Abstract
Jianghua Kucha (JHKC) is a special tea germplasm that has a certain degree of bitterness. Alkaloids (theacrine, caffeine, theobromine, and theophylline) are one of the bitter substances in tea. However, simultaneous comparison of bitterness thresholds at different temperatures and taste active values (TAVs) of alkaloids in JHKC are unclear. Furthermore, as a special purine alkaloid of JHKC, the interaction between theacrine and bitter taste receptors is not clear. In this study, comprehensive analysis of bitterness thresholds and TAVs of alkaloids in JHKC as well as theacrine-bitter taste receptor interaction were carried out. These results showed that thresholds of caffeine, theobromine, theophylline, and theacrine at 15°C were significantly lower than those at 25 °C and 45 °C. Docking simulation results showed that theacrine had the best binding capability with TAS2R14. TAS2R14 amino acid residues Q2667.38, S652.60, and W893.32 may play a key role in docking simulation of theacrine, and hydrogen bonding and π-π interaction was the main interaction.
Introduction
Tea [Camellia sinensis (L.) O. Kuntze] is a widely consumed beverage plant worldwide, mainly through different processing methods to make different beverages (Wu et al., 2021). The typical taste of tea includes bitter, astringent, sweet, and umami. Kucha (Camellia sinensis) is a special tea plant resource in China that was first found in Yunnan province and mostly distributed in the adjoining areas of Guangxi, Guangdong, Jiangxi, and Hunan Provinces (Wang et al., 2020). Jianghua Kucha [JHKC, Camellia sinensis var. assamica (CSA) cv. Jianghua] is a type of Kucha that grows in the Nanling Mountain region of Hunan Province. Some of these JHKC resources have a noticeably bitter taste, and alkaloids are one of the main causes of tea bitterness. The alkaloid content is about 3–5 % of the dry matter of fresh leaves, but alkaloids are extremely bitter, with a low bitterness threshold, and are one of the main contributors to the bitter taste of fresh leaves (Ye et al., 1999). Our previous study (Wu et al., 2022) demonstrated that JHKC contains a special purine alkaloid (theacrine), compared to common tea cultivars, such as Fudingdabai (FDDB), Yunkang 10 (YK10), and Zhuyeqi (ZYQ).
Theacrine has been reported to have a specific bitter taste and it has a lower bitterness threshold than caffeine. The bitterness thresholds of theacrine at 25 °C and 45 °C have been investigated (Shi et al., 2022). It is generally accepted that the thresholds of taste substances are affected by temperature (Ma et al., 2023). However, bitterness thresholds of the four major alkaloids (theacrine, caffeine, theobromine, and theophylline) in tea with different temperatures are not clear. Furthermore, bitterness thresholds of substance molecules were different which may result from the different ways in which they interact with bitter taste receptors in the oral cavity. Humans have 25 bitter taste receptors that recognize a wide range of bitter substances, and one bitter substance can also bind to multiple bitter receptor proteins to produce bitter taste perception (Bayer et al., 2021). Caffeine could activate these bitter taste receptors including TAS2R7, TAS2R10, TAS2R14, TAS2R43, and TAS2R46 (Meyerhof et al., 2010). For theobromine, no data regarding its role as TAS2R agonist have been available (Liszt et al., 2018). However, as a special purine alkaloid of JHKC, the interaction between theacrine and bitter taste receptors is not clear. The molecular docking method is a computer simulation of placing a small ligand molecule in the binding region of a receptor macromolecule, and then a computer prediction of the receptor and ligand binding forces and binding modes to find the lowest energy conformation of the ligand when it binds in its active region (Santos et al., 2019). The binding affinity and conformation of the bitter molecule and the receptor can be rapidly predicted. Therefore, it is necessary to analyze the way theacrine interacts with bitter receptors by molecular docking, which is beneficial to explore the bitter taste mechanism of theacrine.
To comprehensively understand bitterness thresholds influenced by temperature and the mechanism of the differences in the bitterness thresholds of these four alkaloids. in this work, we investigated the bitterness thresholds of theacrine, caffeine, theobromine, and theophylline at 15 °C, 25 °C, and 45 °C, as well as molecular docking of four alkaloids with 25 human bitter taste receptors and for the first time completed molecular docking of theacrine with TAS2R14. This study provides novel insight into the profile of bitterness thresholds affected by temperature and intrinsic bitterness threshold difference mechanisms of four alkaloids in tea.
Materials and Methods
Materials Epigallocatechin gallate (EGCG ≥ 98 %), gallocatechin gallate (GCG ≥ 98 %), epicatechin (EC ≥ 98 %), gallocatechin (GC ≥ 98 %), catechin (C ≥ 98 %), epicatechin gallate (ECG ≥ 98 %), and (-)-epigallocatechin (EGC ≥ 98 %) were purchased from Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). Theacrine was obtained from USA GlpBio Technology Co., Ltd. (Glpbio, USA). Caffeine, theobromine, and theophylline were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). N, N-Dimethylformamide, methanol, and acetic acid of LC–MS grade were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). JHKC -37-2 [Camellia sinensis var. assamica (CSA) cv. Jianghua], Fudingdabai [FDDB, Camellia sinensis var. sinensis (CSS)], Yunkang 10 (YK10, CSA), and Zhuyeqi (ZYQ, CSS) tea samples were produced from tea plantations of the Tea Research Institute, Hunan Academy of Agricultural Sciences, Changsha, Hunan, China. One bud with two leaves was picked from the tea plants on April 7–13, 2022, frozen in liquid nitrogen, and then vacuum freeze-dried.
Evaluator selection and training After reviewed and approved by the Biomedical Ethics Committee of Hunan Agricultural University, the group rigorously screened the 19 (11 female, 8 male) evaluators recruited for the study, including bitter taste sensitivity. Ten healthy evaluators (5 female, 5 male) were finally selected as sensory evaluators. These 10 evaluators were certified and trained as tea evaluators and signed an informed consent form before the experiment.
