2013 Volume 30 Pages 125-143
2013 Volume 30 Pages 125-143
Both the FDA (U.S. Food and Drug Administration) and ICH (International Conference on Harmonisation) have urged the incorporation of Quality by Design (QbD)1) into the manufacture of pharmaceutical products2).
The performance of many pharmaceutical manufacturing processes and the performance of some pharmaceutical products requires a knowledge of powder properties. Under the principles of QbD it is possible to adjust processes to account for variations in powder properties. These adjustments, in turn, require knowledge of the relation between powder properties and manufacturing performance. This relation between powder properties and performance is often not well understood; thus, the required information is not collected.
In this paper, particle-particle and particle-surface interactions are considered to be a source of product variability. As particle size effects are intertwined with particle adhesion effects this topic is also considered. From the discussion below, it can be seen that the surface chemistry of particles can vary due to mechanical treatment, crystallization solvent, and surface contamination. Variations in surface chemistry affect interparticle adhesion and thus may lead to process or product performance changes. Issues concerning the role of interparticle adhesion that are related to tableting and dry powder inhalers are discussed in some detail.
It is clear that a deeper understanding of the powder state and the establishment of appropriate analytical tools will be required to fully implement QbD. Improvements in particle sizing technologies, improvements powder sampling procedures and measurements of particle surface properties will be required. It is hoped that this paper will stimulate thought on this issue.
Many pharmaceutical products use powders in their manufacture. Tablets, capsules and dry powder inhalation formulations, for example, require knowledge of powder properties in order to optimize their pharmaceutical performance and manufacture. The optimal design of dry powder inhalers is, in particular, challenging.
Both the FDA (U.S. Food and Drug Administration) and ICH (International Conference on Harmonisation) have urged the incorporation of Quality by Design (QbD)1) into the manufacture of pharmaceutical products2).
The use of QbD principles offers additional flexibility in the manufacture of products but requires a rather detailed knowledge of material and process properties and their impact on product performance. Traditionally manufacture of drugs has assumed material properties are constant (or held to exacting specifications). Manufacturing processes are similarly held to specific specifications. It is assumed if process and material properties are held constant then product quality will be the same. Traditional manufacturing is performed in batches with the quality of each batch assessed at the end of the manufacturing process. Traditional manufacturing has focused greatly on the chemical purity of materials. Certainly, chemical purity is essential to pharmaceutical production but does not, in itself, assure adequate product performance. Quality by design offers additional flexibility by allowing for variable material properties, variable processing conditions and continuous manufacture. Quality by design does, however, require a detailed scientific knowledge of the important quantities affecting material properties and their impact on manufacturing conditions. Some factors which limit the full implementation of quality by design in industry include limitations in current analytical technology and a detailed understanding of the powder state.
In this paper, current limitations in analytical technologies that affect understanding of pharmaceutical product performance are discussed. Some particle properties which may affect product or process performance are also discussed.
Physical chemistry generally classifies materials as being solids, liquids or gases. Traditional analytical methods are usually adequate to provide characterization of the intrinsic properties of such materials (purity, density, crystalline state, etc.). Pharmaceutical materials are generally well characterized with regards to such properties as various compendial tests address such issues (e.g. chemical purity, etc.). From a theoretical perspective, statistical mechanics, at least in principle, can account for the intrinsic properties of solids, liquids and gases based on fundamental knowledge of intermolecular forces and molecular size and shape.
Powders are composed of solid particles with spaces between the particles that contain a gas (most often air). Materials are generally considered to be powders when particles are less than 40μm. If the particles are of greater size then the material is usually considered to be granular.
The fundamental forces acting on particles in a powder are adhesion, friction and gravitational forces. For granular materials, gravitational forces dominate whereas for powders adhesion and frictional forces dominate. Adhesion and frictional forces are of great importance for fine powders and less so for granular materials. These forces depend on particle surface chemistry and particle mechanical properties.
