2019 年 25 巻 4 号 p. 539-544
2019 年 25 巻 4 号 p. 539-544
The protective effect of glass bottles with different levels of transparency (clear or amber glass) on the quality of extra virgin olive oil was evaluated after exposure to sunlight or storage in clear glass bottles covered with aluminum foil for 5 weeks. Oils stored in clear glass bottles had significantly higher values of all quality parameters (free fatty acids, peroxide value, and K270), and lower pigment (chlorophylls and carotenoids) and phenolic contents than the same oils stored in covered or amber glass bottles. Oils stored in covered glass bottles showed no deterioration, whereas oils stored in amber glass bottles contained primary oxidation products. Sunlight exposure produced acrolein in the oils stored in clear glass bottles, but not to the same extent in oils stored in covered and amber glass bottles. These results show that low transparency packaging provides protection against sunlight-induced oxidative deterioration of extra virgin olive oil during storage.
Olive oil is probably the most well known component of the Mediterranean Diet. Its consumption is increasing around the world, with the European Union being the leading producer, consumer, and exporter of olive oil. Recently, olive oil has become popular in most Asian countries as a premium edible oil with beneficial health effects (Capogna and Gomez, 2016). Extra virgin olive oil (EVOO) is the juice obtained from olive drupes by mechanical procedures and is not subjected to further treatment or chemical additions. Therefore, EVOO is the highest grade of olive oil. It has free fatty acids, expressed in terms of oleic acid content, at not more than 0.80%, and its other characteristics must meet standards specified by the International Olive Council (IOC, 2018). EVOO has high biological value because of its oleic acid content, which is considered a healthy fat, and also minor components such as volatile compounds and several antioxidants. Its main antioxidants are phenolic compounds, which play an important role in preventing certain chronic human diseases (Visioli and Galli, 2002).
Because of consumer demand, EVOO needs to maintain high quality throughout its shelf-life. Packaging can directly affect olive oil quality by protecting it from both oxygen and light. Light plays an important role in the quality and stability of edible oils (Sohail et al., 2010), and exposure to light causes significant deterioration in oil quality. Exposure of vegetable oils to light induces photo-oxidation through the action of natural photosensitizers such as chlorophylls, which are present in the highest concentration in EVOO among other types of vegetable oil (Sena et al., 2017). Photosensitizers can form singlet oxygen from triplet oxygen in the presence of light. Singlet oxygen then forms free radicals from unsaturated fatty acids (Min and Boff, 2002). This leads to the production of hydroperoxides and eventually to carbonyl compounds, resulting in the development of undesirable off-flavors in oils (Skibsted, 2000). An earlier study has demonstrated that olive oil oxidation proceeded slowly in plastic bottles covered with aluminum foil, faster in diffused light, and even faster in indirect sunlight (Kiritsakis and Dugan, 1984).
Olive oil producers, suppliers and consumers need to pay attention to the exposure of olive oil to diffused light and direct sunlight during processing, transportation, handling, and storage. Fluorescent light in the range of 300–700 nm has been reported to cause lipid oxidation (Sattar and deMan, 1975). Sunlight contains higher intensities in the lower wavelength range, which appears to be more conducive to lipid oxidation than fluorescent light (Luby et al., 1986). Unfortunately, olive oils are often exposed to sunlight in open markets, fluorescent lighting in supermarkets and daylight on a sunny windowsill of the home kitchen, which may result in light-induced deterioration of the oil and the production of off-flavors, color defects, and loss of minor nutrients.
Most olive oil consumers purchase olive oil in bottles rather than other types of packaging, and the nature of the packaging has a notable influence on olive oil quality. Generally, amber glass bottles are used to protect olive oil from light. However, the protective effect of colored glass bottles remains unclear. Therefore, the objective of this study was to evaluate the protective effect of glass bottles with different levels of transparency on the quality of olive oil stored with exposure to sunlight in an attempt to simulate a storage condition in the home.