In order for the evaluators to recognize and quantify the intensity of bitter taste, they were trained to identify and differentiate between different qualities of oral sensations in a sensory analysis experiment. Evaluators gave informed consent to participate in the sensory testing for this study and had no known history of taste disorders and attended a three-week weekly training session. Evaluators were trained to assess the taste of aqueous solutions of the following standard taste compounds in bottled water: sucrose (4 g/L) for sweetness, EGCG (0.75 g/L) for astringency, monosodium glutamate (0.3 g/L) for freshness, and quinine hydrochloride (0.03 g/L) for bitterness. In order to minimize the intake of toxic compounds by sensory evaluators during the sensory evaluation process, sensory evaluators were required to spit out each sample after sensory evaluation was completed.
Sensory evaluation of tea infusions One g of JHKC, FDDB, ZYQ, and YK10 tea powder was weighed and added to 100 mL of boiling water at 100 °C, extracted for 5 min, filtered, and cooled to room temperature until use. Each sample was replicated three times. The sensory evaluation method for tea taste quality was described in detail as in a previous study, with minor adjustments (Yang et al., 2018). In short, the tea infusions were used for bitterness taste evaluation, which was conducted by a trained team of 10 panelists (23–38 years old, 5 males and 5 females) from Hunan Agricultural University (Changsha, China). All subjects had signed a declaration of informed consent. Panelists were requested to follow the rules including no eating, drinking, or smoking at least 60 min before the testing. They were also asked not to wear strong cosmetics that may disturb their perception of the samples. The taste strengths of 0, 0.01, 0.02, 0.03, 0.04, and 0.05 g/L quinine dihydrochloride reference solutions were assigned as bitter scores of 0, 2, 4, 6, 8, and 10, respectively.
Quantification of catechins and alkaloids Four samples were ground using a tube mill (IKA; Staufen, Germany), 20 000 rpm for 20 min. Ten milliliters of hot water (100 °C) was added to 0.1 g of tea powder and extracted at 100 °C for 5 min. Then the tea infusion was centrifuged at 4 °C, 5 000 rpm for 20 min.
The samples were quantified by Prominence LC-20A (Shimadzu Corporation, Kyoto, Japan), referring to a previous method with modification (Huang et al., 2022). The chromatographic conditions were as follows: Welch Welchrom-C18 (5 µm, 4.6 × 250 mm); UV detection at 278 nm; mobile phase A, ultrapure water; mobile phase B, N, N-Dimethylformamide: methanol: acetic acid = 39.5: 2: 1.5; flow rate, 1 mL/min; temperature, 35 °C; injection volume, 20 µL; gradient elution, 14–23 %B (0–13 min), 23–36 %B (13–28 min), 36 %B (28–31 min), 36–14 %B (31–34 min) 14 %B (34–43 min). Seven catechins and four alkaloids levels were expressed as mg/g. The samples were analyzed using three biological replicates.
Evaluation of recognition threshold of caffeine, theacrine, theophylline, and theobromine with different temperature conditions The recognition threshold of four alkaloids was determined by a trained team as mentioned above paragraph 2.2. In the first session, panelists received a brief instruction to familiarize them with the method used to determine the recognition threshold.
3-Alternative Forced Choice (3-AFC) was used to determine the recognition threshold of four alkaloids according to the International Organization for Standardization (ISO) 13301 (ISO, 2018), with some modifications. Deionized water was used as the control group, and the test solution was an aqueous solution of the test compound. Both the test solution and the control were labeled with a three-digit code.
A series of gradient concentration solutions of four alkaloids was supplied. All samples were presented to sensory evaluator for testing in a sequence from low to high concentration. Such as, 0.01, 0.02, 0.04, 0.09, 0.17, 0.35, 0.69 mmol/L theacrine aqueous solutions; 0.03, 0.07, 0.14, 0.27, 0.55, 1.10, 2.20 mmol/L caffeine aqueous solutions; 0.05, 0.10, 0.21, 0.42, 0.84, 1.67, 3.34 mmol/L theophylline aqueous solutions; and 0.05, 0.10, 0.21, 0.42, 0.83, 1.67 mmol/L theobromine aqueous solutions. Considering the influence of tea drinking temperature on bitterness threshold, each group would determine the recognition threshold at 15°C, 25°C, and 45°C of these four alkaloids.
Establishment of bitterness concentration intensity function model of four alkaloids and time-intensity curves of the bitterness of theacrine The 0.2, 0.4, 0.6, 0.8, and 1 mmol/L of theacrine, the 0.6, 1, 1.4, 1.8 and 2 mmol/L of caffeine, the 1, 2, 3, 4 and 5 mmol/L of theophylline, and the 0.75, 1, 1.25, 1.5 and 1.75 mmol/L of theobromine were prepared. The reference solution was quinine hydrochloride (refer to paragraph 2.2). Concentration-bitterness intensity curves of theacrine, caffeine, theophylline, and theobromine bitterness were developed based on sensory scores and concentration data.
To establish a time-intensity curve of the bitterness of theacrine, panelists as mentioned above paragraph 2.2 were trained to assess the taste of aqueous solutions of the following standard taste compounds in bottled water: sucrose (4 g/L) for sweetness, EGCG (0.75 g/L) for astringency, monosodium glutamate (0.3 g/L) for freshness, and quinine hydrochloride (0.03 g/L) for bitterness, with the use of nose clips by panelists.
The taste strengths of 0, 0.01, 0.02, 0.03, 0.04, and 0.05 g/L quinine dihydrochloride reference solutions were assigned as bitter scores of 0, 2, 4, 6, 8, and 10, respectively. 1 000 µmol/L of theacrine was prepared solution used as a mother solution, which was diluted into 950 µmol/L, 750 µmol/L, 550 µmol/L, 350 µmol/L and150 µmol/L solution, respectively, and left to be used. 5 mL of theacrine solution was taken orally for 20 seconds and then spat out, and the intensity of bitterness was recorded every 20 seconds until 200 seconds. After that, the mouth was rinsed three times with water, and the soda crackers were chewed and waited for 5 min before tasting the next sample. All review solutions were configured from purified water, sensory training was conducted in a separate, specific sensory evaluation room maintained at 25 ± 2 °C. To minimize the intake of toxic compounds by sensory evaluators during the sensory evaluation, who were asked to spit out the samples after the sensory evaluation of each sample was completed.
