2021 Volume 27 Issue 4 Pages 639-646
2021 Volume 27 Issue 4 Pages 639-646
Ascorbic acid (AsA) is an essential nutrient for humans, but its accumulation in the body is often problematic. Although it has been reported that the ingestion of apples increases AsA in the body, the details remain unknown. If true, it would decrease AsA deficiency in humans. Here, we investigated the effects of apple juice intake on the absorption and accumulation of AsA in the body of osteogenic disorder Shionogi (ODS) rats, a mutant strain of Wistar rats. These model animals that lack AsA biosynthetic capacity were fed a mixture of an AsA solution and apple juice. We examined the AsA concentration in plasma, urine, and the mucosa of the small intestine. We observed that apple juice intake increased the amount of AsA in rats. This mechanism seems to be related to absorption from the small intestine. This study may contribute to research on enhancing AsA absorption efficiency.
Ascorbic acid (AsA), one of the five major nutrients for the human body, is a type of vitamin. AsA acts as a strong antioxidant (Rose et al., 1993) as well as a cofactor that helps the construction of collagen fibers (Peterkofsky, 1991), lipids, such as cholesterol (Takahashi et al., 2014), and enzymes important for the synthesis of catecholamines, such as adrenaline (Amano et al., 2014). Furthermore, it is a promoter of iron absorption (Mackenzie and Garrick, 2005). The estimated average requirement of AsA in Japanese adults is 83.4 mg/day, which has been set as a threshold for cardiovascular disease prevention and effective antioxidant effects in terms of the primary prevention of lifestyle-related diseases (Levine et al., 2001). However, according to the 2019 National Health and Nutrition Examination Survey, the AsA intake among people between the ages of 1 and 49 years does not meet this estimated average requirementi). Therefore, there is a social need to elucidate methods of enhancing the absorption and accumulation of AsA in the body.
The absorption rate of AsA is 90% for amounts up to approximately 200 mg/day, but it is known to be 50% or less at 1 g/day, or more according to a previous study examining in vitro excretion (Tsujimura et al., 2006). It was found that taking more than 1 g of AsA per day was redundant, as the absorption rate was reduced and the surplus was only excreted (Levine et al., 1996; Kallner et al., 1981; Melethil et al., 1986). Therefore, to increase the amount of available AsA in the body, it is crucial to understand how the absorption capacity can be enhanced. However, there are few reports on methods for enhancing the absorption of water-soluble vitamins, as opposed to fat-soluble vitamins; the absorption of fat-soluble vitamins is expected to increase with the consumption of foods with high triglyceride contents.
According to the Standard Tables of Food Composition in Japan 2015 (Seventh Revised Edition), the AsA content of apples is 4 mg/100 g of apple; thus, apples are not considered a good source of AsA. However, plasma, liver, and adrenal AsA concentrations were higher in rats and guinea pigs fed an apple-containing diet (Renee and Rene, 1991). In the report by Renee and Rene (1991), the administration method, dose, and period of apple intake were unclear; therefore, the mechanism of the enhancement of AsA absorption could not be assessed. The aim of the present study was to elucidate the effects of apple intake for enhancing AsA retention in vivo. We used a mutant strain of Wistar rats, osteogenic disorder Shionogi (ODS/ShiJcl-od/od), which, similar to humans and guinea pigs, lack L-glono-γ-lactone oxidase activity, the final step in AsA synthesis. As a result, ODS rats are unable to synthesize AsA (Mizushima et al., 1984). Therefore, we chose them as the best model to study the internal delivery of AsA.
AsA is mostly absorbed in the small intestine, and the absorption occurs via a sodium-dependent vitamin C transporter (SVCT) (Tsukaguchi et al., 1999). In humans, after absorption in the small intestine, AsA is sent to the liver and transported to tissues and organs via the plasma. AsA transporters are expressed in small intestinal epithelial cells. SVCT is also involved in the resorption of AsA in the proximal tubules of the kidney (Corpe et al., 2010). From these facts, it is considered that an increase in apple juice (AJ) absorption in the small intestine would also increase AsA absorption and accumulation in the body.