Materials EVOO from the cultivar Nevadillo blanco was obtained by dual-phase decanter centrifugation using industrial processors (Kishimoto and Kashiwagi, 2018). The fatty acid composition of the EVOO was: palmitic (C16:0; 16.24%), palmitoleic (C16:1; 1.61%), heptadecanoic (C17:0; 0.11%), stearic (C18:0; 1.72%), oleic (C18:1n-9; 65.91%), cis-vaccenic (C18:1n-7; 3.66%), linoleic (C18:2n-6; 8.92%), α-linolenic (C18:3n-3; 1.08%), arachidic (C20:0; 0.32%), eicosenoic (C20:1n-9; 0.32%), and behenic (C22:0; 0.11%). The amount of α-tocopherol in EVOO was 27.9 mg per 100 g of oil, its moisture content < 0.1%, and dissolved oxygen content 4.2 ppm. The contents of trace metals in 100 g of EVOO were: iron < 0.10 mg, copper < 0.01 mg, manganese < 0.01 mg, and chromium < 0.05 mg. The medium-chain triglyceride oil (The Nisshin OilliO Group, Ltd., Tokyo, Japan) was purchased from a market. Acrolein (purity > 95%) was purchased from Tokyo Kasei Industry (Tokyo, Japan).
Sunlight storage conditions Three samples (200 g) of oil for each storage condition were weighed into 250-mL clear glass bottles and 250-mL amber glass bottles with the headspace occupied by air. To shield some of the samples from sunlight, three samples in clear glass bottles were covered completely with aluminum foil. To simulate typical light exposure at the consumer level, the oil samples were then placed on a south-facing windowsill in the laboratory of Shodoshima Healthyland Co., Ltd. (Kagawa, Japan) at a room temperature of 26 ± 2 °C (Anwar et al., 2007) for a period of five weeks through August to September, 2018 (the mean value of cumulative sunshine per week in Shodoshima was 55.7 ± 12.5 hours). For each set of experiments an equal amount of oil (10 g) was taken from the same bottle and analyzed weekly.
Analytical procedures Free fatty acids, peroxide values, K270, and total phenolic contents of the oil samples were measured using an OxiTester (CDR; Ginestra Fiorentina, Italy) (Kamvissis et al., 2008). Preliminary confirmation of the free fatty acids determined using the OxiTester method was conducted by comparing the results for oil samples over a wide range of values with those from the official analysis method (Gucci et al., 2012; Kishimoto, 2019). Samples of the oils were added to prefilled cuvettes for analysis. The volumes of oil used were 0.5–2.5 µL for measuring free fatty acids, 0.5–2.5 µL for peroxide value, 10–100 µL for total phenolic content, and 10 µL for K270.
The contents of chlorophyll and carotenoid pigments, which are reported in mg/kg of oil, were determined using a UV-1700 spectrophotometer (Shimadzu, Kyoto, Japan) following the method described by Minguez-Mosquera et al. (1991). One gram of oil sample was dissolved in 10 mL of iso-octane. Then, the absorption was recorded at 670 nm for chlorophylls and 470 nm for carotenoids. The contents were calculated using the following equations:
Chlorophylls (mg/kg) = (A670 × 106)/(613 × 100 × d),
Carotenoids (mg/kg) = (A470 × 106)/(2 000 × 100 × d),
where A is the absorption and d is the path length of the cell (1 cm).
