2021 Volume 27 Issue 1 Pages 151-159
2021 Volume 27 Issue 1 Pages 151-159
The crude polysaccharides were extracted from the seeds of Toona sinensis using deionized water, and further purified with DEAE-Sepharose CL-6B column to afford STSP-1, STSP-2, STSP-3 and STSP-4. The high efficiency gel filtration chromatography (HPGFC) analysis showed that the average molecular weight of polysaccharides (STSP-2, STSP-3) were 21.4 kDa and 43.7 kDa, separately. Monosaccharide analysis detected arabinose, rhamnose, glucosamine, galactose, glucose, xylose, mannose, glucuronic acid and galacturonic acid. Moreover, their in vitro anticoagulant activities were evaluated by activated partial thromboplastin time (APTT), thrombin time (TT) and prothrombin time (PT) assays. In comparison with the control group(saline), STSP-3 could prolong PT and TT significantly, but not APTT. STSP-3 exhibited potent anticoagulant activity and would be explored as a natural potential anticoagulant.
The World Health Organization registered 17.5 million of deaths in 2012 as consequence from cardiovascular diseases. Cardiovascular diseases such as myocardial infarction, stroke, deep-vein thrombosis, and pulmonary embolism are major causes of mortality in modern society (Kang et al., 2016).
Heparins (Wu et al., 2012), are a family of highly suiphated acidic mucopolysaccharides that play an important role as anticoagulants in the treatment of thrombotic disease (Fachina and Verli, 2008; Chourasia et al., 2014). However, they also accompanied with some side effects of causing thrombocytopenia and bleeding (Wu et al., 2012; Ekanayake et al., 2008). Therefore, it is necessary to look for the natural anticoagulant component in order to reduce adverse reactions, less side effects, low toxicity (Yang et al., 2014; Cai et al., 2010) in the treatment and prevention of thrombotic diseases.
Toona sinensis, belonging to Meliaceae family, is a kind of perennial deciduous tree commonly found in China, whose seeds have been used in traditional Chinese medicine to treat gastroduodenal ulcer and chronic gastritis (Xing, 2010; Peng et al., 2019; Wang et al., 2016). Modern researches have reported that Toona sinensis possessed various pharmacological activities including anti-tumor effects, antioxidant effects, anti-diabetic effects, anti-inflammatory effects, antibacterial and antiviral effects (Peng et al., 2019), and the polysaccharides from Toona sinensis leaves improved CCl4-induced mice liver injury (Cao et al., 2019). However, there are few reports on the anticoagulant effect of polysaccharides from Toona sinensis seeds. In this study, we attempted to investigate the molecular weights and monosaccharide compositions, as well as the anticoagulant activity of the polysaccharide fractions isolated from the Toona sinensis seeds. Different polysaccharide fractions were obtained by ion exchange chromatograph. Through aPTT, PT and TT analysis to evaluate the effect of polysaccharide fraction on its anticoagulant in vitro, it can be used as a natural anticoagulant for research and development.
Materials and reagents Toona sinensis seeds were purchased from a medicine store, Bozhou, Anhui, China. DEAE Sepharose CL-6B was purchased from Pharmacia Company (Uppsala, Sweden). The standard monosaccharides were purchased from Sigma (St. Louis, MO, USA). Then normal human blood was provided from the central blood station (Wuhu, China). APTT reagent, PT reagent, TT reagent and the calcium chloride (0.025 M) were purchased from Shanghai Sun Biotech Co., Ltd (Shanghai, China), and other chemicals reagents (analytical grade) were from Sinopharm Chemical Reagent (Shanghai, China)
Extraction and purification of polysaccharides in the Toona sinensis seeds The Toona sinensis seeds were dried at 70°C, crushed, and sieved through an 80-mesh screen to obtain a fine powder. Effectiveness of extraction and the polysaccharides content were examined using phenol–sulfuric acid colorimetric method (Yi et al., 2020). The dried fine powder was extracted with twenty-five times volumes of distilled water at 83 °C for 92 min. After centrifuging to remove debris fragments (4000 r/min, 20 min), the solution was concentrated in a rotary evaporator, then mixed with four volumes of 95% ethanol and kept at 4 °C in refrigerator for 24 h. The precipitates were collected by centrifugation and washed successively with 80% ethanol, 99% ethanol and acetone, then lyophilized. Protein was eliminated with the static polyamide adsorption method (Zhang, et al., 2015a) and monitored with a UV-vis spectrophotometer (Shimadzu Corporation, Japan) at a wavelength of 200–400 nm. After removal of the protein, the pigment was removed with the active carbon method (Zhang, et al., 2015b), and the amount of active carbon method was determined.
