2019 年 25 巻 4 号 p. 597-605
2019 年 25 巻 4 号 p. 597-605
The used of insects as protein sources is growing rapidly for a variety of reasons, including global food security, sustainability, nutritional value and animal welfare. The nutritional composition and functional properties of extracted proteins from two cricket species (A. domesticus and G. bimaculatus) were investigated. Both cricket species contained high amounts of proteins (60–70% dry weight) with all essential amino acids and 10–23% lipids. The amount of omega-3 and omega-6 fatty acids and several minerals, such as P, Na and Ca were also high. Proteins were isolated from both cricket species by solubilisation at pH 11.0–12.0, followed by pH 4.0 isoelectric precipitation. The cricket proteins have high water capacity, moderate foaming capacity and stability with high emulsion activity. Therefore, they certainly could be further utilized as ingredients in various food types that could be an alternative nutritional source for both direct consumption and processing for protein extracts.
Almost 2 000 species of insects are known to be edible and have always been a part of the human diet. Ants, bees, beetles, caterpillars, cicadas, crickets, dragonflies, flies, grasshoppers, leafhoppers, locusts, planthoppers, termites and wasps, are the most commonly eaten insects group (Jongema, 2017). Approximately 113 countries in Asia, Africa, Australia and the Americas, consider edible insects to be a well-appreciated food (van Huis et al., 2013). Insect-consuming behaviour differs in various regions depending on the cultural practices and the availability of the insect. Recently, eating insects has become not only an alternative source of proteins that offers a cheap and sufficient nutrient intake in the human diet in developing countries but also very popular in developed countries that concerns about healthy food (Hanboonsong et al., 2013). Edible insects are the new trend on the food market worldwide because they are not only a healthy food source and that is highly nutritious that is rich in protein, healthy fats, vitamins, fibre and minerals with low carbohydrates but also are more environmentally friendly to raise than other livestock protein sources (Banjo et al., 2006; Zielińska et al., 2015; Payne et al., 2016).
In Thailand, eating insects is not new. Thai people have had a long history and tradition of using over 200 different insect species as food. Crickets, bamboo caterpillars, silkworm pupae, grasshoppers and giant water bugs are commonly eaten and sold regularly in Thailand (Yhoung-Aree, 2010; van Huis et al., 2013). Before now, most edible insects are collected from nature depending on specific species' behaviour and life cycles. An increasing demand for edible insects has resulted in insect farming worldwide. Crickets are the most common species farmed. Recently over 20 000 cricket farms have been established in Thailand. However, only two species of edible crickets, field crickets (Gryllus bimaculatus (Orthoptera: Gryllidae)) and house crickets (Acheta domesticus (Orthoptera: Gryllidae)), are farmed economically (Halloran et al., 2016). The challenges in addition to developing rearing technologies are the need to maintain the quality of the crickets and consequently cricket flour for nutritional value, hygiene and sanitation. This present work has been performed to determine the amino acid profile, fatty acid profile, nutritional composition, and mineral content of A. domesticus and G. bimaculatus. In addition, protein solubility and functional properties were also investigated. This information would help to perceive the plausible of using insects as health food and to record information of the nutritional contents of these two commercial insect species.
Sample preparation Frozen adult house crickets (A. domesticus) and field crickets (G. bimaculatus) were purchased from a local farm in Kanchanaburi Province, Thailand. Frozen crickets were thawed and then air-dried at 60 °C for 48 h. Dried samples were ground to powder and stored at 4 °C in metallic vacuum-sealed bags prior to analysis.
Proximate analysis The nutritional composition was determined following standard methods recommended by the Association of Official Analytical Chemists (AOAC, 1990). Briefly, samples were placed in a crucible and kept in an oven at 105 °C for 6 h. Then, the samples were cooled in a desiccator and weighed to determine moisture content. Fat content was determined after petroleum ether extraction in a Soxhlet apparatus for 1 h. Ash was determined Determination of ask was done by kindle the sample in a muffle furnace at 550 °C overnight. The Kjeldahl method applying a nitrogen conversion factor of 6.25 was used to determine the protein content. Fibre content was determined through the use of the Fibretherm system. Subtraction of the sum of the moisture, total fat, crude protein, fibre and ash from the total weight of the sample was used to calculate the carbohydrate content.
