2019 年 25 巻 4 号 p. 563-568
2019 年 25 巻 4 号 p. 563-568
Vortex-assisted dispersive liquid–liquid microextraction (VA-DLLME) coupled with a high performance liquid chromatography–diode array detector (HPLC-DAD) was applied for the determination of phenolic acids (gallic, ferulic, and syringic) in vegetable oils. A deep eutectic solvent (DES) – a green extraction solvent – was synthesized by stirring choline chloride and urea at 80 °C until clarification. The experimental parameters affecting extraction efficiency, such as volume of extraction solvent, ionic strength, and extraction time were all optimized. Under optimal conditions, good linearities with the phenolic acids were achieved in the range 0.1 to 10 µg g−1. The limits of detection varied between 0.010 and 0.021 µg g−1, and the limits of quantification between 0.035 and 0.071 µg g−1. The proposed DES-VA-DLLME-HPLC-DAD method is simple, time-saving, eco-friendly, and can be successfully applied to the extraction of phenolic acids from soybean, peanut, and blending vegetable oils with recoveries ranging from 75.6% to 103.4%.
Vegetable oils can be classified into soybean, peanut, rapeseed, palm, sesame, olive, sunflower, and cottonseed oils (amongst others) according to the raw material used. Phenolic derivatives are essential components in vegetable oils because of their influence on the organoleptic characteristics of the oils and their beneficial properties (Christodouleas et al., 2012). Phenolic acids are naturally occurring phytonutrients that possess one or more aromatic rings and hydroxyl groups (Zhang et al., 2019). Their physiological activity comes from their phenylene or phenolic hydrocarbon structures. The quantity of the phenolic compounds in oleaginous plants and their processed foods have received widespread attention because of their antibacterial, anti-cancer, anti-virus, anti-inflammatory, antioxidant, hypolipidemic, and hypoglycemic effects (Alu'datt et al., 2017; Robbins, 2003).
Abbott et al. (Abbott et al., 2003) synthesized the first deep eutectic solvent (DES) in 2003. A DES comprises of at least two components (hydrogen bond acceptor and donor) which are able to form a new eutectic phase by relying on the hydrogen bonds. The melting point of the DES is lower than that of each of the individual components (Shishov et al., 2017). As an alternative to ionic liquids, DESs are easy to prepare, inexpensive, and greener than ionic liquids (Li and Row, 2016). The solvents also have other beneficial characteristics, e.g. high thermal stability, low volatility, low melting point, selective dissolution ability, and so on. Due to these advantages, DESs have good prospects for application in the field of food sample extraction and separation (Bajkacz and Adamek, 2018; Cvjetko Bubalo et al., 2016). Although deep eutectic solvents as a new class of green solvents have aroused widespread interest in many fields of science and technology, the application of DESs in analytical chemistry is still in its infancy (Shishov et al., 2017).
Dispersive liquid–liquid microextraction (DLLME) is a miniaturized liquid–liquid extraction technique involving an aqueous phase, disperser solvent, and extraction solvent (Rezaee et al., 2006). Fine droplets are produced by the rapid injection into the aqueous sample of small amounts of a mixture of extraction solvent (immiscible with water) and disperser solvent (miscible with water) (Mansour and Danielson, 2018). However, vortex-assisted (VA) extraction may be a low-cost and effective way of eliminating the use of a disperser solvent in traditional DLLME (Rezaee et al., 2010). High-speed rotation can cause an extraction solvent to decompose into fine droplets. Because of their short diffusion distances and large specific surface areas, these fine droplets of extraction solvent can efficiently extract target analytes in vortex-assisted microextraction without using organic disperser solvents (Mansour and Danielson, 2018).
The aim of this study is to develop a simple, rapid, and eco-friendly analytical method of determining the phenolic acid content of vegetable oils. A deep eutectic solvent, vortex-assisted dispersive liquid–liquid microextraction, and a high performance liquid chromatography (HPLC)–diode array detector (DAD) are all involved and combined to develop the new method of extraction and analysis. Important variables related to the extraction process, e.g. the volume of extraction solvent required, ionic strength, and extraction time are all optimized to improve the extraction efficiency and sensitivity of the method. The method is subsequently validated and its analytical performance is also evaluated.
Reagents and materials The gallic acid (98%), ferulic acid (98%), syringic acid (98%), choline chloride (99%), and urea (99%) were bought from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The sodium chloride (99%) was bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and HPLC-grade methanol from SK Chemicals Co., Ltd. (Ulsan, Korea). Ultra-high purity water was generated using a Milli-Q purification system. The soybean, peanut, and blending vegetable oils used were obtained from local supermarkets.