Effects of theacrine addition on the bitterness of tea soups Three g of JHKC, FDDB, ZYQ, and YK10 tea powder were weighed and added to 300 mL of boiling water at 100 °C, extracted for 5 min, filtered, and set aside. 150 µmol/L, 550 µmol/L, and 950 µmol/L theacrine solution was added to JHKC, FDDB, ZYQ, and YK10 tea soups, respectively, and the ratio of the bitter theacrine solution to the tea broth solution was 1:10. Bitter reference solution and bitter scores were used as described in paragraph 2.2, and the sensory reviewers took 3–5 mL of the solution to be tested, took it orally for 10 seconds, and then spat it out. The evaluator evaluated and scored the bitterness of the original tea broth and the mixed solution, and each sample was replicated three times.
Molecular docking simulation of interactions between four alkaloids and human bitter receptor proteins The AlphaFold protein structure databasei) was used to download 25 human bitter taste receptor models (Jumper et al., 2021; Varadi et al., 2022). Molecular structures of four alkaloids were downloaded from the Small Molecule Databaseii). In this study, a human bitter taste receptor was as a protein receptor, while a kind of four alkaloids was as a small molecule ligand, these ligands were formatted by openbabel (O’Boyle et al., 2011). Preprocessing of bitter taste receptors and small molecule ligands, such as dehydrogenation, hydrogenation, and charge calculation, were carried out by Autodock Tools 1.5.7 (Olson Laboratory, Scripps Research Institute, USA) and the number of rotatable bonds was set during semi-flexible docking of small molecule ligands, and the output format was pdbqt for backup.
Lattice docking was performed using the Autogrid subroutine in the Autodock software package, blind docking was mainly used in this study, where the docking box was wrapped around the whole protein receptor and a ligand, the output format was gpf, and its coordinate sites were shown in Table S1. The molecular docking was done by genetic algorithm (GA) to find the best binding site of protein receptor and small molecule ligand. The docking parameters were set, the number of docking was set to 50 times and other parameters were the default values of this program, the output format was dpf, and the docking result file in dlg format can be obtained after running the Autodock program. The conformations with optimal docking energies were selected and the interactions of four alkaloids with bitter taste receptor residues were analyzed by Discovery Studio 2021 (Dassault Biosystems, San Diego, CA, USA). To obtain detailed protein-ligand interactions, 2D/3D plots of the optimized docked conformations were visualized with Discovery Studio 2021.
Statistical and data analysis All data were indicated as mean ± standard deviation and analyzed with Statistical Package for Social Sciences Version 26.0 (SPSS Inc., Chicago, IL, USA). The significant differences among groups were tested by one-way analysis of variance (ANOVA) and multiple comparisons. Bitterness threshold analysis was assessed using Sigma Plot 10.0 (Systat Software Inc, San Jose, CA). The histogram was performed by OriginPro software (version 9.5.1, OriginLab Inc., USA).
Results and Discussion
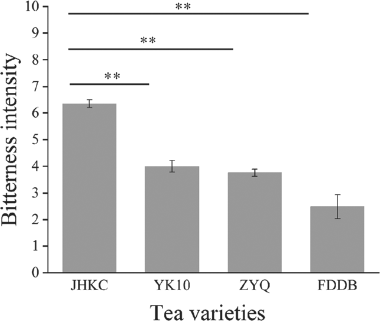
Quantification and identification of catechins and alkaloids for the bitter taste of tea infusion JHKC has a noticeably bitter taste, compared to FDDB, YK10, and ZYQ tea cultivars. Hence, these tea samples were scored for bitterness using standard sensory evaluation methods (Yang et al., 2018). The result was shown in Fig. 1. The result of the sensory review indicated that JHKC had a significantly higher (p < 0.01) bitter taste rating than YK10, ZYQ, and FDDB. It is well known that alkaloids in tea present a bitter taste (Scharbert and Hofmann, 2005; Wang et al., 2022) and catechins are also tea main components that produce bitter and astringent (Liu et al., 2023; Narukawa et al., 2010; Jiang et al., 2022). In order to further identify key catechins and alkaloids influencing the bitterness of tea infusion, 4 alkaloids and 7 catechins of all tea samples were analyzed by HPLC. The HPLC chromatograms were shown in Fig. 2, purine alkaloids and catechins in tea infusions were well separated.
The concentrations and taste active values (TAVs) of these bitter compounds were listed in Table 1. The important compounds in JHKC, YK10, ZYQ and FDDB tea samples were EGCG (40.30–94.09 mg/mL), caffeine (26.53–34.86 mg/mL), ECG (8.22–27.91 mg/mL), EGC (13.09–55.67 mg/mL), theobromine (1.60–7.23 mg/mL), theophylline (0.07–0.58 mg/mL), and GCG (0.40–7.23 mg/mL). Theacrine was detected only in JHKC, presented at a level of 14.47 ± 1.61 mg/g. The total alkaloids content of JHKC was significantly higher than FDDB, YK10, and ZYQ (p < 0.05). The total catechins content of JHKC was significantly higher than FDDB and YK10 (p < 0.05). Catechins and alkaloids were reported to have bitter taste. (Chen et al., 2022; Yan and Tong, 2023). High content of total alkaloids and total catechins in JHKC may be a reason for the higher bitter taste scores.