Apples have various health benefits, and this study explored a new possible benefit of apples – increasing AsA in the body. Therefore, the purpose of this study was to clarify whether the ingestion of AJ enhances the accumulation of AsA in the body. In addition, as it becomes clear that apples are effective in preventing lifestyle-related diseases and improving the development of functional foods, it is expected that apples could be used more effectively to extend the healthy life expectancy. It is thus expected that the sale and consumption of apples will increase more than ever, which would benefit people involved in apple production and sales, and greatly improve public health.
Chemicals and Samples AsA, metaphosphoric acid, ethylenediaminetetraacetic acid, phosphate-buffered saline, dinitrophenylhydrazine, and sulfuric acid were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Pentobarbital was purchased from Nacalai Tesque (Kyoto, Japan). Apples (Malus domestica Borkh. cv Fuji) were purchased from a grocery store.
Preparation of samples The apples used in the experiment were squeezed by a juice extractor (GP-E1503, Green Power, Korea), followed by filtration (No. 2, Advantec Co., Tokyo, Japan). The prepared cloudy AJ was diluted with ultrapure water to 0.2%, 2%, and 20% concentrations. The amount of AsA in the resulting AJ was determined by high-pressure liquid chromatography using a Shodex Asahipak NH2P-50 4D 5-µm column (4.6 × 150 mm, Shodex, Tokyo, Japan). AsA was converted to dehydroascorbic acid (D-AsA) by adding homocysteine to the diluted AJ. The protein was precipitated with metaphosphoric acid, centrifuged (1 000 × g, 4 °C, 10 min), and the resulting supernatant was used for measurements. Based on the measurements, a deficiency was added so that the final concentration of AsA was 2 g/L. This was used as the AJ administered to the rats. For the control, we used drinking water and the AsA solution with a concentration of 2 g/L (prepared with ultrapure water).
Experimental design In the three types of experiments performed, the animals were 4-week-old male ODS rats (CLEA Japan, Tokyo, Japan) that were individually housed in a temperature- and humidity-controlled room (23 °C ± 2 °C, 55% ± 7% relative humidity) under a 12-h light/dark cycle. An AsA-deprived diet (AIN-93G, CLEA Japan) and water containing AsA (2 g/L) were provided ad libitum until 6 weeks of age.
In the first experiment, we focused on the effects of various AJ concentrations on the absorption of AsA in plasma and urine. Twenty rats were divided into four groups (n = 5 for each group): the control group, 0.2% AJ group, 2% AJ group, and 20% AJ group. Furthermore, the 0.2%, 2%, and 20% AJ groups were given the same water, and additionally, 0.2%, 2%, and 20% AJ, respectively.
In the second experiment, we tested the effects of AJ on AsA absorption in several tissues. Ten rats were divided into two groups (n = 5 for each group): the control group and AJ group. The control group was provided with the same water and diet content as in experiment 1. The AJ group was provided with water containing AsA (2 g/L) and 2% AJ.
In the third experiment, we investigated further the effects of AJ on AsA absorption in each part of the small intestine and on AsA uptake by the everted sac. Ten rats were divided into two groups (n = 5 for each group): the control group and AJ group. Both groups were provided with a diet and water with the same contents as in experiment 2; however, it was provided ad libitum until 10 weeks later.
The AsA solution with and without AJ was changed every 2 to 3 days, and the intake volume was measured. In a preliminary experiment, there was no significant change in the concentration of AsA when the mixture was stored for 14 days in an animal room.
The 24-h urine samples were collected before and after experiments for 2 weeks in tubes. All urine samples were immediately stored at −80 °C until analysis.
After the experiment, the rats were dissected under deep anesthesia with pentobarbital. Plasma samples were collected from the heart, gently mixed with ethylenediaminetetraacetic acid, and centrifuged at 3 000 × g for 15 min at 4 °C. The obtained plasma supernatants were used for further analysis. Afterward, rats were systemically perfused with ice-cold phosphate-buffered saline through the left ventricle to wash out the remaining plasma cells, and tissues of interest were collected and stored at −80 °C until analysis.