Mineral analysis The ground crickets were digested with HNO3 and HCl in a microwave digestion system as described above. Inductively coupled plasma-optical emission spectroscopy (ICP-OES, Optima 8000, Perkin Elmer, USA) was used to determine the amounts of calcium, sodium, potassium, phosphorus, magnesium, iron, copper, manganese and zinc in the samples. Standard calibration solutions with concentrations between 0.5 and 10 ppm were prepared by consecutive dilutions of the stock solution of the elements investigated (Chem Lab, Zedelgem, Belgium).
Amino acid analysis The amino acid profile was performed using a Biochrom30+ amino acid analyser (Pharmacia-Biotech, Buckinghamshire, UK) followed the slightly modified method of Udomsil et al. (2011). Briefly, the ground samples were hydrolysed in a microwave oven (Anton Paar GmbH, Graz, Austria) with 12 M HCl containing 1% phenol. Subsequently, precipitates after acid evaporation, were dissolved and filtered through a 0.22-mm membrane filter. Determination of cysteine and methionine contents was done by hydrolysis after oxidation with performic acid. Tryptophan analysis was performed after alkaline hydroxylation of the samples with 4.2 N NaOH. The samples were then hydrolysed as described and analysed for amino acid composition. Ninhydrin was used for post-column derivatisation with photometric detection at 570 nm (440 nm for Proline). The amino acids were identified and quantified by comparison with standards (A6282, A6407 Sigma-Aldrich, USA).
Fatty acid analysis Fatty acid composition was analysed by gas chromatography, GC-7980A (Agilent Technology, USA) following the method of Bonturi et al. (2015). Total lipids of the ground samples were extracted with chloroform:methanol (2:1 v/ v). Subsequently, fatty acid methyl esters (FAMEs) of the total lipid extract were prepared by transesterification in methanolic KOH solution (5% w/v) with the addition of a 10% BF3-methanol solution (Sigma-Aldrich). Gas chromatography was performed using a Supelco SPTM-2560 fused silica capillary column (100 m × 250 µm × 0.2 µm) (Sigma-Aldrich). The oven temperature was programmed to start at 70 °C for 4 minutes, increase to 175 °C for 27 min, increase to 215 °C for 17 min and finally increase to 240 °C for 10 min. Identification and quantification of FAMEs were accomplished by comparing their retention times to those of FAME standards (Sigma-Aldrich).
Protein extraction Aqueous protein extraction was carried out following the method of Föste et al. (2015). Briefly, a pH-dependent extraction of the cricket proteins was done by adjusting the pH of the extracts from 5 to 12 using 1 N HCl or 1 N NaOH. The suspensions were stirred for 1 h at 30 °C, 40 °C, or 50 °C. Separation of soluble proteins from the insoluble residue was performed by centrifugation at 10 000×g for 20 min at 4 °C. Then, the protein concentration of the supernatants was determined using the Lowry method (Lowry et al., 1951). Afterwards, the protein isolated at the optimal pH value and temperature was precipitated by adjusting the pH from 3 to 5.5 with 1 N HCl. Then, the proteins were centrifuged at 10 000 ×g for 20 min at 4 °C. The obtained white precipitates were dried in a hot air oven at 60 °C for 1 h and stored at 4 °C until further analyses.
Protein solubility Solubility of the cricket proteins was tested using a slightly modified method of Hall et al. (2017). Briefly, the extracted proteins were dissipated in buffers of pH 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0 and 12.0. Each mixture was stirred at room temperature for 30 min and centrifuged at 10 000 ×g for 15 min at 4 °C. The protein contents of the supernatant and total protein in each sample were determined using Lowry method. Protein solubility was calculated as a percentage.
Water holding capacity (WHC) WHC was determined according to Mishra and Rai (2006) with some modifications. The proteins were weighed in pre-weighed plastic centrifuge tubes and distilled water was added. Samples were placed at room temperature for 30 min. The mixture was then centrifuged at 10 000×g for 30 min. Then, the supernatant was carefully decanted, the new mass of the samples was recorded and WHC was calculated.