Table 1. Quantitative results of alkaloids and catechins among four tea samples.
| NO. |
Compounds |
Bitterness threshold (mg/L) |
Concentration (mg/g)c |
Taste active value, TAVd |
| JHKC |
YK10 |
ZYQ |
FDDB |
JHKC |
YK10 |
ZYQ |
FDDB |
| 1 |
Theobromine |
123.42b |
2.37 ± 0.0532c |
7.23 ± 0.16a |
5.69 ± 0.46b |
1.60 ± 0.05d |
0.192 |
0.586 |
0.461 |
0.130 |
| 2 |
Theophylline |
163.11b |
0.19 ± 0.0029b |
0.58 ± 0.02a |
0.19 ± 0.01b |
0.07 ± 0.01c |
0.012 |
0.035 |
0.011 |
0.004 |
| 3 |
Theacrine |
16.05b |
14.47 ± 1.61a |
n.d. |
n.d. |
n.d. |
9.015 |
- |
- |
- |
| 4 |
caffeine |
97.10a |
31.14 ± 0.79b |
30.25 ± 0.69b |
34.86 ± 2.63a |
26.53 ± 0.85c |
3.207 |
3.116 |
3.590 |
2.732 |
|
Total alkaloids |
- |
48.17 ± 1.97a |
38.06 ± 0.87b |
40.73 ± 3.07b |
28.20 ± 0.91c |
- |
- |
- |
- |
| 5 |
GC |
165.39a |
12.17 ± 0.36a |
0.76 ± 0.06c |
1.54 ± 0.09b |
0.51 ± 0.01c |
0.736 |
0.046 |
0.093 |
0.031 |
| 6 |
EGC |
499.22a |
15.03 ± 0.47c |
13.09 ± 0.19c |
55.67 ± 4.19a |
22.01 ± 0.47b |
0.301 |
0.262 |
1.115 |
0.441 |
| 7 |
C |
249.63a |
15.52 ± 0.40a |
1.93 ± 0.06b |
1.57 ± 0.10b |
0.57 ± 0.01c |
0.622 |
0.077 |
0.063 |
0.023 |
| 8 |
EC |
156.74a |
6.62 ± 0.93a |
5.19 ± 0.09b |
10.35 ± 0.77b |
6.06 ± 0.24b |
0.422 |
0.332 |
0.660 |
0.387 |
| 9 |
EGCG |
87.09a |
63.12 ± 1.87a |
65.30 ± 1.89a |
57.72 ± 2.13b |
40.30 ± 0.84c |
7.248 |
7.498 |
6.634 |
4.627 |
| 10 |
GCG |
178.76a |
7.23 ± 0.18a |
1.16 ± 0.11b |
0.81 ± 0.13c |
0.40 ± 0.03d |
0.405 |
0.065 |
0.045 |
0.022 |
| 11 |
ECG |
79.63a |
27.91 ± 0.94a |
19.87 ± 0.69b |
15.37 ± 0.95c |
8.22 ± 0.11d |
3.505 |
2.496 |
1.931 |
1.033 |
|
Total catechins |
- |
147.60 ± 3.13a |
107.31 ± 2.98c |
143.05 ± 11.61a |
78.06 ± 1.66d |
- |
- |
- |
- |
b Shows the actual measured threshold value.
c Different letters indicate significant differences among four tea groups (JHKC, YK10, and ZYQ and FDDB) according to one-way ANOVA (Tukey’s multiple comparison) (p < 0.05).
n.d.= not detected.
d TAV means the ratio of the concentration of a taste active compound to its taste threshold, also known as the Dose over threshold, DoT.
And five compounds with TAVs > 1 were found, they were followed by theacrine (9.02), EGCG (4.63–7.50), caffeine (2.73–3.60), and ECG (1.03–3.50) and EGC (1.12). In addition, caffeine, EGCG, and ECG were the substances with TAV > 1 in all four tea samples. Theacrine with TAV > 1 was presented only in JHKC, and EGC with TAV > 1 was presented only in ZYQ. The TAV depends on the intensity and concentration of taste of a particular compound. The TAV is greater than 1 means that the compound contributes significantly to the taste of tea infusion, generally, compounds with higher TAV are considered to be the major contributors to the overall taste (Chen et al., 2022). Therefore, these compounds were considered significant contributors to the bitter taste of fresh tea leaves.
Correlation analysis between concentrations of catechins, alkaloids, and intensity of bitterness in tea infusions was analyzed (Table 2). The results showed that concentrations of total alkaloids, theacrine, GC, C, GCG, and ECG were significantly positively correlated with bitterness intensity (p < 0.01).
Table 2. Correlation coefficients between concentrations of alkaloids, catechins, and the intensity of bitterness in tea infusions.
|
Bitterness intensity |
| Total alkaloids |
0.905** |
| Theobromine |
0.057 |
| Theophylline |
0.133 |
| Theacrine |
0.897** |
| Caffeine |
0.338 |
| Total catechins |
0.494 |
| GC |
0.914** |
| EGC |
0.279 |
| C |
0.928** |
| EC |
0.023 |
| EGCG |
0.232 |
| GCG |
0.932** |
| ECG |
0.954** |
Note: **means p < 0.01.
Based on above these correlations and TAVs of each component to the bitter taste. We considered that theacrine, caffeine, GC, C, GCG, ECG, and EGCG were found to be possible contributors to the bitter taste of tea infusions.
Bitterness thresholds of theacrine, caffeine, theophylline, and theobromine at different temperature conditions Bitterness thresholds of theacrine, caffeine, theophylline, and theobromine were determined at different temperatures, the results showed that theacrine, caffeine, and theophylline had the highest thresholds at 25 °C and the lowest thresholds at 15 °C, while the threshold of theobromine was gradually increased with the increase of temperature (15 °C, 25 °C, 45 °C) (Fig. 3, Table S2). It is generally accepted that the thresholds of taste substances are affected by temperature; cold temperatures inhibit thresholds of bitter, sweet, and umami in humans, while high temperatures enhance them (Ma et al., 2023). Yahiro et al. (2008) investigated that the higher the temperature the weaker the bitterness of quinine hydrochloride, which was similar to the property of theobromine we studied. However, the influence mode of temperature on threshold were not applicable to specific compounds (Talavera et al., 2007), such as the properties of theacrine, caffeine, and theophylline in our study. Our study showed that theacrine had the highest threshold at 25 °C (71.5 µmol/L), the second highest at 45 °C, and the lowest threshold at 15 °C. Shi et al. (2022) studied the thresholds of theacrine at 25 °C and 45 °C, and found that the bitterness threshold of theacrine at 45 °C was higher than 25 °C, which were not quite the same as our findings. The reason for different outcomes may be that the perception of bitterness varies greatly among individuals and is influenced by gender, age, taste preference, and daily life (Chrysanthou et al., 2016).