All experiments in this study received prior approval from the Animal Care Advisory Committee of Aomori University of Health and Welfare (No. 11012, 13008, 14006). The animals were maintained in accordance with the guidelines on animal experiments at the Aomori University of Health and Welfare.
AsA measurement Tissues were homogenized in 14-fold of 5.4% metaphosphate, and centrifuged at 1 000 × g for 10 min at 4 °C. Plasma and urine were mixed with equal volumes of 10% metaphosphate, and centrifuged at 1 000 × g for 10 min at 4 °C. To convert AsA to D-AsA, 0.2% 2,6-dichloroindophenol was added to the supernatant solution as an oxidant. This was used as a sample for measurement. The samples were placed in individual tubes, and 5% metaphosphoric acid was added. Dinitrophenylhydrazine was added, and the solutions were incubated in a 37 °C water bath for 3 h. Upon completion of treatment, 85% sulfuric acid was added dropwise to each tube in an ice bath. The solution was allowed to stand for 30 min before color measurements were performed with a spectrophotometer equipped with a 530-nm filter.
Uptake experiment using everted sacs Modified Krebs Ringer Bicarbonate Solution (KRB) and KRB with AsA (AJ (+) KRB, final concentration: 10%) were prepared. The inverted intestine was prepared with a modification of Muto's method (Muto, 1992). The removed small intestine was separated from the gastric side, and divided into three equal parts: the duodenum, jejunum, and ileum. We measured the weight of each part. We used 1 g of each part to measure the amount of AsA uptake. The end of the large intestine was ligated, and a gastric sonde was inserted to invert the intestine. The inverted intestine was filled with approximately 1 mL of AJ (−) KRB. The part of the gastric sonde containing the silicon tip was removed to separate the intestine from the inverted intestine preparation device. Two to three inverted intestines were prepared from one animal, and the AsA uptake was measured for each of them.
The prepared inverted intestine was placed in 9 mL of AJ (−) KRB or AJ (+) KRB solution, and incubated at 37 °C for 1 h. The test tube was always filled with O2 gas. After the incubation, the inverted intestine was immediately removed from the test tube, and we collected the external and internal fluids with a syringe. Moreover, we compared the amount of AsA transferred from the inverted intestine external fluid to the internal fluid among groups by measuring the AsA concentration. The collected solutions were stored at −80 °C until the time of AsA concentration measurement.
Statistics analysis The results are shown as the mean ± standard deviation (SD), and were analyzed using SPSS 22.0 (IBM, Chicago, USA). Significant differences among the three or four groups were evaluated by ANOVA and Tukey's test or Welch's t-test. Differences were considered to be significant when p < 0.05.
Effects of AJ concentrations on plasma and urinary AsA absorption To examine the effects of AJ on plasma levels, AsA and AJ were administered orally to rats, and the effects were compared to those of AsA alone. The effects of 2 weeks of oral administration of 0.2%, 2%, and 20% AJ on the AsA plasma concentration in rats are shown in Fig. 1A. The total AsA intake for 2 weeks in each group is shown in Fig. 1B, and the effects of AJ intake at different concentrations on the plasma AsA concentration, corrected for AsA intake, are shown in Fig. 1C.

Effects on AsA concentration in the plasma after 2 weeks of oral administration of 0. 2%, 2% and 20% apple juice in rats. (A) Total AsA intake for 2 weeks in each group; (B) Effect of different apple juice concentrations on plasma AsA concentration, corrected for AsA intake; (C) The control group received 2% AsA solution. Plasma was processed as described in the methods section, and the AsA content was determined using the hydrazine colorimetric method. Values are presented as mean ± SD (n = 5). ND, Not Detected. In Fig. A and C, multiple comparison was made without the control group because AsA concentration was not detected in it.
The plasma AsA concentration corrected for intake in the 2% AJ group increased when compared to the control group. There was no significant increase in plasma AsA concentration in the 0.2% and 20% AJ groups.