DES-VA-DLLME procedure
Preparation of the DES Choline chloride is an excellent hydrogen bond acceptor to use to synthesize a DES (Shishov et al., 2017). Furthermore, different proportions of urea (as hydrogen bond donor) can be mixed with the choline chloride to obtain DESs with different melting points. In this study, choline chloride and urea were mixed in a molar ratio of 1 : 2 in order to prepare an extraction solvent with a lower melting point. This mixture was then heated to 80 °C in a water bath until clarification to obtain the choline chloride–urea eutectic solvent required.
DES-VA-DLLME procedure A 1 g sample of vegetable oil was transferred to a 10-mL centrifuge tube and 10 mg of NaCl and 100 µL of DES added in succession. The mixture was then stirred for 2 min using a vortex agitator. The resulting cloudy solution containing fine droplets of DES was then centrifuged at 3 500 rpm for 2 min. Subsequently, the 50 µL of DES sediment at the bottom of the centrifuge tube could be readily collected using a microsyringe and diluted with 50 µL of methanol before HPLC-DAD analysis. The procedure of DES-VA-DLLME is illustrated in Fig. 1.
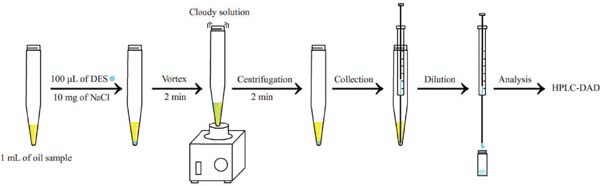
Schematic representation of the DES-VA-DLLME extraction procedures.
Chromatographic analysis A HPLC system (Agilent 1260) equipped with a DAD detector and Agilent Eclipse Plus C18 column (250 × 4.6 mm, 5 µm) were purchased from Agilent Technologies (Waldbronn, Germany). The column temperature was set to 20 °C. The mobile phase consisted of methanol and 0.1% phosphoric acid solution with a flow rate of 0.5 mL min−1. The elution gradient was 30%–70% methanol (0–10 min) and 70%–30% (10–20 min). The injection volume was 20 µL. The successful separation of the gallic, ferulic, and syringic acids was achieved with the retention times of 6.3, 13.8, and 11.7 min, respectively (detection wavelengths of 270, 325, and 270 nm, respectively).
Optimization of the extraction conditions In order to optimize the extraction conditions, the effect on extraction efficiency of using different DES volumes, ionic strengths, and extraction times were investigated. All experiments were carried out in triplicate and the results averaged.
Effect of extraction solvent volume In liquid–liquid microextraction procedures, the volume of extraction solvent used is a critical variable that has a significant effect on extraction performance (Karadaş and Kara, 2017). In our experiments, the volume of extraction solvent was varied over the range from 50 to 200 µL (Fig. 2).

Effect of DES volume on extraction recovery of the phenolic acids.
As Fig. 2 shows, the extraction efficiency increased as the volume of the extraction solvent was increased from 50 to 100 µL. However, the extraction efficiency decreased when the volume of the extraction solvent exceeded 150 µL. The three phenolic acids had good recovery levels when the volume of extraction solvent was 100 µL, and the standard deviations among the repetitions were also favorable. Hence, 100 µL was selected as the optimal volume.
Effect of ionic strength Ionic strength can affect the viscosity and mobility, as well as phase separation, and hence the extraction efficiency (Viñas et al., 2014). The effect of ionic strength was evaluated by adding different amounts of NaCl (up to 25 mg). As shown in Fig. 3, recovery increased as the amount of NaCl added increased from 0 to 5 mg, and remained high over the range 5–15 mg. Further increasing the ionic strength caused the recovery rates to decrease. According to the literature (Liu et al., 2017), salting-out effect may increase the extraction efficiency in the study, while salting-in effect reduced the extraction efficiency. Therefore, 10 mg of NaCl was selected for the subsequent experiments.

Effect of ionic strength on extraction recovery of the phenolic acids.
Effect of extraction time Extraction time is another important variable to optimize if an effective transfer of target analyte from the sample to the extraction solvent is to be achieved. Here, the vortex time used in the VA extraction process is considered to be the extraction time (Fernández et al., 2016). This variable was investigated over the range from 1 to 2.5 min (Fig. 4).