Foam capacity and stability Foam capacity was determined by the method of Hall et al. (2017). The proteins were dispersed in distilled water and equilibrated by a magnetic stirrer for 10 min at room temperature. A blender was used to aerate the protein mixture. Foam capacity after aeration was expressed as percentage volumetric increase. Volumes before and after aeration were recorded. Foam stability was calculated as the percentage of foam remaining after 30, 60, and 90 min.
Emulsifying properties The proteins in phosphate buffer pH 7.0 were mixed with 100% pure canola oil and homogenized at 18 000 ×g for 1 min. Aliquots were diluted into 0.3% SDS. To ensure homogeneity, the tubes were inverted six times, and the turbidity was measured at 500 nm using a Beckmann UV-Visible spectrophotometer (Irvine, USA). Emulsifying activity index (EAI) was expressed and emulsion stability index (ESI) was determined by measuring the light absorbance at a wavelength of 500 nm of aliquots from the emulsion at 30, 60, and 90 min after emulsion formation (Hall et al., 2017).
Gel formation Visual observation of gelation was performed according to the method of Yi et al. (2013). Cricket protein supernatant solutions were heated to 85 °C for 10, 20 and 30 min in a water bath. Visual observation was used to determine the gel formation. A gel was considered if the liquid did not move upon turning the tube.
Statistical analysis Each cricket sample was performed independently in triplicate (n = 3). Each chemical analysis was also analysed in triplicate from the three independent cricket samples. Data in triplicate are given as the mean ± standard deviation (SD). Data on proximate nutrient composition and mineral content, amino acid content, fatty acid composition and functional properties were analysed by using Student's t-test. Data on protein extraction and protein precipitation were analysed by ANOVA, followed by Tukey's multiple comparisons. The p-values < 0.05 were considered to indicate statistical significance.
Nutritional Composition and Mineral Content The protein content of A. domesticus was higher than that of G. bimaculatus (Table 1), while G. bimaculatus contained higher levels of lipid and fibre than A. domesticus (p < 0.001). The results indicated that protein was the major component of the nutrient composition of the crickets. It has been shown that the protein contents of edible insects are in the range of 10–85% of dry matter. The variation is due to the orders and species of the insects (van Huis et al., 2013; Rumpold and Schluter, 2013; Ghosh et al., 2017). The requirement to be called high protein food according to WHO/FAO, is a 10 g/100 g edible part (Food and Agriculture Organization, 2007). Therefore, these crickets can be an attractive alternative protein source. The results also showed that these crickets contained low fat contents compared with other edible insects (Rumpold and Schluter, 2013; Ghosh et al., 2017). High fat content considered to hinder the process ability and the food products shelf life, therefor, the low fat content makes these crickets interesting for food industries. Recently, there have been many reports on the proximate compositions of edible insects. The variation originates not only from differences between species but also from the developmental stages and different feed. The measuring methods can also be the cause of the variation. However, the overall nutrient composition of these two species of crickets (Table 1) surpassed that of conventional livestock with the same weight.
| A. domesticus | G. bimaculatus | |
|---|---|---|
| Components | % dry matter | |
| Moisture | 6.3 ± 0.04 | 3.0 ± 0.03*** |
| Protein | 71.7 ± 0.5 | 60.7 ± 0.4*** |
| Lipid | 10.4 ± 0.1 | 23.4 ± 0.1*** |
| Ash | 5.4 ± 0.3 | 2.8 ± 0.06*** |
| Fibre | 4.6 ± 0.2 | 10.0 ± 0.3*** |
| Carbohydrate | 1.6 ± 0.1 | 0.1 ± 0.01*** |
| Mineral content | mg/100g dry matter | |
| Calcium (Ca) | 149.75 ± 7.16 | 105.14 ± 9.31** |
| Sodium (Na) | 101.44 ± 7.80 | 88.84 ± 20.43 |
| Potassium (K) | 389.92 ± 1.38 | 321.71 ± 6.21** |
| Phosphorus (P) | 899.33 ± 36.19 | 702.02 ± 6.35** |
| Magnesium (Mg) | 136.58 ± 4.92 | 72.94 ± 2.64*** |
| Iron (Fe) | 8.83 ± 3.88 | 7.16 ± 1.28 |
| Copper (Cu) | 4.86 ± 0.35 | 3.86 ± 0.18* |
| Manganese (Mn) | 4.40 ± 0.08 | 3.40 ± 0.13*** |
| Zinc (Zn) | 19.61 ± 0.83 | 14.39 ± 2.29* |
Values are expressed as means ± standard deviations of triplicate analyses.