The lyophilized STSP was redissolved in distilled water, filtered with a 0.45 µm filter and centrifuged. The microfiltration supernatant was taken to was added to a DEAE Sepharose CL-6B column(1.6 × 60 cm) equilibrated with 0.02 M phosphate buffer (pH 6.0). After washing the sample with the same buffer, the column was eluted stepwise with different concentration of NaCl solution (0, 0.1, 0.2, and 0.5 M) at a flow rate of 1.0 mL/min. The eluents were collected in test tubes (5 mL/tube) by automatic fraction collector, and monitored by the phenol-sulfuric acid Method using glucose as the standard (Dubois et al., 1956), and the elution curves were plotted. The eluate of each major fraction was pooled, concentrated, and then dialyzed (molecular weight cut off: 14 000 Da) against deionized water for 3 days to remove NaCl to afford STSP-1, STSP-2, STSP-3 and STSP-4.
The homogeneity of STSP-1, STSP-2, STSP-3 and STSP-4 was determined by HPGFC method according to the subsequent description (determination of molecular weight).
UV and FT-IR spectral analysis STSP-1, STSP-2, STSP-3 and STSP-4 were made up solution (0.2 mg/mL) by distilled water, respectively, and then scanning the fractions of STSP on the 1280 UV-vis spectrophotometer (Shimdau, Japan) from 200-400 nm to observe the ultraviolet spectrum (Liao et al., 2018).
The polysaccharide components (2 mg) were pressed with KBr powder, and then analyzed the polysaccharide components by FT-IR spectral within 4000–400 cm−1 (Yang et al., 2012).
Determination of molecular weight The molecular weight of STSP-1, STSP-2, STSP-3 and STSP-4 were determined by high-efficiency gel filtration chromatography (HPGFC) (Chen et al., 2013). The sample solution was loaded onto HPLC (Waters 1525, USA) equipped with Ultrahydrogel™ Linear columns (300 × 7.8 mmid ×2) and waters 2410 refractive index detector. The chromatographic conditions were as follows: (1) mobile phase: 0.1 M NaNO3; (2) flow rate: 0.9 mL/min; (3) column temperature: 45 °C; (4) injection volume: 20 µL. The sample were dissolved in mobile phase solution and passed through a 0.45 µm filter. Dextran standards with different molecular weights (MW135350, MW36800, MW9750, MW2700, MW180) were used for calibration curve.
Monosaccharide composition analysis Monosaccharide composition was determined by dissolving samples in 1 mL 12 M H2SO4 at 35 °C for a half hour followed by diluting the solution to 1 M H2SO4 which was kept at 100 °C for 2 h. The hydrolytes were diluted to appropriate concentration, and analyzed by high performance anion exchange chromatography (HPAEC) as described by Xie (Xie et al., 2013).
Anticoagulant activity assay Measurements of plasma activated partial thromboplastin time (APTT), prothrombin time (PT), and thrombin time (TT) were performed by using semi-automatic coagulometer (MTN-II, Changchun Man Te Nuo industrial Co., Ltd, Changchun, China) as described(Cai et al., 2016) and reagent Kit (Sun Biotechnology Co., Ltd, Shanghai, China) instructions. In the study, human plasma was obtained from the blood of healthy volunteers without a history of bleeding or thrombosis. In brief, the platelet-poor-plasma was incubated with STSP or heparin for 3 min at 37 °C. Fifty microliters of the incubated plasma was mixed with 50 µL of STSP in the clotting cup, and the coagulation was started by addition of CaCl2 (25 mM, 50 µL) after mixed 50 µL of ellagic acid, 50 µL of thrombin and 50 µL of thromboplastin into the incubated plasma for APTT, PT and TT assay, respectively. The anticoagulant activity was expressed as the clotting time.