Effect of vortex time on extraction recovery of the phenolic acids.
The vortex promotes the distribution and equilibration of the target compounds between the DES and oil solution. The generation of fine DES droplets greatly increased the interfacial area available for mass transfer. As shown in Fig. 4, the extraction efficiency gradually increased with extraction time over the range 1–2 min. However, there were no improvements in the extraction efficiencies when the extraction time was prolonged further, probably because equilibrium had already been reached (Farhadi et al., 2009). Therefore, a 2 min vortex time was selected for subsequent experiments carried out in this study.
Method validation The performance characteristics of the DES-VA-DLLME-HPLC-DAD method, including its linearity, limit of detection (LOD), limit of quantitation (LOQ), and recovery rates were subsequently evaluated. Experiments were carried out using fortified oils in which the concentration of the phenolic acids ranged from 0.1 to 10 µg g−1 and the results used to generate calibration curves. The LOD and LOQ values were calculated as the amounts of phenolic acid required to obtain signal-to-noise ratios of 3 and 10, respectively. Recovery rates were evaluated by spiking the phenolic acids into vegetable oils at 0.1, 1, and 10 µg g−1 levels, and calculated by comparing the obtained amounts with those added. As shown in Table 1, high coefficients of determination (R2 > 0.99) were obtained for each calibration curve. The LOD values of the phenolic acids ranged from 0.010 to 0.021 µg g−1, while the LOQ values ranged from 0.035 to 0.071 µg g−1. The results in Table 2 shows that the mean extraction recoveries of the phenolic acids were in the range 75.6% to 103.4%. The number of phenolic hydroxyl groups and methoxy groups of gallic, ferulic, and syringic acids may affect the interaction between phenolic acids and DES, thus affecting the extraction recoveries. In addition, the extraction recoveries may be affected by instrument fluctuations, operational errors and matrix effects. The relative standard deviations (RSDs) ranging from 1.9% to 13.1% were evaluated by repeated extractions (n = 3). These results demonstrate that the method is suitable for the determination of phenolic acids in vegetable oils.
| Oil | Phenolic acid | Calibration curve (µg g−1) | R2 | LOD (µg g−1) | LOQ (µg g−1) |
|---|---|---|---|---|---|
| Soybean | Gallic | y = 274.232x + 2.867 | 0.999 | 0.01 | 0.035 |
| Ferulic | y = 428.958x − 1.238 | 0.999 | 0.014 | 0.048 | |
| Syringic | y = 341.836x + 4.201 | 0.997 | 0.015 | 0.051 | |
| Peanut | Gallic | y = 249.568x + 1.832 | 0.999 | 0.016 | 0.055 |
| Ferulic | y = 436.884x + 2.889 | 0.997 | 0.016 | 0.053 | |
| Syringic | y = 372.843x + 4.167 | 0.996 | 0.021 | 0.071 | |
| Blending | Gallic | y = 263.422x + 3.569 | 0.999 | 0.016 | 0.046 |
| Ferulic | y = 409.613x + 2.421 | 0.999 | 0.019 | 0.062 | |
| Syringic | y = 371.258x − 4.611 | 0.998 | 0.02 | 0.068 |
| Oil | Spiked level (µg g−1) |
Gallic acid | Ferulic acid | Syringic acid | |||
|---|---|---|---|---|---|---|---|
| Recovery (%) |
RSD (%) |
Recovery (%) |
RSD (%) |
Recovery (%) |
RSD (%) |
||
| Soybean | 10 | 76.9 | 7.7 | 77.0 | 5.1 | 82.1 | 10.5 |
| 1 | 80.0 | 3.4 | 80.4 | 12.4 | 100.2 | 8.6 | |
| 0.1 | 97.4 | 7.9 | 82.0 | 2.1 | 76.3 | 5.0 | |
| Peanut | 10 | 78.0 | 1.9 | 77.4 | 3.0 | 90.0 | 11.3 |
| 1 | 80.4 | 9.9 | 86.8 | 3.9 | 102.1 | 7.7 | |
| 0.1 | 89.9 | 9.3 | 91.1 | 13.1 | 103.4 | 9.2 | |
| Blending | 10 | 78.1 | 8.6 | 75.6 | 7.1 | 95.2 | 10.6 |
| 1 | 79.2 | 1.9 | 79.4 | 12.4 | 87.9 | 11.1 | |
| 0.1 | 86.2 | 3.0 | 94.1 | 12.6 | 85.5 | 6.7 | |
Analysis of real samples The new method was used to determine the phenolic acid contents of soybean, peanut, and blending oils. The phenolic acids of interest were found in the three vegetable oils. Table 3 shows the mean concentrations of gallic, ferulic, and syringic acids in the vegetable oils.