Flash gas chromatography electronic nose analysis To examine the volatile organic compounds of the oil samples, the headspace (gas mixture) prepared in a temperature-controlled vial was analyzed using the HERACLES II electronic nose (Alpha MOS; Toulouse, France) (Kishimoto et al., 2017). The HERACLES II was equipped with a non-polar column (MXT-5: 10 m × 180 µm diameter) and a polar column (MXT-WAX: 10 m length × 180 µm diameter) working in parallel to produce two chromatograms simultaneously. It was also equipped with an HS100 auto-sampler (CTC Analytics AG; Zwingen, Switzerland) to automate sample incubation and injection. An alkane mixture (from n-hexane to n-hexadecane) was used to convert retention times into Kovats indices for calibration. For analysis, an aliquot of oil (2.0 g) was placed in a 20-mL vial and then sealed with a magnetic cap. The vial was placed in the auto-sampler, which was placed in the HERACLES's shaker oven and incubated for 15 min at 60 °C with shaking at 500 rpm. A syringe was used to sample 5 mL of the headspace, and this was injected into the gas chromatograph. The oven temperature was initially 40 °C (held for 10 s), then increased to 250 °C at 1.5 °C/s and held at this temperature for 60 s. The total separation time was 120 s. Data were acquired and processed using AlphaSoft software v14.5 (Alpha MOS). The AroChemBase module (Alpha MOS) was used to identify the volatile compounds.
Quantification of acrolein To determine the amount of acrolein in each oil sample, a standard curve was established (Kishimoto and Kashiwagi, 2018). Samples of medium-chain triglyceride oil containing different concentrations of acrolein were prepared and subjected to flash gas chromatography electronic nose analysis. The amounts of acrolein in the EVOO samples after exposure to sunlight were determined using the standard curve.
Statistical analyses The data are presented as means ± standard deviations from three replicates. The data were analyzed by one-way analysis of variance followed by the Tukey-Kramer test in Microsoft Excel. Differences between mean values with p < 0.05 were considered statistically significant.
The free fatty acids in EVOO result from the hydrolysis of triacylglycerols. The free fatty acid contents of EVOO samples stored under different conditions with exposure to sunlight are shown in Fig. 1A. The free fatty acids of EVOO stored in clear glass bottles increased gradually as the exposure time to sunlight increased, but did not exceed the 0.80% standard for EVOO (IOC, 2018). Aluminum foil laminate can prevent light-induced lipid oxidation in foods by completely blocking light transmission (Emmons et al., 1986; Luby et al., 1986). In the present study, no significant increase in free fatty acid content was observed in EVOO stored in clear glass bottles covered with aluminum foil. For EVOO stored in amber glass bottles, a slight increase in free fatty acids was observed after exposure to sunlight for 5 weeks. These observations suggest that sunlight can induce oxidative degradation of EVOO and that amber glass bottles can protect against hydrolysis of triacylglycerols upon exposure to sunlight.

Changes in free fatty acid (A), peroxide value (B), and K270 (C) of EVOO during storage with exposure to sunlight (○: clear glass bottles; ▴: amber glass bottles; ●: clear glass bottles covered with aluminum foil). a–cFor the 5-week values, mean values with different letters are significantly different (p < 0.05).
The peroxide value is widely used as an indicator of fat oxidation, which is often used for monitoring peroxide formation during the initial stages of lipid oxidation. In the present study, before exposure to sunlight, the peroxide value of the EVOO was 8.9 meq O2/kg, a value lower than the 20.0 meq O2/kg limitation in the standard for EVOO (IOC, 2018). However, the EVOO samples stored in the clear and amber glass bottles exhibited higher peroxide values after exposure to sunlight for 1 week, exceeding the limit in the standard; therefore, this oil could not be classed as EVOO (Fig. 1B). The level of oxidation of EVOO stored in amber glass bottles was lower than that in clear glass bottles. The peroxide values of EVOO stored in clear glass bottles covered with aluminum foil did not increase significantly during storage. Generally, during the beginning of storage, the peroxide value increases as a consequence of the action of primary oxidation. After a period of storage, the peroxide value progressively decreases due to the degradation of primary oxidation products into secondary oxidation products. However, the peroxide values of EVOO samples stored in clear and amber glass bottles gradually increased without sudden changes (Fig. 1B), as observed in other studies (Kiritsakis and Dugan, 1984; Fekarurhobo et al., 2009; Sohail et al., 2010). These observations suggest that amber glass bottles are not sufficient to protect against primary oxidation of EVOO upon exposure to sunlight.
