing temperatures used (Fig. 2). To identify the effects of freezing temperature on soymilk separation, we determined the weight ratios, protein and lipid contents, and 11S/7S ratios of the upper and lower layers (Table 1). The protein content was only significantly decreased in the lower layer of the sample processed at −80°C and the other components of soymilks did not differ significantly at any temperature. Thus, the physicochemical properties of the fractions obtained from the raw soymilk by freeze-thawing were almost identical between all of the freezing temperatures used.

Effect of freezing temperature on the freezing curve of raw soymilk.
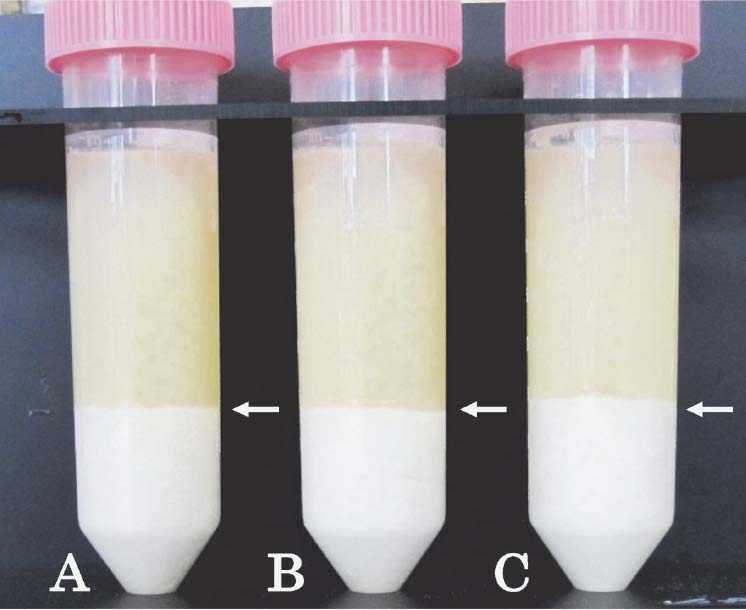
Effect of freezing temperature on the freeze-thaw fractionation of raw soymilk.
Shown are the results from freezing temperatures of (A) −10°C; (B) −30°C; and (C) −80°C. Arrows indicate the boundary between the upper and lower layers in each sample.
| Temperature | Weight ratio (%) | Protein content (%) | Lipid content (%) | 11S/7S ratio | ||||
|---|---|---|---|---|---|---|---|---|
| (°C) | Upper | Lower | Upper | Lower | Upper | Lower | Upper | Lower |
| −10 | 61.7 | 38.3 | 4.2 | 10.2a | 0.5 | 8.6 | 0.3 | 1.6 |
| −30 | 62.8 | 37.3 | 4.3 | 10.3a | 0.5 | 9.0 | 0.3 | 1.8 |
| −80 | 61.4 | 38.6 | 4.4 | 9.9b | 0.6 | 8.6 | 0.3 | 1.7 |
Upper, upper layer; Lower, lower layer. Values are means of triplicate experiments.
There have been many studies regarding the effects of freezing rate on food quality. The ice crystals formed by slow freezing are large, whereas those formed by rapid freezing are small (Ueno et al., 2004). Therefore, the process of slow freezing causes accelerated destruction of the food structure, resulting in a decrease of food quality (Alvarez et al., 1997; Ngapo et al., 1999). Conversely, rapid freezing is appropriate for the preservation of food by freezing because of the minimal associated decrease in food quality. However, in this study, we found that the physicochemical properties resulting from the freeze-thaw fractionation of raw soymilk were unaffected by the freezing rate.
Effects of freezing temperature on the particle size distribution of soymilk After freeze-thawing, the raw soymilk was separated into two layers, and the particle size distribution of the lower layer was measured. Differences in particle size distributions among lower layers obtained by various freezing temperatures were estimated using a laser diffraction particle size analyzer (Fig. 3). In comparison with the layers from samples frozen at −10°C, which had a particle size distribution of 0.3 to 200 µm with a 5 µm mode diameter, the samples frozen at −30°C had a particle size distribution of 0.3 to 100 µm with a 4 µm mode diameter, while those frozen at −80°C had a particle size distribution of 0.3 to 50 µm with a 3 µm mode diameter. These results show that the particle size of the lower layer increased as the freezing temperature was elevated.

Particle size distributions of the lower layers at different freezing temperatures.
In raw soymilk, proteins have been reported to interact with lipid emulsions to form lipid/protein complexes (Ono et al., 1991; Guo et al., 1997). In addition, the formation of a lower layer from raw soymilk during the freeze-thaw process has recently been reported to arise from an increase in disulfide and/or hydrophobic interactions among proteins that have been concentrated by freezing (Morita and Shimoyamada, 2013). Therefore, we concluded that the particle sizes of the lower layers were increased through elevated concentration mediated by the formation of larger ice crystals.
However, the protein components of the lipid/protein complexes in raw soymilk are liberated when heated (Ono et al., 1996; Guo et al., 1997), and the interactions among proteins themselves are so weak that it is easy to solubilize the soymilk by heating (Morita et al., 2015). Accordingly, after the lower layer samples were heated and the particle size distributions were measured again (Fig. 4), the heated lower layers showed similar particle size distributions among all freezing temperatures tested.

Particle size distributions of the lower layers at different freezing temperatures after heating.
Effects of freezing temperature on the coagulability of soymilk and the breaking stress of tofu curds To determine the effects of freezing temperature on the coagulability of the samples fractionated by the freeze-thawing of soymilk, we added various concentrations of calcium chloride to the heated lower layers, and then analyzed the degree of precipitation. We found that the level of precipitate increased as the amount of calcium chloride added increased, and that the degree of increase was similar among all of the freezing temperatures tested (Fig. 5).

Effects of calcium chloride on precipitation in the lower layers at different freezing temperatures.
Furthermore, to verify the physical properties of the tofu curds made from the lower layer of the freeze-thaw fractionated soymilk, we determined the breaking stress of the tofu curds (Fig. 6). This increased slightly but not significantly as the freezing temperature increased. Therefore, the effect of freezing temperature on the physical properties was considered to be slight but not significant, and it may be considered that the particle size distributions were equivalent by heating, whereas the interactions among proteins varied only slightly.

Breaking stress of tofu curd made with 0.3% GDL from the lower layers at different freezing temperatures.
Values represent means ± standard error of triplicate experiments.
In conclusion, the results from the investigation of the effects of freezing temperature on the freeze-thaw fractionation of soymilk suggested that the physicochemical properties of the upper and lower layers fractionated by the freeze-thawing of soymilk were similar among the freezing temperatures tested. On the other hand, differences in particle size caused by the freezing rate were observed and were thought to relate to changes in the amount of protein binding to the emulsion surface. However, these protein particles were readily dissociated by heating, resulting in almost equivalent measures of soymilk calcium coagulability and the texture of the produced tofu curds.
Overall, our results suggest that it might be possible to utilize this freeze-thaw fractionation method at various freezing temperatures. The data obtained in this study might be useful for the development of this freeze-thaw fractionation method at a wide variety of production sites.