Typical powders are composed of particles that vary in shape and size. Unlike solids and liquids powders are not characterized by a single density (at fixed T and P). The powder bulk density can be modified by various processes acting on the powders and can be affected by past history. Powders, in contrast to liquids and gases, are nonergodic. Following a single particle over a long period of time will not allow one to assess the overall behavior of the powder as it would for a molecule in a liquid or gas. Simulation of non-ergodic systems is clearly more difficult and may be beyond current computer capabilities. An “accurate” description of particle size is part of the description of a powder.
As the physics of various shaped and sized particles interacting in a gaseous medium is very complex, a detailed fundamental knowledge of powder behavior lags considerably behind that for ordinary matter.
Because, full implementation of quality by design for many pharmaceuticals depends to a substantial degree on a fundamental knowledge of properties of powders, the full implementation of quality by design has not yet been achieved. Full implementation would enhance the ability to trouble shoot problems that occur during development or manufacture.
In this paper, several areas that limit a more complete understanding of powder behavior are identified and discussed. The properties discussed here are generally related to interparticle interactions. It is hoped that this paper will inspire others to make advances in at least some of the identified areas. It is also important to recognize the current limits of current science and technology when addressing product development challenges.
Particle size affects the behavior of powders in a number of ways. Some of these ways are discussed below.
The mixing of materials is an important operation3). Mixtures containing particles of significantly different sizes segregate by size during transport. The ability to obtain uniform mixtures of materials is thus affected by the relative sizes of the components. Hence, it is generally desirable to have the sizes of the component materials be similar. Particle size and interparticle adhesion affect mixing performance.
The flowability of powders is affected by particle size3–5). In the case of small particle sizes, where the friction and adhesion forces between particles are important, flow decreases with decreasing particle size. The opposite is true when gravitational forces dominate. Jallo and co-workers, in particular, have considered the relation between interparticle adhesion and a measure of flow-ability5).
Low dosage tablets require that drug material be distributed amongst a significant number of particles. If the average number of particles per unit dose is too small then content uniformity problems will result. The normal rules of statistical selection are responsible for the variation in content. The frequent use of volume weighted distributions in the pharmaceutical industry deemphasizes the importance of particle numbers in sampling.
Granulation times and the amount of liquid used for granulation are affected by surface area and hence the particle size6). The packing density of powders is affected by the particle size and particle size distribution7). The tabletting properties of the powder are governed, in part, by the ability of the powder to densify under pressure. Both packing density and particle size thus influence the tabletting process. Granulation may affect the nature of the particle surfaces by promoting contamination of surfaces thus changing the strength of interparticle adhesion.
Products for pulmonary delivery require aerosolized particles in the 2–5μm range. Interparticle adhesion and adhesion to container walls is also influenced, in part, by particle size8). The choice of formulation materials affects the ability to deliver particles of the desired respirable size.
The dissolution rate of materials9) depends on surface area. Powders consisting of small particles have greater specific surface areas. Control of particle size may be of particular importance to those developing formulations from poorly soluble compounds. Processing times for the dissolution of materials incorporated into liquid formulations may also be important.
Particle shape also affects powder behavior in the above listed areas. The effects of particle shape are less well studied are not considered in detail in this paper.
3.2 Particle size analysisThe measurement of particle size and shape is difficult for both technical and philosophical reasons. Allen10) and Etzler11) have previously provided more complete discussions of particle size analysis. Iococca12), Rawle13) and Moschwitzer14) have more recently discussed particle size analysis.
For spherical particles the particle size can be unambiguously be assigned as the particle diameter or radius. It is also possible to assign unambiguous sizes to other regularly shaped particles such as cubes but it is not usual to do so. In the case of cubes, edge length could be used as a measure of size.
For particles of irregular shape it is not possible to assign sizes unambiguously. In this instance, it is typical to express particle size as equivalent spherical diameters. Any measurement of length, area, volume or mass may be used to specify the equivalent diameter. Equivalent spherical diameters for irregularly shaped particles determined in different ways are not numerically equal. For particles that are compact but nonetheless not spherical (e.g. icosahedra) the various measures may give results that do not differ greatly from each other. Needles, disks and flakes are, however, particularly problematic. In this instance, various measurements of size may greatly differ. For this reason it is necessary to be clear with regard to the method used for particle size analysis. It is also necessary to consider the appropriateness of a particular particle size method to the problem at hand.
