2015 Volume 21 Issue 6 Pages 869-873
2015 Volume 21 Issue 6 Pages 869-873
An osteoclast is a large multinucleated cell that is formed by mononuclear preosteoclast fusion. Dendritic cell-specific transmembrane protein (DC-STAMP) is one of the key regulators of osteoclast cell-cell fusion. When tea extract was added to RAW264.7 cells stimulated by nuclear factor kappa-B ligand (RANKL), the mRNA expression of DC-STAMP was enhanced while that of tartrate-resistant acid phosphatase (TRAP), an osteoclast differentiation marker, was suppressed. These observations indicated that increased DC-STAMP mRNA in RAW264.7 cells by tea extract occurred independently of the RANKL-mediated signaling pathway in osteoclastogenesis. Real-time PCR analysis indicated that epigallocatechin gallate (EGCg) might be one of the stimulating factors. Moreover, the increase in DC-STAMP mRNA induced by tea extract tended to be higher than that induced by a reconstituted mixture of the four major tea catechins. On the other hand, the increase in DC-STAMP mRNA was also observed in RANKL-unstimulated macrophage-like RAW264.7 cells. These results suggest that tea extract modulates the expression of DC-STAMP mRNA not only in osteoclastic cells but also in the other cell types.
Tea extract is known to have various bioactivities (Cabrera et al, 2006). Some reports show that tea extract has a positive effect on bone (Shen et al, 2009; Shen et al, 2011), for example, by inhibiting osteoclast differentiation (Lee et al, 2010; Lin et al, 2009; Yun et al, 2004) and promoting osteoclastic apoptosis (Nakagawa et al, 2002). The main active component driving these effects has been identified as catechins, especially epigallocatechin gallate (EGCg) (Crespy & Williamson, 2004; Jung & Ellis, 2001; Katiyar & Mukhtar, 1997; Khan et al, 2006). EGCg is found in high levels in tea leaves; however, it is difficult to extract with water and is thus found in low amounts in tea (Tokunaga & Goto, 1997).
Bone is maintained by the balance between bone formation by osteoblasts and bone resorption by osteoclasts (Karsenty et al, 2009). Osteoclast differentiation consists of differentiation to preosteoclast, multinucleation, and activation and apoptosis; and is under the careful control of various molecules. One of these molecules is dendritic cell-specific transmembrane protein (DC-STAMP), which is essential for cell fusion during multinucleation (Yagi et al, 2006). It has been shown that osteoclast bone resorption efficacy increases with multinucleation (Kukita et al, 2004; Yagi et al, 2005).
In this study, we evaluated the effect of a hot water extract of freeze-dried tea sample as a model tea beverage on DC-STAMP mRNA expression in RAW264.7 cells.
Reagents and preparation of tea extract (-)-Epigallocatechin gallate (EGCg), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECg) and (-)-epicatechin (EC) were purchased from Nagara Science Co., Ltd., Gifu, Japan.
The tea extract was prepared from dried tea leaves, cultivated in Mie Prefecture, Japan, by grinding the leaves into powder, extracting in hot water at 80°C for 20 min, filtering with No.1 filter paper (ADVANTEC, Tokyo, Japan), and freeze-drying the filtrate.
Determination of the catechin contents of tea extract by HPLC The EGCg, EGC, ECg, and EC contents of the tea extract were measured by HPLC under the following conditions (Yamaguchi et al, 1997): column, Develosil ODS-HG-5 (4.6 × 150 mm)(Nomura Chemical Co., Ltd., Aichi, Japan); guard column, Develosil ODS-HG-5 (4 × 10 mm) (Nomura Chemical Co., Ltd); flow rate, 1 mL/min; injection volume, 10 µL; absorbance detection, 230 nm UV wavelength; column temperature, 40°C; eluents, (A) 0.1% phosphoric acid in H2O, (B) 60% eluent A and 40% acetonitrile; gradient conditions, 20% B (0 to 10 min), 20% to 64% B (10 to 40 min); sample preparation, the tea extract was dissolved in extraction eluent (0.1% phosphoric acid in H2O, 50% acetonitrile).