Data means were compared with A. domesticus using Student's t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Some main minerals (Na, Mg, P, K, and Ca) and trace minerals (Mn, Fe, Cu, Zn) contents in both cricket species were different (Table 1). Most of mineral contents of A. domesticus were higher than that of G. bimaculatus (p < 0.05). The result was comparable to those in previous studies (van Huis et al, 2013; Rumpold and Schluter, 2013). The results showed that phosphorus was the highest, followed in order by potassium, calcium and sodium. Phosphorus is essential for ATP and nucleic acid synthesis (RNA and DNA) and protein production, which is particularly phosphorus rich. Calcium, phosphorus, and magnesium are important constituents of bone. The calcium concentration of the crickets in this study was higher than that in milk (90–130 mg/100 g), suggesting that crickets could be used as an alternative source of calcium. The data obtained from this study were consistent with data from other edible insect species that they contained more iron, zinc and calcium, which are essential for human health, than conventional food such as beef, pork, and chicken (Yang et al., 2014; Ghosh et al., 2017). The mineral composition showed that crickets have the potential to provide good sources of dietary minerals.
Amino Acid Composition The quality of protein from these edible insects as related to the human diet has to be assessed through the amino acid content. All essential amino acids (EAAs) were present in both A. domesticus (42.7%) and G. bimaculatus (40.4%) as shown in Table 2. This result indicated that the EAA contents of both crickets were comparable to those of egg, chicken, pork and beef (dry matter), which are considered the main protein sources of the human diet. The high lysine and threonine contents in both A. domesticus and G. bimaculatus could help supplement cereal-based diets, which are generally low in lysine and threonine (Mokrane et al., 2010). Glutamic acid and glutamine were the most abundant amino acids in both crickets. The results of this study are similar to those of previous studies that showed that glutamic acid is dominant in several insect species (Rumpold and Schluter, 2013; Ghosh et al., 2017). A strong umami flavour in edible insects might be from the rich glutamic acid. Arginine was also found in high amounts in both crickets. In humans, arginine is considered to be an essential amino acid since the synthesis is generally not enough for growth and development in children. The cricket amino acid profiles were found to have low amounts of methionine, tryptophan and cysteine. In most edible insects lysine and tryptophan have been reported to be low. However, the low or limiting amino acids vary according to insect species and their diets (Ghosh et al., 2017). Our results demonstrated that both A. domesticus and G. bimaculatus could be used as dietary amino acid supplements that provide gratifying amounts of the essential amino acids for human health.
| Amino acid | Studied species | |
|---|---|---|
| A. domesticus | G. bimaculatus | |
| Valine | 4.50 ± 0.03 | 3.50 ± 0.03*** |
| Isoleucine | 2.90 ± 0.10 | 2.35 ± 0.07 |
| Leucine | 3.80 ± 0.14 | 3.88 ± 0.08 |
| Lysine | 3.22 ± 0.08 | 2.89 ± 0.07** |
| Threonine | 1.65 ± 0.05 | 1.67 ± 0.09 |
| Phenylalanine | 2.38 ± 0.00 | 2.24 ± 0.05 |
| Methionine | 0.98 ± 0.03 | 0.86 ± 0.04 |
| Histidine | 1.72 ± 0.02 | 1.57 ± 0.03 |
| Tryptophan | 0.43 ± 0.03 | 0.27 ± 0.02*** |
| Arginine | 3.92 ± 0.05 | 3.47 ± 0.05* |
| Asparagine + Aspartic acid | 4.61 ± 0.23 | 2.87 ± 0.16*** |
| Glutamine + Glutamic acid | 6.45 ± 0.05 | 6.77 ± 0.07 |
| Serine | 1.59 ± 0.09 | 1.32 ± 0.13 |
| Glycine | 2.60 ± 0.15 | 3.31 ± 0.26** |
| Alanine | 3.67 ± 0.05 | 4.69 ± 0.10*** |
| Cystine | 0.40 ± 0.00 | 0.38 ± 0.00 |
| Proline | 3.04 ± 0.03 | 2.81 ± 0.06** |
| Tyrosine | 2.71 ± 0.10 | 2.77 ± 0.05 |
| EAA | 21.58 ± 0.28 | 19.23 ± 0.04** |
| NEAA | 28.97 ± 0.48 | 28.40 ± 0.22 |
| Total | 50.55 ± 0.20 | 47.63 ±0.27*** |
Values are expressed as means ± standard deviations of triplicate analyses.