Table 1 shows the changes in urinary AsA concentration at the start of the intake of 2% AJ (Day 0) and 2 weeks later (Day 14). The control group received a 2% AsA solution. There was no significant difference in urinary excretion before and after feeding in each group. The rate of increase in urinary AsA concentration from the start of feeding to 2 weeks later was 69.2% ± 0.039% in the control group and 1.8% ± 0.017% in the 2% AJ group, showing a remarkable difference. There was no significant difference in body weight at the time of dissection between groups (data not shown).
| AsA Concenttation(µg/ml) | Day 0 | Day 14 | Rate of increase(%) |
|---|---|---|---|
| Control group | 0.039 ± 0.003 | 0.066 ± 0.006 | 69.2 ± 0.039 |
| AJ group | 0.057 ± 0.016 | 0.058 ± 0.007 | 1.8 ± 0.017 |
Effects of AJ on AsA in various tissues Thyroid and eye AsA concentrations were significantly higher in the AJ group than in the control group. There were no significant differences in the AsA concentrations in other tissues and relative weights. Moreover, there was no significant difference in the amount of AsA intake between the groups (Fig. 2B). As in experiment 1, the AsA concentration in the plasma of the AJ group tended to be higher than that of the control group (data not shown).
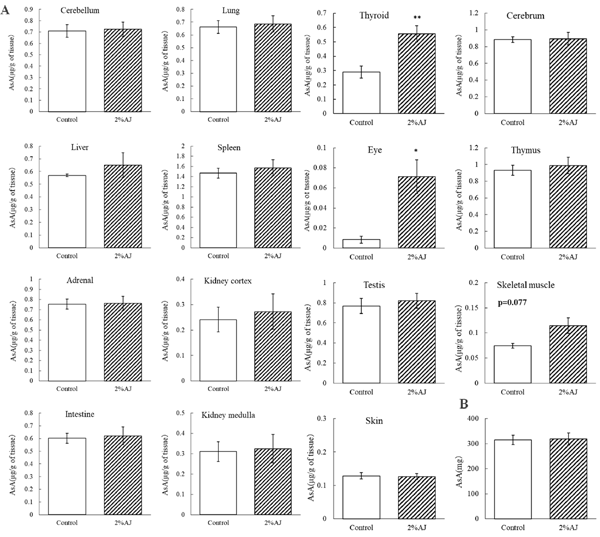
Effect of apple juice intake on AsA concentration in the tissues of rats (A). Total AsA intake for 2 weeks in each group (B). Tissues were processed as described in the methods section, and AsA concentration was determined by the hydrazine colorimetric method. Values are presented as mean ± SD (n = 5). Asterisks indicate significant differences compared to the control (*p < 0.05; **p < 0.01).
Effects of AJ on AsA concentrations in each part of the small intestine and on AsA absorption in the everted sac Figure 3A shows the AsA concentration in each part of the small intestine. There was no statistically significant difference between the control group and the AJ group in the AsA concentration in the duodenum, jejunum, and ileum. In addition, no statistically significant difference was observed in the AsA concentration between the duodenum, jejunum, and ileum of the AJ group. Similarly, there was no statistically significant difference in the AsA concentration between the duodenum, jejunum, and ileum in the control group. However, in both the AJ and control groups, the duodenum and ileum tended to have higher AsA concentrations than the jejunum. There was no significant difference in the intake of AsA among individuals (Fig. 3B).
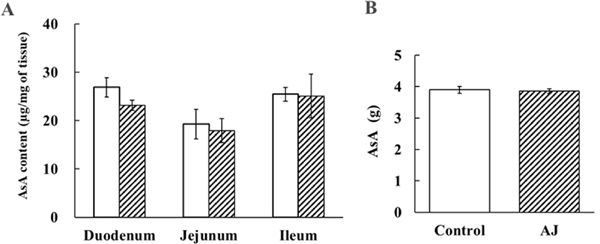
Effects on AsA concentration in each part of small intestine (duodenum, jejunum, and ileum) after 10 weeks of oral administration of 2% AJ in rats (A). Total AsA intake for 10 weeks in each group (B). The AsA concentration was quantified by the hydrazine colorimetric method. Values are presented as mean ± SD (n = 5). (□), control group; (▨), AJ group.