Cell culture RAW264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). Cells were maintained in α-minimum essential medium (α-MEM) (Sigma-Aldrich Japan, Tokyo, Japan) supplemented with 10% fetal bovine serum (FBS) (BioWest, Nuaillé, France) and incubated under 5% CO2 at 37°C. For osteoclast differentiation, RAW264.7 cells were plated at 5.0 × 104 cells/well in 12-well plates and cultured for 24 h. Then, the medium was changed to α-MEM supplemented with 10% FBS in the presence of 50 ng/mL of receptor activator of nuclear factor kappa-B ligand (RANKL; Cell Signaling Technology Japan, K.K., Tokyo, Japan) alone or in combination with 40 mg/L of tea extract. The tea extract and catechins were diluted in dimethyl sulfoxide (DMSO) prior to being added to the medium; the final concentration of DMSO in the medium was 1/1000.
RNA isolation and quantitative RT-PCR Total RNA was isolated from RAW264.7 cells using the NucleoSpin® RNA Kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Reverse transcription reactions were performed using ReverTra Ace® qPCR RT Master Mix (TOYOBO Co., Ltd., Osaka, Japan) according to the manufacturer's instructions. Real-time PCR for the genes encoding tartrate-resistant acid phosphatase (TRAP), DC-STAMP and GAPDH was performed using the LightCycler™ Nano System (Roche Diagnostics K.K., Tokyo, Japan) and THUNDERBIRD® SYBR® qPCR Mix (TOYOBO Co., Ltd.) according to the manufacturers' protocols. The PCR conditions were as follows: 40 cycles of denaturation at 95°C for 15 s each, and 1 min of amplification at 60°C. All reactions were normalized to the housekeeping gene GAPDH. Relative differences in the PCR results were evaluated using the comparative cycle threshold method. The following primer sets were used: mouse DC-STAMP: forward, 5′-CTAGCTGGCCTGGACTTCATCC-3′, reverse, 5′-TCATGCTGTCTAGGAGACCTC-3′; mouse TRAP: forward, 5′-CGACCATTGTTAGCCACATACG-3′, reverse, 5′-TCGTCCTGAAGATACTGCAGGTT-3′ and mouse GAPDH: forward, 5′-GGCACAGTCAAGGCTGAGAATG-3′, reverse, 5′-ATGGTGGTGAAGACGCCAGTA-3′.
Immunostaining RAW264.7 cells were plated at 1.5 × 104 cells/cm2 and cultured for 24 h. The medium was then changed to α-MEM supplemented with 10% FBS in the presence of 40 mg/L of tea extract and incubated for 5 days. Cells were stained as follows: cells were fixed with 4% formaldehyde diluted in Tris-buffered saline (TBS; 137 mM NaCl, 2.68 mM KCl, 25 mM Tris-HCl, pH 7.4) for 30 min at room temperature and then rinsed three times in TBS. Next, the cells were treated with 0.1% Triton X-100 in TBS for 10 min at room temperature and then rinsed three times in TBS. The cells were then blocked with 2% BSA in TBS for 60 min at room temperature. Next, the blocking solution was aspirated and anti-mouse DC-STAMP polyclonal antibody (4 µg/mL; Trans Genic Inc., Ltd., Fukuoka, Japan) diluted in TBS was added to the cells. The cells were incubated for 60 min at room temperature, rinsed three times in TBS, and then goat anti-rabbit IgG H&L (HRP) (Abcam, Abcam plc, Cambridge, UK) diluted 500-fold in TBS was added to the cells. The cells were incubated for 60 min at room temperature and rinsed three times in TBS. Finally, 3,3′-diaminobenzidine (DAB) solution (6 mg/mL DAB and 0.03% hydrogen peroxide in TBS) was applied to the cells, and then incubated at room temperature for 5 min.
Statistical methods All data are presented as means ± standard deviation (SD). Statistical analysis was performed using the two-tailed unpaired Student's t-test and Tukey's test using Excel statistics.