Concentration-taste intensity curves of four alkaloids and time-intensity curves of the bitterness of theacrine Concentration-bitterness intensity curves were established for four alkaloids (Fig. 4). In this study, we performed sensory evaluation of theacrine 0–1 mmol/L, caffeine 0–2 mmol/L, theophylline 0–5 mmol/L and theobromine 0–1.75 mmol/L solutions at 25 °C. The concentration-bitterness intensity functions curves of the four alkaloids were well fitted to the cubic function (Table S3). The results showed that the cubic function model was reliable and accurate. These alkaloids were only bitterness, and at 1 mmol/L, the bitterness scores followed the order theacrine > theophylline > theobromine > caffeine. This was different from the bitter threshold ordering at 25 °C: theacrine (71.50 ± 11.64 µmol/L) < caffeine (578.86 ± 39.91 µmol/L) < theobromine (685.08 ± 49.59 µmol/L) < theophylline (746.50 ± 89.19 µmol/L) (Table S2).

The time-intensity curve of the bitterness of theacrine was drawn (Fig. 5). In different concentrations of theacrine solution in the human oral cavity for 20 seconds, the bitterness feeling was the strongest, which gradually decreased. A low concentration of theacrine solution had a shorter bitterness feeling in the human oral cavity and disappeared in about 120 seconds. With the increase of concentration of theacrine solution, the bitter feeling of theacrine solution in the human oral cavity was gradually prolonged, and the disappearance time increased with the concentration gradient. The bitter feeling of the theacrine solution (1 000 µmol/L) was maintained for 200 s not gone yet.

Evaluation of theacrine addition on the bitterness of tea soups To clarify the effect of theacrine on the bitterness of tea soups, different amounts of theacrine were added to JHKC, FDDB, ZYQ, and YK10 tea soups. As shown in Fig. 6, 150 µmol/L, 550 µmol/L, and 950µmol/L of theacrine concentrations were correspond to bitterness sensory review scores of 2.34, 5.9, and 7.78, respectively. With the increase of theacrine concentration, the bitterness of JHKC, FDDB, ZYQ, and YK10 tea soups also increased. Comparing with tea (not added theacrine), a low concentration of 150 µmol/L of theacrine had no significant effect (p > 0.05) on the bitterness of FDDB and YK10 tea soups, while a higher concentration of 550 µmol/L and 950 µmol/L of theacrine had a significant effect (p < 0.05). Notably, a low concentration of 150 µmol/L theacrine solution had a significant effect (p < 0.05) on the bitterness of JHKC and ZYQ tea soups. Our results implied that theacrine may be responsible for the strong bitter taste of JHKC tea.

At the same time, it could be found that the scores and contribution of theacrine bitterness in different tea varieties of JHKC, FDDB, ZYQ, and YK10 were different, which might be related to the complexity of the tea soup system.
Molecular docking simulation of four alkaloids with human bitter taste receptor proteins Bitterness perception is transduced by type 2 taste receptors (TAS2R), which are categorized as a family of T-class GPCRs (Xu et al., 2022). Humans have 25 bitter taste receptors that recognize thousands of bitter substances (Grau-Bové et al., 2022). Molecular docking is an advantageous tool to visualize the binding sites of proteins and small molecules. In this study, blind docking was used to perform molecular simulations of four alkaloids with 25 bitter taste receptors. According to the best binding energy (affinity) ranking of each docking, when the binding energy is less than 0, it indicates that the ligand can spontaneously bind to the receptor; it is generally accepted that when the conformations of the ligand and receptor are stable, the lower the energy, the greater the likelihood of binding (De León et al., 2021). As shown in Fig. 7, the red color indicated lower binding affinity and better docking conformation, so the docking effect of TAS2R14 was found to be superior to that of other bitter taste receptors. It is worth noting that docking simulation is just a simulation result. TAS2R46 was the first to resolve the structure of the bitter taste receptor to date (Xu et al., 2022), and we downloaded model of TAS2R46 from the Protein Data Bank (PDB)iv) with PDB code 7XP6 (Xu et al., 2022), docking simulations were done, the binding affinity of TAS2R46 and caffeine was −3.5 kcal/mol. The result showed that the binding affinity value of the known structural receptor TAS2R46 (−3.5 kcal/mol) was similar to our modeled result (−3.41 kcal/mol) on docking with caffeine and TAS2R46 created by alphafold2i) under the same conditions. Therefore, caffeine was valid in docking with the model created by alphafold2i) in our study, similarly, docking simulations of other three alkaloids were also validated. The previous investigation showed that caffeine activated bitter taste receptors including TAS2R7, TAS2R10, TAS2R14, TAS2R43 and TAS2R46 (Meyerhof et al., 2010). For theobromine, no data regarding its role as TAS2R agonist have been available (Liszt et al., 2018). These results were consistent with our findings. Scores of the docking results of TAS2R14 with the four alkaloids were listed Table S4. Affinity ranking with the bitter taste receptor TAS2R14 was, theacrine (−6.1 kcal/mol) > theophylline (−5.6 kcal/mol) > caffeine (−5.5 kcal/mol) > theobromine (−5.4 kcal/mol). However, sensory evaluation bitterness scores followed the order at 1 mmol/L, theacrine > theophylline > theobromine > caffeine. That could be mainly due to bitter score is a comprehensive impact of interaction between 25 humans bitter taste receptors and alkaloids, and TAS2R14 was just one of these receptors with the most stable docking conformation.