We analyzed whether AsA absorption in the small intestine was enhanced by AJ using everted sacs. The internal AsA concentration in the duodenum, jejunum, and ileum of the AJ group was significantly increased by 1.25-fold, 1.20-fold, and 1.23-fold, respectively, when compared to the control group. On the other hand, there was no significant difference in the rate of increase between the different regions of the small intestine (Fig. 4A). The internal AsA concentration in the reversed intestinal fluid in the duodenum, jejunum, and ileum of the AJ group was significantly increased by 1.27-fold, 1.33-fold, and 1.42-fold, respectively, when compared to the control group. Furthermore, there was no difference in the rate of increase between the different regions of the small intestine (Fig. 4B).
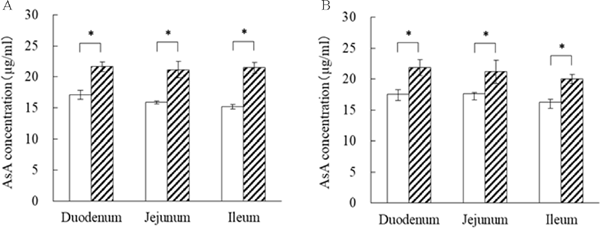
Effect of Apple juice on AsA uptake by everted sacs. 2% AJ and/or 2 g/L AsA solution were added to the mucosal side of the everted sacs of rat duodenum, jejunum, and ileum. The sacs were incubated at 37 °C for 60 min. (A) Group without AJ on the mucosal side; (B) Group with added AJ on the mucosal side. Serosa solutions were recovered and subjected to the hydrazine colorimetric method to determine AsA content. Two to three inverted intestines were prepared from one animal, and each had AsA uptake measured. Values are presented as mean ± SD (n = 13). Asterisks indicate significant differences compared to control (*p < 0.05). (□), control group; (▨), AJ group.
AsA plays a variety of important roles. However, since it is a water-soluble vitamin, sufficient AsA absorption and accumulation in the body are difficult to achieve. In Japan, the AsA intake among people between the ages of 1 and 49 years does not meet the estimated average requirementi). In particular, it has been reported that elderly people have a higher requirement than young adults for maintaining AsA in the body (Brubacher et al., 2000). In Japan, where the population is aging, the establishment of a method for retaining AsA in the body is highly desired. However, there are still no reports on such methods. There has been a report that the consumption of AJ significantly increases the concentration of AsA in the body (Renee and Rene, 1991). However, the lack of data on an accurate administration method, dosage, and period for apple intake makes it difficult to understand how AsA absorption can be enhanced. Here, we aimed to elucidate the effects of apple intake for enhancing AsA retention in vivo. Rats lacking the ability to synthesize AsA ingested a mixture of an AsA aqueous solution and AJ, and the AsA concentrations in their plasma, urine, and various tissues were examined.
The plasma AsA concentration corrected for intake in the 2% AJ group tended to be higher than that in the control group. There was no significant increase in plasma AsA concentration in the 0.2% and 20% AJ groups. We found that the 2% AJ group had a higher AsA concentration than the control group while the 20% AJ group did not show a significant difference. This may be due to the effect of the sugar concentration in AJ. Although we cannot be certain, because we did not measure the blood glucose in rats in this study, long-term intake of highly concentrated AJ as drinking water may have caused hyperglycemia. In humans, it has been reported that diabetic patients have significantly lower plasma AsA levels than healthy individuals, because of increased consumption of AsA due to hyperglycemia (Jae et al., 2000). Therefore, the 20% AJ group may have increased AsA consumption due to hyperglycemia, and as a result, the plasma AsA levels may not have increased.