Statistical analysis All the experiments were repeated three times in parallel. All the results were presented as mean ± standard deviation (SD) and statistical analysis was performed with Minitab15 software.
Purification of STSP Extraction of crude water-soluble polysaccharides yielded approximately 5.08% w/w of dried powder. The protein content in polysaccharide sample was eliminated by the static polyamide adsorption method. As seen in Fig. 1, the protein removal rate of crude STSP was increasing, and the loss of polysaccharide was also increasing with the increase of polyamide powder dosage. When the amount of polyamide was 1.4 to 1.8 g, the rate of protein removal rate was reduced, which could be due to the polysaccharide adsorption has reached the saturation effect. So the appropriate polyamide powder mass was 1.4 g with the protein removal rate and polysaccharide retention rate reached 68.15% and 88.09%, respectively.
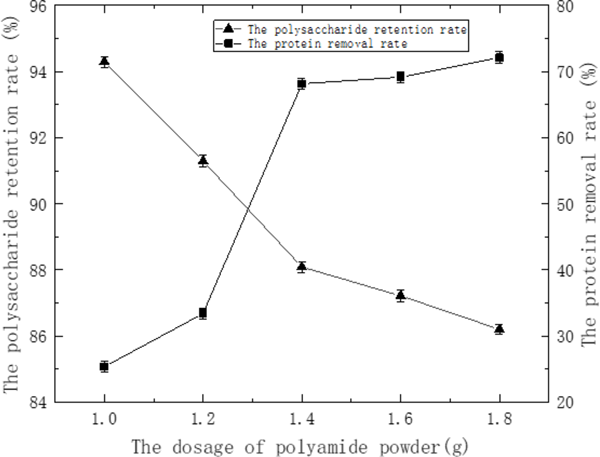
Effect of the dosage of polyamide on the polysaccharide retention rate and the protein removal rate of CSTSP
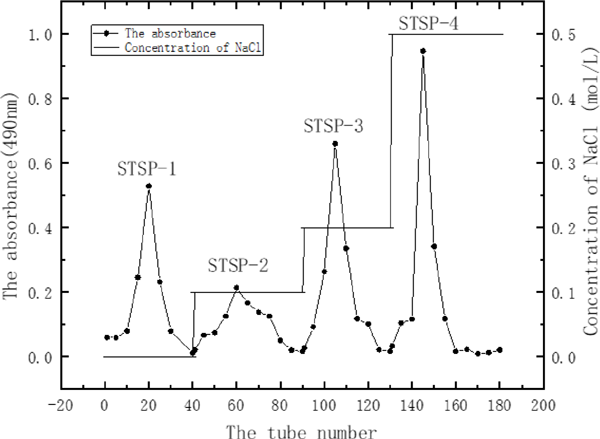
The elution curve of STSP on DEAE Sepharose CL-6B column
Pigments will not only bring difficulties to the separation of polysaccharides, but also interfere with the analysis of polysaccharides in the subsequent experiments, such as the determination of molecular weight and the anticoagulant activity detection, so it must be removed from crude extract before further study (Zha et al., 2012). The decolorization of the polysaccharides were monitored according to the active carbon method.
As seen in Fig. 2, the decolorization rate increased while the recovery of polysaccharide decreased gradually with the increase of the dosage of activated carbon. Comprehensive the decolorization rate and polysaccharide retention rate two aspects, the most appropriate the amount of active carbon was chosen to be 2.0% with the decolorization rate and polysaccharides retention rate reached 65.14%, 64.19%, respectively. The pigments could not be completely removed, UV and FT-IR spectrum analysis The UV spectral analysis of STSP fractions were shown in Fig. 4. No absorption was observed at 260 nm or 280 nm for STSP-1, STSP-2, STSP-3, STSP-4, indicating that there was no protein and nucleic acid in the STSP fractions. At the same time, it showed that the polyamide method has a better effect on the deproteinization on STSP.