Data means were compared with A. domesticus using Student's t-test. * p < 0.05, ** p < 0.01, *** p < 0.001. EAA = essential amino acids; NEAA = non-essential amino acids
Fatty Acid Composition The fat content of A. domesticus and G. bimaculatus was similar to that reported for orthopteran species (Table 3). Comparable amount was found for all fatty acids from both species, indicated that the fatty acid profile is influenced by feed and environmental factors (Ghosh et al., 2017). Saturated fatty acids (SFAs) are the predominant fatty acids found in both cricket species, followed in order by monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). SFAs are one of the dietary factors that have a great negative effect on LDL cholesterol. In addition, MUFAs and PUFAs have been shown to decrease plasma cholesterol concentrations. The major SFAs found in A. domesticus and G. bimaculatus were palmitic (C16:0) and stearic acid (C18:0), respectively, while the dominant unsaturated fatty acid was oleic (C18:1). Essential alpha-linolenic acid (an omega-3 fatty acid) and linoleic acid (an omega-6 fatty acid) were also present in both cricket species. These essential fatty acids are important for children and infants for their development (van Huis et al, 2013). The results revealed that cricket lipids are rich in healthy lipids, including omega-3 and omega-6 fatty acids. The fatty acid content in the lipids of edible insects has been reported to be better to that in the lipids of conventional food (e.g., chicken, pork and beef) (Ghosh et al., 2017). Cricket lipids can provide nutrition by supplying essential fatty acids and energy.
| Fatty acid | Studied species | |
|---|---|---|
| A. domesticus | G. bimaculatus | |
| C10:0 Capric acid | 0.011 ± 0.00 | 0.008 ± 0.00 |
| C11:0 Undecylic acid | 0.003 ± 0.00 | 0.004 ± 0.00 |
| C12:0 Lauric acid | 0.028 ± 0.00 | 0.045 ± 0.00*** |
| C14:0 Myristic acid | 0.107 ± 0.00 | 0.271 ± 0.02 |
| C15:0 Pentadecanoic acid | 0.009 ± 0.00 | 0.036 ± 0.00 |
| C16:0 Palmitic acid | 5.870 ± 0.31 | 9.260 ± 0.77** |
| C17:0 Heptadecanoic acid | 0.078 ± 0.00 | 0.101 ± 0.01** |
| C18:0 Stearic acid | 1.830 ± 0.09 | 2.730 ± 0.25** |
| C20:0 Arachidic acid | 0.125 ± 0.01 | 0.212 ± 0.01*** |
| C21:0 Heneicosanoic acid | 0.005 ± 0.00 | 0.008 ± 0.00 |
| C22:0 Behenic acid | 0.064 ± 0.01 | 0.049 ± 0.01 |
| C24:0 Lignoceric acid | 0.024 ± 0.01 | 0.037 ± 0.02 |
| C14:1 Myristoleic acid | 0.019 ± 0.02 | 0.007 ± 0.00 |
| C16:1 Palmitoleic acid | 0.153 ± 0.01 | 0.295 ± 0.07 |
| C18:1n-9 trans Elaidic acid | 0.031 ± 0.00 | 0.044 ± 0.01 |
| C18:1n-9 cis Oleic acid | 3.900 ± 0.24 | 9.400 ± 2.20* |
| C20:1 cis-11-Eicosenoic acid | 0.024 ± 0.01 | 0.084 ± 0.05 |
| C22:1n-9 Erucic acid | 0.014 ± 0.01 | 0.020 ± 0.01 |
| C18:2n-6 trans Linolelaidic acid | 0 | 0.011 ± 0.01 |
| C18:2n-6 cis Linoleic acid | 1.170 ± 0.36 | 1.390 ± 0.73 |
| C20:2 cis-11,14-Eicosadienoic acid | 0.194 ± 0.04 | 0.173 ± 0.07 |
| C22:6n-3 Docosahexaenoic acid | 0 | 0 |
| C18:3n-6 cis Linolenic acid | 0.007 ± 0.00 | 0.062 ± 0.01* |
| C18:3n-3 Linolenic acid | 0.011 ± 0.00 | 0.014 ± 0.01 |
| C20:3n-6 cis-8,11,14-Eicosatrienoic acid | 0.011 ± 0.00 | 0.077 ± 0.08 |
| C20:3n-3 cis-11,14,17-Eicosatrienoic acid | 0.006 ± 0.00 | 0 |
| C20:4n-6 Arachidonic acid | 0 | 0.005 ± 0.00 |
| C20:5n-3 cis-5,8,11,14,17-Eicosapentaenoic acid | 0.057 ± 0.01 | 0.070 ± 0.05 |