| Sample | Gallic acid (µg g−1) | Ferulic acid (µg g−1) | Syringic acid (µg g−1) |
|---|---|---|---|
| Soybean oil | 0.401 ± 0.022 | 0.461 ± 0.012 | 0.225 ± 0.029 |
| Peanut oil | 0.312 ± 0.039 | 0.161 ± 0.015 | 0.372 ± 0.024 |
| Blending oil | 0.218 ± 0.027 | 0.332 ± 0.033 | 0.274 ± 0.030 |
The concentrations of the gallic, ferulic, and syringic acids were in the ranges 0.218–0.401, 0.161–0.461, and 0.225– 0.372 µg g−1, respectively. The gallic and ferulic acid concentrations in soybean oil were higher than those in peanut oil, while the syringic acid concentration was lower. According to the literature (Ferrone et al., 2018), soybean and peanut oils both contain ferulic acid and it is more abundant in soybean oil compared to peanut oil. This agrees with the results of this study. However, it should be noted that the phenolic acid content of such oils can be affected by a variety of factors, e.g. the degree of ripening, irrigation, climate, extraction and storage processes, etc. (Zeineb et al., 2008).
Comparison with other methods The proposed DES-VA-DLLME method has several advantages over other extraction techniques with respect to the determination of phenolic acids in vegetable oils. Modern extraction techniques focus on finding solutions that minimize the amount of solvent used (Farid et al., 2012). In Table 4, we make a comparison of our method with other reported methods (Bakar et al., 2012; Delgado-Zamarreño et al., 2007; Reboredo-Rodríguez et al., 2014; Sin et al., 2006).
| Method | Volume of n-hexane diluent (mL) | Extraction solvent | Linearity range (µg g−1) |
LODs (µg g−1) |
Ref. |
|---|---|---|---|---|---|
| LLE-HPLC-PDAa | 25 | 3×50 mL, acetonitrile | 10–500 | 2–4 | (Sin et al. 2006) |
| LLE-MEKCb | 75 | 3×50 mL, methanol | 10–1000 | 0.27–1.47 | (Delgado-Zamarreno et al. 2007) |
| LLME-CEc | 2 | 2×300 µL, methanol | 0.1–30 | 0.02–0.16 | (Bakar et al. 2012) |
| USAEME-HPLC-DADd | 6 | 2×250 µL, methanol/water mix | 0.07–3.33 | 0.01–0.47 | (Reboredo-Rodriguez et al. 2014) |
| DES-VA-DLLME-HPLC-DAD | 0 | 1×100 µL, DES | 0.1–10 | 0.01–0.02 | This work |
As can be seen from the table, our method uses micro-amounts of a green extraction solvent (DES) as extraction solvent. Thus, its adoption would greatly reduce the volume of traditional toxic extraction solvents used and it also avoids the use of organic diluent solvents. In addition, the proposed DES-VA-DLLME method uses a simple physical way of dispersing the extraction solvent and does not use a dispersive solvent. The proposed method also quickly reaches extraction equilibrium (within 2 min). The linearity range and LOD values of the new method are comparable to those of the previously reported methods. In conclusion, the DES-VA-DLLME-HPLC-DAD method is simple, time-saving, eco-friendly and can be used to quantify the phenolic acid content of vegetable oils.
A vortex-assisted dispersive liquid–liquid microextraction method coupled with a high performance liquid chromatography– diode array detector method was developed for the determination of phenolic acids (gallic, ferulic, and syringic) in vegetable oils. In this study, microliters of a choline chloride–urea eutectic solvent were used as the extraction solvent, replacing the toxic organic extraction solvents traditionally used. The DES-VA-DLLME-HPLC-DAD approach is simple, time-saving, eco-friendly and shows good linearity and limits of detection and quantification with different oils. Due to its excellent characteristics, the new method has many potential applications in various fields such as food analysis, environmental analysis, and others.
There are no conflicts of interest to declare.
Acknowledgements This study was supported by the <National Key Research and Development Program of China> under Grant <number 2017YFD0400206>; <Key project of Shanxi Key R&D Program of China> under Grant <number 201703D211001-06>; <Shanxi Agricultural University Science and Technology Innovation Fund> under Grant <numbers 2017YJ35, zdpy201709>.