On the other hand, it was found that the binding of different alkaloids to the TAS2R14 was different after visualization by Discovery Studio 2021 software. The binding sites of the TAS2R14 structure with four alkaloids were shown in Fig. 8, and 3D and 2D plots were shown respectively. The interaction between TAS2R14 and caffeine (Fig. 8A), TAS2R14 amino acid residue F175ECL2 formed a π-π interaction with caffeine. In the interaction between TAS2R14 and theacrine (Fig. 8B), TAS2R14 two amino acid residues (Q2667.38 and S652.60) formed hydrogen bonds with theacrine, meanwhile residue W893.32 formed a π-π interaction with theacrine. In the interaction of TAS2R14 with theobromine (Fig. 8C), TAS2R14 amino acid residue Y159ECL2 formed a π-π interaction with theobromine. In the interaction of TAS2R14 with theophylline (Fig. 8D), TAS2R14 amino acid residue S155ECL2 formed a hydrogen bond with theophylline, and amino acid residue Y159ECL2 formed a π-π interaction with theophylline. Based on these above investigations, it can be identified that there were two main interaction forces between TAS2R14 and these four alkaloids, mainly hydrogen bonding and π-π interaction.

Conclusions
Within our knowledge, this study reported simultaneous comparison of bitterness thresholds of caffeine, theacrine, theophylline, and theobromine at different temperature conditions (15 °C, 25 °C, and 45 °C), and revealed their binding mechanisms with the bitter taste receptor TAS2R14 screened out of 25 human receptors for the first time. In this study, thresholds of caffeine, theobromine, theophylline, and theacrine at 15 °C were significantly lower than those at 25 °C and 45 °C. Concentration-taste intensity curves results displayed that the bitterness scores followed the order, theacrine > theophylline > theobromine > caffeine at 1 mmol/L and 25 °C. Addition experiments showed that theacrine contributed more to the bitterness of JHKC and ZYQ than FDDB and YK10. Docking results showed that theacrine had the best binding affinity to TAS2R14 compared to other three alkaloids (theophylline, theobromine, and caffeine), which was −6.1 kcal/mol. In the interaction between TAS2R14 and theacrine, Q2667.38 and S652.60 amino acid residues were mainly hydrogen-bonded to the theacrine, and amino acid residue W893.32 interact with theacrine through π-π interaction. This study provides profiles of the bitterness of alkaloids and the interaction mechanisms between TAS2R14 and alkaloids, and will enrich the chemical theory of tea taste.
Acknowledgements This work was supported by the Hunan Agricultural Science and Technology Innovation Funds of China (2022CX33), the Key Laboratory Open Funds of Ministry of Agriculture and Rural Affairs of China (TZDZW202204) and the Postgraduate Scientific Research Innovation Project of Hunan Agriculture University (2023XC100).
CRediT authorship contribution statement Ruixue Xing: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft. Tianchen Cai: Methodology, Formal analysis, Investigation, Writing - Original Draft. Wenbao Jia: Formal analysis. Qianting Ma: Formal analysis. Xia Yin: Formal analysis. Yangbo Xiao: Investigation. Shujuan Liu: Resources. Shuguang Zhang: Formal analysis, Investigation. Yong Lin: Conceptualization, Formal analysis, Writing - Review & Editing, Funding acquisition. Wenliang Wu: Conceptualization, Writing - Review & Editing, Project administration, Funding acquisition.
Conflict of interest There are no conflicts of interest to declare.
Appendix The appendix is supporting information.
Table S1.
Molecular docking parameters of four alkaloids with 25 human bitter taste receptors
|
Grid box coordinates |
| Receptor id |
Center(X/Y/Z) |
Size(X/Y/Z) |
Spacing# |
| TAS2R1 |
−0.99 X 0.964 X 5.109 |
126 X 90 X 112 |
0.614 |
| TAS2R3 |
1.974 X 13.413 X −1.231 |
104 X 126 X 112 |
0.642 |
| TAS2R4 |
−7.177X 1.968 X −8.685 |
126 X 98 X 126 |
0.575 |
| TAS2R5 |
4.234 X 6.325 X 0.756 |
126 X 84 X 110 |
0.581 |
| TAS2R7 |
1.410 X −1.770 X 3.450 |
126 X 90 X 126 |
0.586 |
| TAS2R8 |
−9.689 X 5.670 X −2.084 |
126 X 90 X 126 |
0.575 |
| TAS2R9 |
1.200 X 6.247 X −2.042 |
126 X 74 X 104 |
0.653 |
| TAS2R10 |
−6.735 X 3.629 X −1.103 |
126 X 108 X 120 |
0.553 |
| TAS2R13 |
−3.615 X 1.936 X −0.418 |
126 X 98 X 118 |
0.542 |
| TAS2R14 |
0.414 X −14.113 X 8.786 |
116 X 126 X 126 |
0.592 |
| TAS2R16 |
−2.212 X 2.691 X −3.573 |
126 X 110 X 126 |
0.525 |
| TAS2R19 |
2.010 X 2.980 X 2.103 |
126 X 100 X 126 |
0.553 |
| TAS2R20 |
2.576 X −2.528 X 0.512 |
126 X 112 X 126 |
0.547 |
| TAS2R30 |
3.163 X −10.330 X 2.038 |
126 X 126 X 126 |
0.558 |
| TAS2R31 |
−5.413 X 0.436 X −0.003 |
98 X 96 X 126 |
0.631 |
| TAS2R38 |
−1.713 X 2.646 X 1.800 |
126 X 88 X 106 |
0.675 |
| TAS2R39 |
−0.241 X 10.602 X −18.943 |
126 X 88 X 126 |
0.814 |
| TAS2R40 |
1.439 X 9.208 X −7.576 |
94 X 94 X 126 |
0.686 |
| TAS2R41 |
−3.854 X 5.013 X −4.446 |
126 X 96 X 104 |
0.619 |
| TAS2R42 |
−7.244 X 9.725 X −2.425 |
124 X 118 X 112 |
0.619 |
| TAS2R43 |
1.715 X 3.805 X 1.337 |
126 X 126 X 126 |
0.525 |
| TAS2R45 |
1.170 X 0.496 X 0.894 |
126 X 118 X 126 |
0.547 |
| TAS2R46 |
−3.783 X 0.137 X −1.111 |
112 X 102 X 126 |
0.642 |
| TAS2R50 |
−9.577 X 3.492 X −3.648 |
118 X 114 X 126 |
0.519 |
| TAS2R60 |
1.474 X 13.439 X 3.704 |
126 X 126 X 126 |
0.603 |
Note: # indicates the size of the docking box
Table S2.