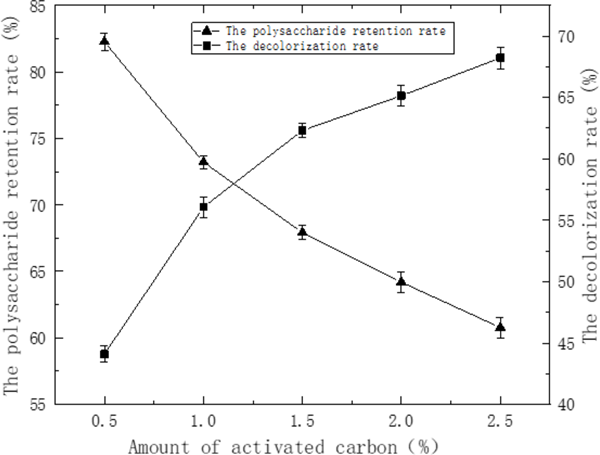
Effect of active carbon content on the polysaccharide retention rate and the decolorization rate of CSTSP
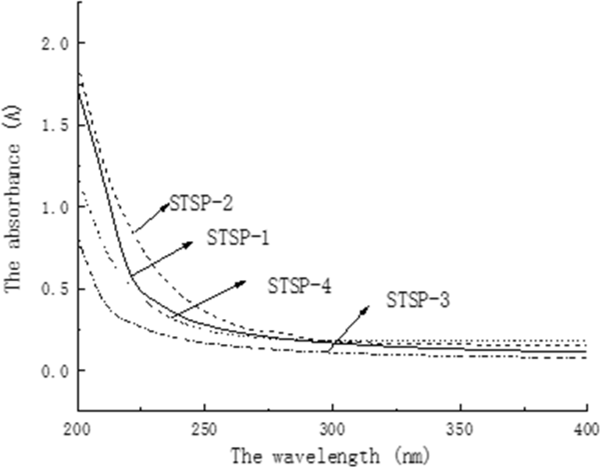
UV-visible spectra of the STSP fractions
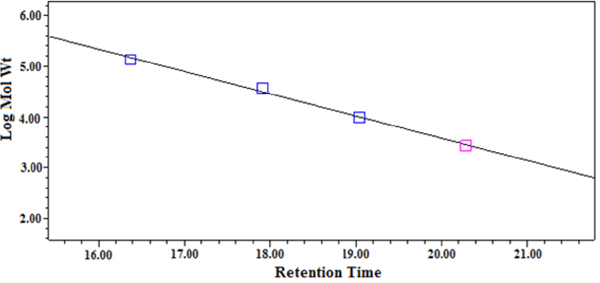
Standard curve of different molecular weight dextran
The FT-IR spectral analysis of STSP fractions were shown in Fig. 5. There were seen that STSP-1, STSP-2, STSP-3, and STSP-4 all have a wide broad strong absorption peak at around 3400 cm−1, indicating that it was the stretching vibration of O-H. There was also a weak peak near 2930 cm−1, which is characteristic absorption peak C-H of sugars, and these two absorption peaks are characteristic absorption peaks of polysaccharides (YI, 2014). A weak absorption peak appeared at 1622–1640 cm−1 and 1385 cm−1, possibly with the presence of a carboxyl group and a carbonyl group. The peak at 1075–1140 cm−1 indicated the presence of pyranose, and the presence of a peak at 870–875 cm−1 indicated the presence of a β-glycosidic bond (Cai et al., 2016) in the polysaccharide.
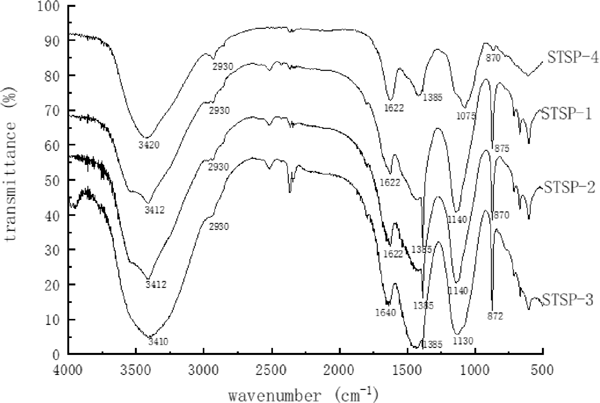
FT-IR spectra of the STSP fractions
Molecular weight of polysaccharide fractions The molecular weight of STSP-2 and STSP-3 were 21.4 kDa (Mp:13.2 kDa) and 43.7 kDa (Mp:36.1 kDa), respectively. However, there were still two peaks in the HPGFC spectra of STSP-1 (Fig. 7a) and STSP-4 (Fig. 7d), indicating their low purity, which needed to be further separated.