| SFA | 8.145 ± 0.35 | 12.761 ± 1.07** |
| MUFA | 4.141 ± 0.25 | 9.850 ± 2.35* |
| PUFA | 1.456 ± 0.33 | 1.802 ± 0.53 |
| Total | 13.742 ± 0.76 | 24.413 ± 2.77** |
| OMEGA 3 | 0.074 ± 0.01 | 0.084 ± 0.04 |
| OMEGA 6 | 1.125 ± 0.35 | 1.545 ± 0.88 |
Values are expressed as means ± standard deviations of triplicate analyses.
Data means were compared with A. domesticus using Student's t-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Protein Extraction and Functional Properties of Cricket Proteins To produce insect protein-rich ingredients the understanding of the quality and characteristics of the crickets in food processing are needed. A similar trend in the protein content extracted from A. domesticus and G. bimaculatus was shown in Fig 1. The protein content of the extracted samples and the extraction yield were affected by pH. The highest extraction yield was observed at pH 12.0 (Fig 1). At various pH, increasing the temperature during protein extraction from 30 to 50 °C did not increase the protein yield significantly. However, at pH 12 extracting the protein at 40 °C gave better yield than at 50 °C. After acid precipitation, the cricket proteins showed their isoelectric point (pI) in the region around pH 4 (Fig 2). The solubility of the extracted proteins from both cricket species was found to be highly dependent on the pH and was highest at pH 12 (data not shown). Data on the solubility were helpful to determine the optimal conditions of protein extraction and fractionation. Using alkaline media, higher extraction yield and protein solubility have been produced from various insect species (Foo et al., 2006; Yi et al., 2013; Buβler et al., 2016). Insect proteins have also been reported to have a pI in the region between pH 4.0 and 6.5. Optimization of extraction parameters such as pH, temperature, run time, and water-to-sample ratio are species specific and required to maximize the yield of soluble insect proteins (Zhao et al., 2016; Józeiak et al., 2016). Knowledge of the protein solubility and the functional properties of cricket protein extracts suggests that crickets could be an important food source and useful for food industrial applications. Moreover, data on the functional properties of the extracted protein from these insects are scarce. A high water holding capacity was observed in both cricket species (Table 4). This showed that cricket proteins could easily be incorporated into aqueous food formulations and it could also contribute to the improvement of selected properties of bakery products. The data from this study suggested that cricket protein preparations exhibited excellent foaming and emulsion properties, while gel formation was not detected (Table 4). In the food industry, foams are used to improve the texture, consistency, and appearance of foods. This relatively high level of emulsion suggests that cricket proteins would be highly desirable for the preparation of comminuted meats and could also play a significant role in many food systems, such as doughs, salad dressings, infant foods, ice cream and coffee whiteners. However, the different functional properties are related to many factors, such as pH, temperature, ionic strength, protein concentration, amino acid content, and protein charge, conformation, and hydrophobicity (Yi et al., 2013; Zhao et al., 2016).