The effect of tea extract on DC-STAMP mRNA expression in RANKL-stimulated RAW264.7 cells In this study, we found for the first time that tea extract enhanced DC-STAMP mRNA expression in RAW264.7 cells. RAW264.7 cells are used as a model for osteoclast formation including DC-STAMP expression. Figure 1 shows that DC-STAMP mRNA expression increased in RAW264.7 cells exposed to tea extract. Expression of DC-STAMP mRNA also increased in the presence of RANKL alone at 24 h, but by 48 h, the increase was larger in the sample with both RANKL and 40 mg/L of tea extract (Fig. 1). Tea extract at 40 mg/L slightly decreased cell proliferation, but showed no cytotoxic effects in the RAW264.7 cells (data not shown).
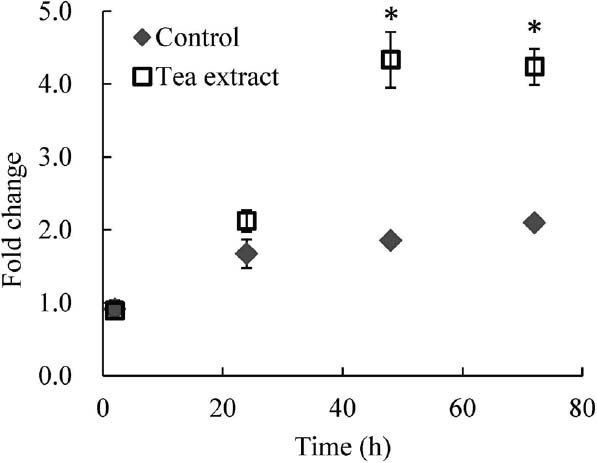
The effect of tea extract on DC-STAMP mRNA expression RAW264.7 cells were treated with RANKL alone (Control) or in combination with 40 mg/L of tea extract (Tea extract). Samples were incubated for 2, 24, 48, or 72 h after the addition of the reagents. Total RNA was extracted, and real-time PCR with primers for DC-STAMP was performed after cDNA synthesis.
n = 3, **P < 0.01 by Student's t-test (between samples at the same time point). All data are means ± SD.
DC-STAMP is a seven-transmembrane protein that has been isolated from dendritic cells, interleukin (IL)-4-induced macrophages, and osteoclasts (Hartgers et al, 2000). Studies have shown that the multinucleation of osteoclasts is completely abrogated in DC-STAMP-deficient mice, both in vivo and in vitro, even when the expression of osteoclast differentiation markers is normal, indicating that DC-STAMP is required only for osteoclast cell-cell fusion rather than differentiation (Yagi et al, 2005). The production of DC-STAMP is stimulated by RANKL, and some studies have shown that other molecules may also regulate DC-STAMP production (Xing et al, 2012). In this study, it was found that tea extract contains a new molecule that can alter DC-STAMP production in RAW264.7 cells. However, treatment of RAW264.7 cells with tea extract significantly inhibited TRAP mRNA expression as previously reported (Lee et al, 2010; Lin et al, 2009; Yun et al,2004); similar to our results (Fig. 2). These results indicated that increased DC-STAMP mRNA in RAW264.7 cells by tea extract occurred independently of the RANKL-mediated signaling pathway in osteoclastogenesis.
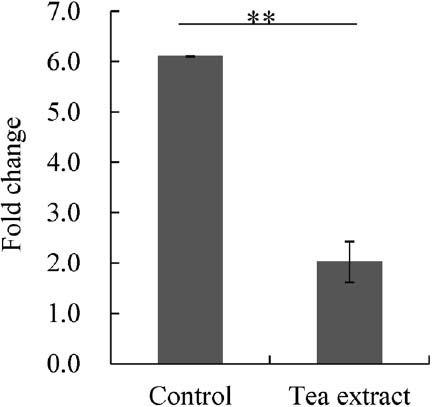
The effect of tea extract on TRAP mRNA expression RAW264.7 cells were treated with RANKL alone (Control) or in combination with 40 mg/L of tea extract (Tea extract). Samples were incubated for 72 h after the addition of the reagents. Total RNA was extracted, and real-time PCR with primers for TRAP was performed after cDNA synthesis. Changes in mRNA transcript levels are represented as fold change relative to unstimulated sample. n = 3, **P < 0.01 by Student's t-test (between samples at the same time point). All data are means ± SD.