Bitterness thresholds of theacrine, caffeine, theophylline and theobromine at 15°C, 25°C and 45°C
|
15°C [µmol/L] |
25°C [µmol/L] |
45°C [µmol/L] |
| Theacrine |
64.35±3.69a |
71.50±11.64b |
66.15±26.99c |
| Caffeine |
416.84±52.21a |
578.86±39.91b |
505.56±61.16c |
| Theophylline |
596.80±78.08 |
746.50±89.19b |
650.93±9.63c |
| Theobromine |
568.91±91.01a |
685.08±49.59b |
780.44±11.17c |
Note: Different letters indicate significant differences among three temperatures (15°C, 25°C, and 45°C) according to one-way ANOVA (Tukey’s multiple comparison) (p < 0.05).
Table S3.
Concentration-bitterness intensity curves function of theacrine, caffeine, and theophylline and theobromine
| compounds |
Cubic functions# |
R2 |
| Theacrine |
Y=26.117X3−34.729X2+17.746X−0.04 |
0.945 |
| Caffeine |
Y=0.378X3−1.326X2+1.841X−0.004 |
0.818 |
| Theophylline |
Y=0.026X3−0.181X2+1.426X−0.005 |
0.976 |
| Theobromine |
Y=1.045X3−0.750X2+0.476X−0.046 |
0.874 |
Note: # Y is the bitterness intensity, and X is the concentration of each component (mmol/L).
Table S4.
Binding affinity results of TAS2R14 with four alkaloids
|
Caffeine |
Theacrine |
Theobromine |
Theophylline |
| Binding affinity (kcal/mol) |
−5.5 |
−6.1 |
−5.4 |
−5.6 |
References
- Bayer, S., Mayer, A. I., Borgonovo, G., Morini, G., Di Pizio, A., and Bassoli, A. (2021). Chemoinformatics View on Bitter Taste Receptor Agonists in Food. Journal of agricultural and food chemistry., 69, 13916–13924. doi: 10.1021/acs.jafc.1c05057
- Chen, Y., Zhang, Y., Chen, G., Yin, J., Chen, J., Wang, F., and Xu, Y. (2022). Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. NPJ Science of Food., 6, 8. doi: 10.1038/s41538-022-00124-8
- Chrysanthou, A., Pouliou, E., Kyriakoudi, A., and Tsimidou, M. Z. (2016). Sensory threshold studies of picrocrocin, the major bitter compound of saffron. Journal of Food Science., 81, S189–S198. doi: 10.1111/1750-3841.13152
- De León, G., Fröhlich, E., and Salar-Behzadi, S. (2021). Bitter taste in silico: A review on virtual ligand screening and characterization methods for TAS2R-bitterant interactions. International Journal of Pharmaceutics., 600, 120486. doi: 10.1016/j.ijpharm.2021.120486
- Deng, S., Zhang, G., Olayemi Aluko, O., Mo, Z., Mao, J., Zhang, H., Liu, X., Ma, M., Wang, Q., and Liu, H. (2022). Bitter and astringent substances in green tea: composition, human perception mechanisms, evaluation methods and factors influencing their formation. Food Research International., 157, 111262. doi: 10.1016/j.foodres.2022.111262
- Grau-Bové, C., Grau-Bové, X., Terra, X., Garcia-Vallve, S., Rodríguez-Gallego, E., Beltran-Debón, R., Blay, M. T., Ardévol, A., and Pinent, M. (2022). Functional and genomic comparative study of the bitter taste receptor family TAS2R: Insight into the role of human TAS2R5. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology., 36, e22175. doi: 10.1096/fj.202101128RR
- Huang, F., Xiong, L., Li, Y., Liu, Z., Huang, J., and Li, J. (2022). Differential gene expression involved in catechin synthesis of tea leaves (Camellia sinensis) during spring and summer. Food Science., 43, 80–87.
- Iso. (2018). Sensory analysis - Methodology - General guidance for measuring odour, flavour and taste detection thresholds by a three - alternative forced - choice (3-AFC) procedure.