It is also possible to verify the degree of olive oil oxidation by assessing the spectrophotometric absorption at 270 nm (K270). The K270 value indicates the presence of carbonyl compounds, which is correlated to the presence of secondary oxidation compounds (Di Giovacchino et al., 2002). Changes in the K270 value were measured during storage under sunlight exposure (Fig. 1C). Before exposure to sunlight, the K270 of the EVOO (0.138) was lower than the standard for EVOO (0.22) (IOC, 2018). With increasing storage time, exposure to sunlight induced a progressive increase in the K270 for EVOO stored in clear glass bottles and a small increase in the value for EVOO stored in amber glass bottles. The K270 for EVOO stored in amber glass bottles exceeded the limit in the standard after exposure to sunlight for 3 weeks. The K270 in EVOO stored in the clear glass bottles covered with aluminum foil remained within the quality range for EVOO throughout the storage period. These observations suggest that amber glass bottles do not provide sufficient protection to prevent EVOO oxidation on exposure to sunlight.
The color of olive oil is mainly related to the presence of chlorophylls and carotenoids, which are responsible for production of green and yellow coloration, respectively. These pigments affect consumer acceptance of the oil and the oil stability as antioxidants. In the present study, both pigments were stable in EVOO stored in clear glass bottles covered with aluminum foil (Fig. 2A and 2B). The chlorophylls content in EVOO stored in clear and amber glass bottles decreased rapidly after sunlight exposure (Fig. 2A). Small reductions in the carotenoid contents were observed after sunlight exposure for EVOO stored in amber glass bottles. Sunlight exposure resulted in more severe loss of carotenoids from EVOO stored in clear glass bottles than that stored in amber glass bottles (Fig. 2B). Thus, the sensitivity of these pigments to sunlight depended on glass bottles with different levels of transparency, and this may lead to the photo-oxidation of olive oil (Kiritsakis and Dugan, 1985). Phenolic compounds have antioxidant activity and are important in oil stability. Changes in the phenolic contents of EVOO during storage under exposure to sunlight are shown in Fig. 2C. Decreasing trends that were similar to those for the carotenoid contents were observed. Chlorophylls a and b have two main absorption maxima located at 430–460 and 640–670 nm, while the major carotenoids have three main absorption maxima located within 420–480 nm (Moyano et al., 2010). The contents of chlorophylls and carotenoids in EVOO stored in clear glass bottles decreased rapidly owing to their role as photosensitizers (Kiritsakis and Dugan, 1985). In contrast, amber glass can absorb nearly all radiation with a wavelength less than 450 nm (GPI), offering partial protection to carotenoids from sunlight, but not chlorophylls. The decomposition and oxidation processes have more impact on reducing the phenolic content in EVOO than the bottle glass color (Morello et al., 2004; Rizzo et al., 2014). The phenolic content of EVOO during storage may depend on chlorophyll and carotenoid-induced photosensitized oxidation processes. These observations suggest that storing EVOO in amber glass bottles may slightly protect against degradation of carotenoids and phenolic compounds, but not chlorophylls.

Changes in chlorophylls (A), carotenoids (B), and phenolic contents (C) of EVOO during storage with exposure to sunlight (○: clear glass bottles; ▴: amber glass bottles; ●: clear glass bottles covered with aluminum foil). a–cFor the 5-week values, mean values with different letters are significantly different (p < 0.05).