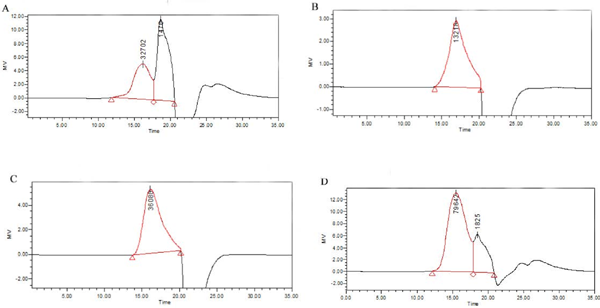
HPGFC spectra of the STSP fractions (STSP-1(A), STSP-2(B), STSP-3(C) and STSP-4(D))
Monosaccharide composition analysis The monosaccharidecompositions of STSP fractions were analyzed by ion chromatography and the analysis results were shown in Table 1. STSP-1 was composed of rhammose, glucosamine, galactose and xylose, of which galactose (57.8%) was the main component. The main constitutes of STSP-2 were galactose (31.65%) and glucose (50.15%). STSP-3 was composed of seven monosaccharides, including arabinose, rhamnose, glucosamine, galactose, glucose, xylose and mannose at a mole percent of 4.36:26.05:3.74:54.79:6.88:0.72:3.45 respectively. Five of the same monosaccharides (arabinose, rhamnose, Glucosamine, galactose and glucose) were detected besides galacturonic acid and glucuronic acid in STSP-4. This result is very close to the studies of Zhang (Zhang et al., 2015c).
| Sample | Molar ratios/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | GluN | Gal | Glu | Xyl | Man | GluA | GalA | |
| STSP-1 | - | 9.42 | 19.92 | 57.8 | - | 12.87 | - | - | - |
| STSP-2 | - | 6.56 | 7.69 | 31.65 | 50.15 | - | 3.94 | - | - |
| STSP-3 | 4.36 | 26.05 | 3.74 | 54.79 | 6.88 | 0.72 | 3.45 | - | - |
| STSP-4 | 16.82 | 17.96 | 1.72 | 54.24 | 2.9 | - | - | 5.56 | 0.7 |
“-” Undetected. Ara (arabinose), Rha (rhamnose), GluN (glucosamine), Gal (galactose), Glu (glucose), Xyl (xylose), Man (mannose), GluA (galacturonic acid), GalA (glucuronic acid)
In vitro anticoagulant activities The anticoagulant activities of the polysaccharides from the Toona sinensis seeds were investigated by the classical coagulation assays APTT, PT and TT, and meanwhile the saline and heparin were used as negative and positive control, respectively. APTT is used to evaluate the coagulation factors including VIII, IX, XI, XII and prekallikrein in the intrinsic blood coagulation pathway. PT is used to characterize the extrinsic coagulation factors, and TT is as an indicator of blood coagulation status that transforming fibrinogen into fibrin (Ye et al., 2012).
As listed in Table 2, STS polysaccharide extended PT and TT but APTT in a dose-dependent manner compared to saline control although activity less than heparin. It is suggesting that the anticoagulant effect is associated to polysaccharide containing uronic acid (Pawlaczyk-Graja, 2018; Yoon et al., 2002). The different anticoagulant effects of polysaccharides are related to their purity, molecular weight and uronic acid content. Meanwhile STSP-3 fractions exhibited a significant extension (p < 0.05) in PT and in TT (p < 0.01) compared to that of saline. Furthermore, PT of STSP-3 at 4 mg/mL was 15.5 ± 1.76 s, which was approximately 1.27-fold longer than that of the saline. Compared with the saline control group, TT increased 1.6-fold with the addition of STSP-3 at 4 mg/mL. Transformation of fibrinogen into fibrin is known to be the last step in coagulation, and TT is a key indicator of this process (Zhao et al., 2016). These results obtained for the anticoagulant activity suggest that STSP-3 may inhibit both the extrinsic and the common coagulation pathways.