The effect of tea catechins on DC-STAMP mRNA expression in RANKL-stimulated RAW264.7 cells aIt is known that many bioactive ingredients are present in tea leaves and extracts, and that catechins are a main component. Thus, we determined the major catechins in tea leaf (EGCg, EGC, ECg and EC) by HPLC. The EGCg, EGC, ECg, and EC contents of the tea extract in this study are shown in Table 1. Among catechins, EGCg is present at the highest concentration in tea leaf; however, as it is difficult to extract with water, it is found at lower levels in tea extract compared to EGC.
| Concentration | ||||
|---|---|---|---|---|
| EGCg | EGC | ECg | EC | |
| % | 17.8 | 19.0 | 3.7 | 3.9 |
To identify stimulating factors, we assessed the effect of these major catechins found in tea leaf on DC-STAMP expression. We found that EGCg at 25 µM increased DC-STAMP mRNA expression, but no such effect was seen with EGC, ECg, or EC at 25 µM (Fig. 3a). Thus, it appears that EGCg is a factor in the increased DC-STAMP mRNA expression in RAW264.7 cells.
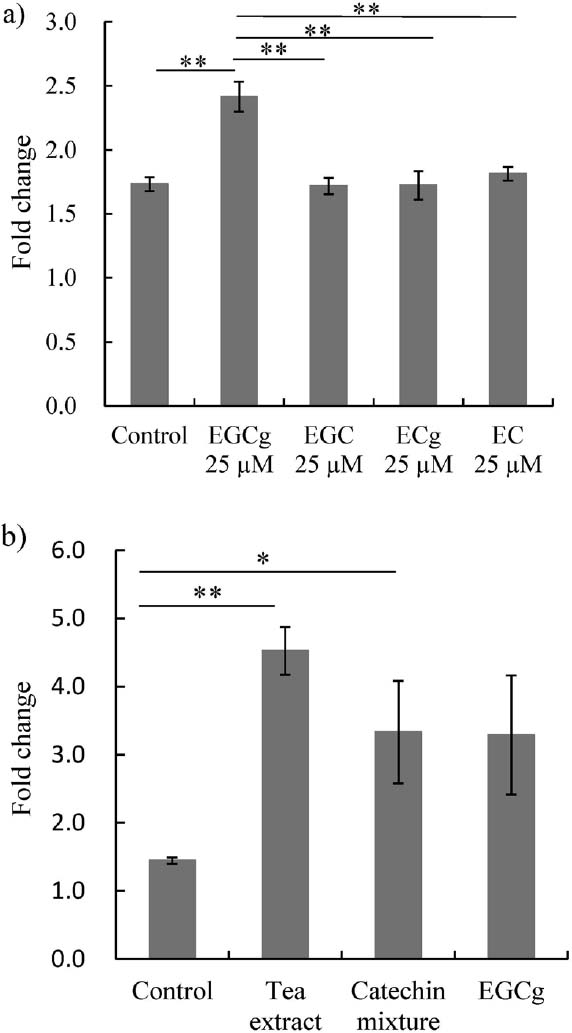
The effect of catechin on DC-STAMP mRNA expression (a) RAW264.7 cells were treated with RANKL alone (Control) or in combination with 25µM EGCg, EGC, ECg, or EC. (b) RAW264.7 cells were treated with RANKL alone (Control) or in combination with 40 mg/L of tea extract (Tea extract), the same contents of EGCg, EGC, ECg and EC as the tea extract (Catechin mixture), or the same content of EGCg as the tea extract (EGCg). Total RNA was extracted 72 h after the addition of reagents, and real-time PCR with primers for DC-STAMP was performed after cDNA synthesis. Changes in mRNA transcript levels are represented as fold change relative to unstimulated sample. n = 3, *P < 0.05, **P < 0.01 by Post-ANOVA comparisons with Tukey's-test. All data are means ± SD.