The amount of AsA in urine tended to increase in a timedependent manner in the control group, but not in the 2% AJ group. On the other hand, the urinary excretion of the control group on day 0 was lower than that of the AJ group. Since there was no significant increase in urinary excretion before and after the feeding in each group, it suggested that AJ intake did not affect AsA excretion. The time-dependent increase in urinary AsA levels in this study was similar to the plasma results. It is known that the AsA level in raw urine depends on the plasma, because AsA is absorbed from raw urine in the proximal tubule and returns to the plasma (Iwama et al., 2011). Therefore, it is possible that ingestion of 2% AJ resulted in the active reabsorption of AsA in the kidneys and the accumulation of a large amount of AsA in the plasma.
The AsA concentrations in the eye and thymus were significantly higher in the 2% AJ group than in the control group. Based on this result, it could be considered that AsA accumulates in an tissue-specific manner after AJ consumption. AsA has been suggested to have a role in maintaining the mucous membrane. In addition, AsA is known to stimulate the immune system by facilitating functions, such as interferon production, enhancing phagocytosis by macrophages, increasing natural killer cell numbers, and enhancing migration ability (Kawada et al., 2013). Since the thymus is involved in immune system activities, such as the differentiation and maturation of lymphocytes, it is considered that AsA accumulation and immune function could be related.
If AsA in AJ is more stable than the AsA in the solution that was used as a control, the AsA concentration in the tissues may have been greater due to the greater AsA concentration. However, as this issue remains unclear based on the results of this study, it is an issue for further investigation.
There was no statistically significant difference in the AsA concentration among the different sites of the small intestine in each group. However, in both the AJ group and the control group, the duodenum and ileum tended to have a higher AsA concentration than the jejunum. It is known that AsA is taken up mostly in the duodenum of the small intestine, followed by the jejunum and ileum (Hierro et al., 2013). Based on this, we considered that the amount of AsA in the duodenum may be higher than that in the jejunum. Furthermore, in an experiment where the amount of AsA uptake into the small intestinal lumen was examined using the everted intestine, AsA uptake from the everted extraintestinal fluid into the everted intestinal lumen was significantly higher in the AJ group at all sites of the small intestine. This result suggested that the small intestine of the AJ group took up more AsA intracellularly than that of the control group, transporting it systemically. As mentioned earlier, there was no accumulation of AsA in the small intestine. In contrast, in the everted intestine, AsA was transferred from the mucosal side to the serosal membrane side. This suggested that AsA in the small intestine is absorbed in vivo and transported to various tissues rather than remaining in the cells of the small intestine. As a result, the amount of AsA in plasma and some tissues may be increased. In addition, we found that the uptake of AsA into the small intestine is enhanced regardless of the presence or absence of AJ in the inverted extracellular fluid. Therefore, these results suggested that AJ is not directly involved in the uptake of AsA from the small intestine, but that it improves the uptake of AsA by enhancing some function of the small intestine. Based on the above results, we found that a control mechanism, which enhances the absorption of AsA in the small intestine, is possibly activated by the ingestion of AJ. According to previous reports, when rats and guinea pigs consume apple-containing feeds, the in vivo AsA concentration increases (Renee and Rene, 1991). Furthermore, reports showed that consuming acerola juice with AsA leads to a higher plasma AsA concentration and a reduced amount of AsA excreted in the urine (Uchida et al., 2011). AsA absorption in the small intestine is carried out by the AsA transporter SVCT1. Experiments using cultured cells have revealed that acerola polyphenols enhance SVCT1 gene expression in Caco-2 cells and promote AsA uptake (Takino et al., 2020). Similarly, the AJ-induced increase in AsA uptake in the inverted intestine observed in the present study may also be influenced by some other component in apple. Further investigation of the relationships with transporters, such as SVCTs, and other factors will be necessary.
In conclusion, we revealed that the consumption of AJ increased the amount of AsA in the body of rats lacking the ability to synthesize AsA. It was also suggested that this mechanism is related to absorption from the small intestine. The findings of this study may provide new approaches and ways of utilizing apples for maintaining and improving health, and may contribute to research on enhancing the absorption efficiency of AsA, which is difficult to maintain in the body.
Conflict of Interest No conflicts of interest to be declared.