Acrolein (2-propenal) can be formed during frying with fats and oils. For instance, the formation of acrolein has been observed in olive oil after heating at high temperature (Fullana et al., 2004; Katragadda et al., 2010; Molina-Garcia et al., 2017; Kishimoto and Kashiwagi, 2018; Kishimoto, 2019). An obvious source of acrolein is the glycerol component of triacylglycerol. When oil is heated at frying temperatures, acrolein can be formed from glycerol via the dehydration process, after triacylglycerol has been hydrolyzed to produce glycerol and three fatty acids. Production of acrolein is undesirable, because it has an irritating odor and a negative impact on olive oil flavor. It is a key volatile compound that is used to evaluate the sensory qualities of lipids that are susceptible to oxidative degradation. Consequently, the formation of acrolein during sunlight exposure should be monitored. Figure 3 shows the changes in the levels of acrolein formed during sunlight exposure. The amount of acrolein formed in EVOO exposed to sunlight increased as the storage time increased, reaching 2 376 ppb after sunlight exposure for 5 weeks. Particularly for EVOO stored in clear glass bottles, a rapid increase was observed at 3 weeks as oxidative degradation occurred (Fig. 1A). The process of oxidation induced by room temperature air forming peroxides is referred to as autoxidation (Lawson, 2013). It is known that acrolein may be formed in autoxidized lipids and in frying oils (Hirayama et al., 1989; Olsen et al., 2005; Pan et al., 2005). The results of the present study suggest that sunlight exposure may induce the formation of acrolein from fatty acids by lipid peroxidation processes. A small amount of acrolein formed in EVOO stored in amber glass bottles exposed to sunlight, and reached levels of 165 ppb after sunlight exposure for 5 weeks. No acrolein was formed in EVOO stored in clear glass bottles covered with aluminum foil. These observations indicate that amber bottles can protect EVOO from the deterioration of sensory qualities induced by sunlight.
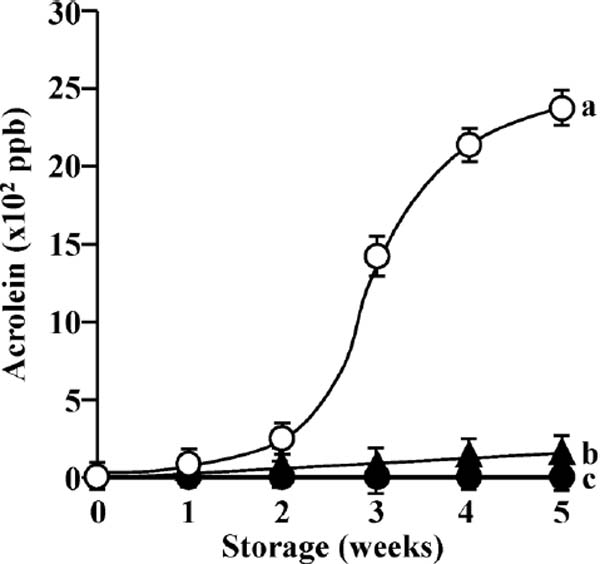
Changes in acrolein formation in EVOO during storage under sunlight condition (○: clear glass bottles; ▴: amber glass bottles; ●: clear glass bottles covered with aluminum foil). a–cMean values with different letters for storage for 5 weeks are significantly different (p < 0.05).
In this study, changes in the oxidative degradation of EVOO after exposure to sunlight were evaluated during storage in glass bottles with different levels of transparency. Storage in clear glass bottles covered with aluminum foil still provided complete protection of the EVOO quality during sunlight exposure. Storage in amber glass bottles did not provide sufficient protection against primary and secondary oxidation of EVOO upon exposure to sunlight, with only partial protection against the degradation of carotenoids and phenolic compounds, but no protection against the degradation of chlorophylls. In particular, amber glass bottles can prevent the deterioration of EVOO sensory qualities induced by sunlight. These results show that sunlight exposure can induce the oxidative degradation of EVOO and that limited transparency of packaging provides protection against this deterioration. These findings will help to select appropriate storage conditions and packaging to maintain the quality of EVOO.
Acknowledgments I thank Gabrielle David, Ph.D. and Philip Creed, Ph.D. from Edanz Group for editing a draft of this manuscript.