Next, we prepared a mixture of four catechins or EGCg alone. These were adjusted to the same catechin content as the tea extract, and their effect on DC-STAMP mRNA expression was assessed. The tea extract showed a slightly stronger effect, although not significantly so, than the mixture of four catechins or EGCg alone. There was no significant difference between the mixture of four catechins and EGCg alone (Fig. 3b). It is possible that an active molecule other than EGCg, EGC, ECg or EC, or that a molecule able to enhance the activity of EGCg, EGC, ECg or EC was present in the tea extract. EGCg, EGC, ECg and EC are the primary catechins in tea leaf (Beecher et al, 1999), but some other minor catechins and tannins also exist. Moreover, it is known that EGCg, EGC, ECg or EC can epimerize and change to (-)-gallocatechin gallate (GCg), (-)-gallocatechin (GC), (-)-catechin gallate (Cg) and (-)-catechin (C) when heated (Ishino et al, 2010). Because the tea extract used in this study was prepared with boiling water, it is possible that GCg, GC, Cg and C were present in the tea extract.
The effect of tea extract on DC-STAMP expression in the absence of RANKL In the absence of RANKL, treatment with 40 mg/L of tea extract or 25 µM EGCg increased the expression of DC-STAMP mRNA to the same levels as the samples containing RANKL (Fig. 3 and 4a). Immunostaining using the anti-DC-STAMP antibody confirmed that treatment with 40 mg/L of tea extract increased the expression of DC-STAMP protein in cells (Fig. 4b). These results might indicate that tea extract accelerates multinuclear formation in RAW264.7 cells with or without RANKL stimulation.
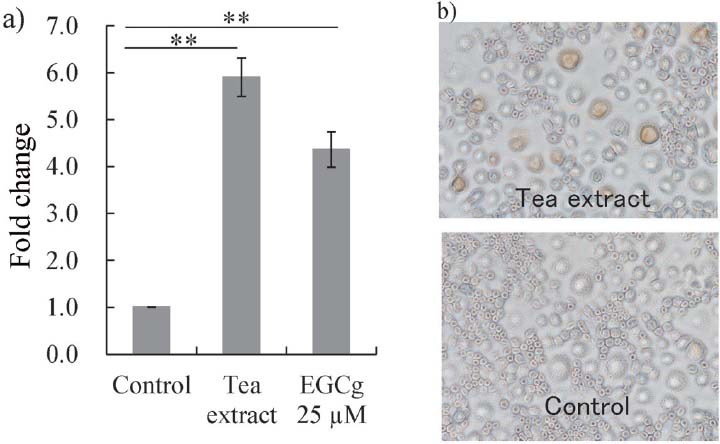
The effect of tea extract or EGCg on DC-STAMP mRNA expression (a) and protein expression (b) in RAW264.7 cells. (a) Untreated RAW264.7 cells (Control), and those treated with 40 mg/L of tea extract (Tea extract) or 25µM EGCg (EGCg 25µM). Total RNA was extracted 72 h after the addition of reagents, and real-time PCR with primers for DC-STAMP was performed after cDNA synthesis. (b) RAW264.7 cells treated with 40 mg/L of tea extract (top panel) and untreated cells (lower panel). Immunostaining with anti-DC-STAMP antibody was performed 5 days after the addition of reagents. **P < 0.01 by Post-ANOVA comparisons with Tukey's-test. All data are means ± SD.
In conclusion, we found that the hot-water tea extract enhanced DC-STAMP mRNA expression in RAW264.7 cells. It is known that DC-STAMP plays a role in the fusion of not only osteoclasts, but also monocytes and macrophages (Yagi et al, 2005; Yagi et al, 2006), and that DC-STAMP-mediated osteoclast fusion increases bone resorption efficiency and contributes to physiological bone mass control (Kukita et al, 2004; Yagi et al, 2005). In this study, treatment with EGCg or tea extract increased DC-STAMP expression in RAW264.7 cells through a mechanism unrelated to osteoclast differentiation by RANKL, suggesting that these substances affect the cell-cell fusion of RAW264.7 cells through an unknown pathway. In the future, we intend to further examine the mechanisms by which these substances control DC-STAMP expression.