| Sample | Concentration | APTT(s) | PT(s) | TT(s) |
|---|---|---|---|---|
| Saline | 0.90% | 35.03±3.55 | 12.17±0.25 | 10.50±0.20 |
| Heparin | 2 ug/mL | 42.3±0.29** | 19.53±0.74** | 16.7±1.59** |
| STSP | 1mg/mL | 34.43±2.20 | 11.97±0.50 | 11.53±0.76 |
| 2mg/mL | 35.73±0.49 | 14.13±0.21* | 12.63±0.57* | |
| 4mg/mL | 36.6±0.6 | 17.97±0.29** | 14.37±0.32** | |
| STSP-1 | 1mg/mL | 36.63±2.21 | 9.63±0.42 | 9.93±0.23 |
| 2mg/mL | 35.77±0.55 | 9.9±0.17 | 10.77±0.35 | |
| 4mg/mL | 36.77±3.67 | 10.33±0.06 | 11.8±0.66 | |
| STSP-2 | 1mg/mL | 33.4±0.1 | 11.23±0.46 | 11.73±0.87 |
| 2mg/mL | 34.9±0.95 | 11.23±0.21 | 11.87±0.58 | |
| 4mg/mL | 36.97±0.55 | 11.47±1.16 | 12.1±2.87 | |
| STSP-3 | 1mg/mL | 29±1.80 | 9.97±0.23 | 11.0±1.12 |
| 2mg/mL | 34.97±0.45 | 12.78±1.62 | 14.17±1.46** | |
| 4mg/mL | 35.77±0.26 | 15.5±1.76* | 16.8±1.71** | |
| STSP-4 | 1mg/mL | 31.57±1.38 | 10.47±0.21 | 11.13±0.35 |
| 2mg/mL | 33.17±2.02 | 10.53±0.23 | 14.13±0.95** | |
| 4mg/mL | 34.97±9.72 | 10.8±0.17 | 15±0.89** |
The data in the table 3 were showed as mean ± SD (n = 3)
Previous studies indicated that there are many factors that affect the anticoagulant activity of non-sugar sulfated polysaccharides, such as the structure, the configuration of the polymer molecules, molecular weight and branching structures (Lin et al., 2019; Yoon et al., 2003). Our report is the first description of a natural nonsulfated polysaccharide from Toona sinensis seeds with anticoagulant activity.
Anticoagulants can be used to prevent endovascular embolism or thrombosis, stroke or other thrombotic diseases by affecting certain coagulation factors during the process of coagulation. These thrombotic diseases have developed into the main causes of death, so effective anticoagulant drugs are urgently needed. Based on the above data analysis, it can be concluded that STSP-3 has a certain anticoagulant effect in vitro and could be developed as an anticoagulant drug and applied to the treatment of coagulation-related diseases.
In the paper, the polysaccharides were extracted from of Toona Sinensis seeds by hot water extraction. With the application of activated carbon method to remove the pigment from polysaccharides which have deproteinized by polyamide method.
It was concluded that Toona Sinensis seeds contained four major polysaccharide fractions purified by DEAE sepharose CL-6B column chromatography, and STSP-2 and STSP-3 were homogeneous polysaccharides with an average molecular weight of 21.4 kDa and 43.7 kDa, respectively.
In addition, the anticoagulant activity in vitro investigation of STSP-3 that consisted of Ara, Rha, GluN, Gal, Glu, Xyl and Man significantly prolonged the clotting time in TT and PT experiment. The difference in anticoagulant activity for aPTT may be due to the presence of uronic acid in the polymer chain.Our results suggest that STSP-3 might have potential for application in clinical or the food supplements.
Acknowledgements The authors are grateful to Science and Technology Project in Anhui Province (No.1604a0702013) and Research on key research and development projects in Anhui Province (No. 202004a06020022) for their supply of financial.
Compliance with Ethics requirements This article does not contain any studies with human or animal subjects.