- Jiang, Z., Han, Z., Wen, M., Ho, C., Wu, Y., Wang, Y., Xu, N., Xie, Z., Zhang, J., Zhang, L., and Wan, X. (2022). Comprehensive comparison on the chemical metabolites and taste evaluation of tea after roasting using untargeted and pseudotargeted metabolomics. Food Science and Human Wellness., 11, 606–617. doi: 10.1016/j.fshw.2021.12.017
- Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Žídek, A., Potapenko, A., Bridgland, A., Meyer, C., Kohl, S. A. A., Ballard, A. J., Cowie, A., Romera-Paredes, B., Nikolov, S., Jain, R., Adler, J., Back, T., Petersen, S., Reiman, D., Clancy, E., Zielinski, M., Steinegger, M., Pacholska, M., Berghammer, T., Bodenstein, S., Silver, D., Vinyals, O., Senior, A. W., Kavukcuoglu, K., Kohli, P., and Hassabis, D. (2021). Highly accurate protein structure prediction with AlphaFold. Nature., 596, 583–589. doi: 10.1038/s41586-021-03819-2
- Liszt, K. I., Hans, J., Ley, J. P., Köck, E., and Somoza, V. (2018). Characterization of Bitter Compounds via Modulation of Proton Secretion in Human Gastric Parietal Cells in Culture. Journal of Agricultural and Food Chemistry., 66, 2295–2300. doi: 10.1021/acs.jafc.7b01051
- Liu, Z., Ran, Q., Li, Q., Yang, T., Dai, Y., Zhang, T., Fang, S., Pan, K., and Long, L. (2023). Interaction between major catechins and umami amino acids in green tea based on electronic tongue technology. Journal of Food Science., 88, 2339–2352. doi: 10.1111/1750-3841.16543
- Ma, Z., Paudel, U., and Foskett, J. K. (2023). Effects of temperature on action potentials and ion conductances in type II taste-bud cells. American Journal of Physiology. Cell Physiology., 325, C155–C171. doi: 10.1152/ajpcell.00413.2022
- Meyerhof, W., Batram, C., Kuhn, C., Brockhoff, A., Chudoba, E., Bufe, B., Appendino, G., and Behrens, M. (2010). The molecular receptive ranges of human TAS2R bitter taste receptors. Chemical Senses., 35, 157–170. doi: 10.1093/chemse/bjp092
- Narukawa, M., Kimata, H., Noga, C., and Watanabe, T. (2010). Taste characterisation of green tea catechins. International Journal of Food Science & Technology., 45, 1579–1585. doi: 10.1111/j.1365-2621.2010.02304.x
- O’Boyle, N. M., Banck, M., James, C. A., Morley, C., Vandermeersch, T., and Hutchison, G. R. (2011). Open Babel: An open chemical toolbox. Journal of Cheminformatics., 3, 33. doi: 10.1186/1758-2946-3-33
- Santos, L. H. S., Ferreira, R. S., and Caffarena, E. R. (2019). Integrating molecular docking and molecular dynamics simulations. Methods in Molecular Biology., 2053, 13–34. doi: 10.1007/978-1-4939-9752-7_2
- Scharbert, S., and Hofmann, T. (2005). Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. Journal of Agricultural and Food Chemistry., 53, 5377–5384. doi: 10.1021/jf050294d
- Shi, Y., Zhang, S., Sun, K., Wang, X., Jiang, J., Luo, L., and Zeng, L. (2022). Characterization of bitter taste theacrine in Pu-erh tea. Journal of Food Composition and Analysis., 106, 104331. doi: 10.1016/j.jfca.2021.104331
- Talavera, K., Ninomiya, Y., Winkel, C., Voets, T., and Nilius, B. (2007). Influence of temperature on taste perception. Cellular and Molecular Life Sciences., 64, 377–381. doi: 10.1007/s00018-006-6384-0
- Varadi, M., Anyango, S., Deshpande, M., Nair, S., Natassia, C., Yordanova, G., Yuan, D., Stroe, O., Wood, G., Laydon, A., Židek, A., Green, T., Tunyasuvunakool, K., Petersen, S., Jumper, J., Clancy, E., Green, R., Vora, A., Lutfi, M., Figurnov, M., Cowie, A., Hobbs, N., Kohli, P., Kleywegt, G., Birney, E., Hassabis, D., and Velankar, S. (2022). AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Research., 50, D439–D444. doi: 10.1093/nar/gkab1061
- Wang, S., Chen, J., Ma, J., Jin, J., Chen, L., and Yao, M. (2020). Novel insight into theacrine metabolism revealed by transcriptome analysis in bitter tea (Kucha, Camellia sinensis). Scientific Reports., 10, 6286. doi: 10.1038/s41598-020-62859-2
- Wang, Y., Li, C., Lin, J., Sun, Y., Wei, S., and Wu, L. (2022). The impact of different withering approaches on the metabolism of flavor compounds in Oolong Tea leaves. Foods., 11, 3601. doi: 10.3390/foods11223601
- Wu, T., Zou, R., Pu, D., Lan, Z., and Zhao, B. (2021). Non-targeted and targeted metabolomics profiling of tea plants (Camellia sinensis) in response to its intercropping with Chinese chestnut. BMC plant biology., 21, 55. doi: 10.1186/s12870-021-02841-w
- Wu, W., Lu, M., Peng, J., Lv, H., Shi, J., Zhang, S., Liu, Z., Duan, J., Chen, D., Dai, W., and Lin, Z. (2022). Nontargeted and targeted metabolomics analysis provides novel insight into nonvolatile metabolites in Jianghua Kucha tea germplasm (Camellia sinensis var. Assamica cv. Jianghua). Food Chemistry: X., 13, 100270. doi: 10.1016/j.fochx.2022.100270
- Xu, W., Wu, L., Liu, S., Liu, X., Cao, X., Zhou, C., Zhang, J., Fu, Y., Guo, Y., Wu, Y., Tan, Q., Wang, L., Liu, J., Jiang, L., Fan, Z., Pei, Y., Yu, J., Cheng, J., Zhao, S., Hao, X., and Hua, T. (2022). Structural basis for strychnine activation of human bitter taste receptor TAS2R46. Science., 377, 1298–1304. doi: 10.1126/science.abo1633
- Yahiro, Miki., Ezaki, Shu., Takamatsu, Ryuji., and Toko Kiyoshi. (2008). Temperature dependence of bitter taste and output characteristics of taste sensor. Sensors and Materials., 20, 161–169.
- Yang, C., Hu, Z., Lu, M., Li, P., Tan, J., Chen, M., Lv, H., Zhu, Y., Zhang, Y., Guo, L., Peng, Q., Dai, W., and Lin, Z. (2018). Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Research International., 106, 909–919. doi: 10.1016/j.foodres.2018.01.069
- Yan, J., and Tong, H. (2023). An overview of bitter compounds in foodstuffs: Classifications, evaluation methods for sensory contribution, separation and identification techniques, and mechanism of bitter taste transduction. Comprehensive reviews in Food Science and Food Safety., 22, 187–232. doi: 10.1111/1541-4337.13067
- Ye, C., Lin, Y., Su, J., Song, X., and Zhang, H. (1999). Purine alkaloids in Camellia assamica var. kucha Chang et Wang. Europe PMC., 38, 82–86.
- i) https://alphafold.ebi.ac.uk/ (Dec. 18, 2023)
- ii) https://pubchem.ncbi.nlm.nih.gov/ (Jun. 8, 2023)
- iii) https://bitterdb.agri.huji.ac.il/dbbitter.php (Sep. 10, 20 23)
- iv) https://www.rcsb.org (Jan. 17, 2024)