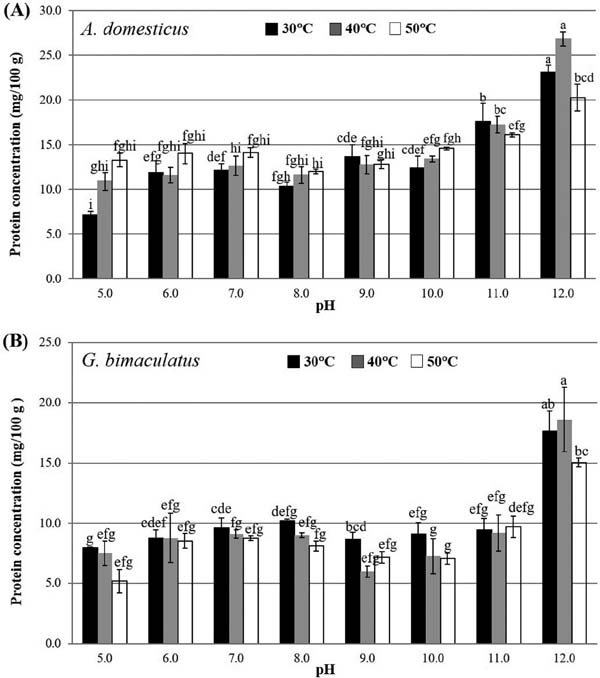
Proteins extracted from A. domesticus (A) and G. Bimaculatus (B) in aqueous pH (5 to 12) and temperature (30, 40 and 50 °C) conditions. Values are expressed as the means ± standard deviations of triplicate analyses. Two-way ANOVA analysis revealed significant main effect of pH and temperature condition (p < 0.001) and significant interaction between the treatment × temperature (p < 0.01). Different superscript letters indicate statistically significant differences (Tukey's test, p < 0.05).

Crude protein extracted from the cricket precipitate, A. domesticus (A) and G. bimaculatus (B). Values are expressed as the means ± standard deviations of triplicate analyses. Different superscript letters indicate statistically significant differences (Tukey's test, p < 0.05).
| Parameters (%) | Studied species | |
|---|---|---|
| A. domesticus | G. bimaculatus | |
| Water holding capacity | 201.99 ± 10.01 | 201.22 ± 5.65 |
| Foam capacity | 26.00 ± 2.00 | 16.67 ± 1.15** |
| Foam stability at 30 min | 88.90 ± 1.41 | 94.86 ± 1.73* |
| at 60 min | 88.38 ± 2.24 | 93.14 ± 0.07* |
| at 90 min | 86.79 ± 2.21 | 92.58 ± 0.91* |
| Emulsion capacity | 7.52 ± 0.24 | 7.19 ± 0.38 |
| Emulsion stability at 30 min | 98.68 ± 0.36 | 98.26 ± 1.41 |
| at 60 min | 97.83 ± 0.93 | 97.69 ± 1.44 |
| at 90 min | 97.36 ± 1.13 | 97.38 ± 0.44 |
| Gel formation | ND | ND |
Values are expressed as means ± standard deviations of triplicate analyses. ND = Not Detected
Data means were compared with A. domesticus using Student's t-test. *p < 0.05, **p < 0.01.
In conclusion, to feed the rapidly increasing population, the use of agricultural land, water and climate change will be major problems in the near future. Edible insects that contain high-quality nutrition are one of the answers. To produce the same amount of protein, crickets need less feed than cattle, pigs and chickens. In this study, we demonstrated that the nutritional composition and functional properties of the isolated proteins were similar in the house cricket and field cricket. They were rich in good-quality protein, with all the essential amino acids. They also contained high-quality fatty acids, such as long-chain omega-3 and omega-6 fatty acids, and were rich in several micronutrients, such as phosphorous, potassium, calcium, iron, zinc, magnesium and manganese. Moreover, information concerning the amino acid profile, solubility, and foaming and emulsion properties of cricket proteins will also enable a greater understanding of the functional properties and how these proteins can be extracted and developed for use as a good source of protein ingredients in food systems.
Acknowledgements This work was supported by Mahidol University and Suranaree University of Technology (SUT). S. Imsoonthornruksa and C. Gosalawit were supported by SUT from the Office of the Higher Education Commission under the NRU Project of Thailand. The authors would like to thank the Scientific Operation Unit (SOU), Research Academic Supports Division of Mahidol University Kanchanaburi Campus for providing research facilities.