Particle size data can be presented in a number of ways10). Data can be presented as either a cumulative, differential, or as a histogram distribution. A histogram plot displays the fraction in a given size interval. A cumulative plot shows the fraction of particles having a size less than a certain value. This plot often has a characteristic sigmoid shape. A differential plot is the first derivative of the cumulative plot. The differential and histogram plots may look similar and should be not confused with one another. When data are presented the type of plot used should be made clear.
Particle size distributions may be based on number, volume or surface area. Only the number distribution represents a census of the particles in the system. The volume or mass distribution reflects the fraction of mass which is composed of particles of a specified size. Similarly, the surface area distribution reflects the fraction of surface area composed of particles of a specified size. Each type of plot is useful. For a given population of particles the various plots may look very different from one another. It is, thus, important to make clear which type of distribution is presented. Many publications in the pharmaceutical literature do not make this distinction clear. It is infrequent that investigators make clear the type of distribution being presented as well as the limitations of the method used. It is even more infrequent that the results of several methods are compared to provide a more complete understanding of the material at hand.
Materials composed of particles of different size will segregate by size during transport and handling. Care must be taken to ensure that size segregation does not occur during the process of providing samples to the laboratory. Laboratories receiving improperly sampled materials will report incorrect results10),15–16).
Instruments used to determine particle size distributions usually have quite high precision. If identical samples are presented to the instrument, then nearly identical particle size distributions will result. It is reasonable for the analyst to conclude that sampling errors in the laboratory have occurred if the particle size distributions of replicate samples significantly differ. It is possible to remove quite small and still representative samples from very large containers, if proper procedures are followed. The spinning riffler is considered to be the gold standard for sampling devices17).
The authors have not found that good sampling practices are consistently applied in the pharmaceutical industry. Conclusions based on poor sampling may delay product development or prevent the proper identification of problems that are, in fact, not related to particle size. For instance, two samples apparently exhibiting different particle sizes may do so because poor sampling practices have been employed. Manufacturing problems thus are mistakenly attributed to particle size when another variable is indeed causing the problem. Sampling issues can be particularly problematic when volume distributions are measured for polydisperse powders. The particles representing the greatest mass (or volume) may, indeed, be rather few in number a thus may be quite sensitive to sampling. The variable numbers of large particles may sometimes lead to variations is dissolution rates. It should be recognized that the rigor to which sampling procedures must be held depends on the nature of the powder and its use in the manufacturing process or its effect on product performance. The authors have encountered powders which are nearly impossible properly sample.
A list of the most common instrument types employed in the pharmaceutical industry is given below.
Microscopy and Laser Diffraction instruments are the industry workhorses. Sieving is probably the third most used particle sizing method but is not suitable for fine pharmaceutical powders. The above list indicates, however, that a variety of particle sizing instruments are used in the pharmaceutical industry. Because a variety of equipment is used it is important to achieve an understanding of the relative performance of various instruments. More detailed discussions of the various techniques have been published in the literature10,15,22). At the present time, light microscopy is often used in conjunction with image analysis and automatic scanning to allow large numbers of particles to be measured. Several commercial instruments combine image analysis and light microscopy for particle size analysis.
The various particle sizing techniques can be generally divided into two classes - ensemble methods and particle counting methods. Particle counting methods assess the size of individual particles. The size data is then pooled into size classes in order to construct a histogram. Microscopy, Light Obscuration, Aerosizer, Nanosight and Malvern Zetasizer are examples of particle counting instruments. Ensemble methods, in contrast, do not assess the size of individual particles but rather assess the nature of the entire or a large portion of the population. Light scattering, Sieving and the Anderson Cascade Impactor are examples of ensemble methods.
The nearly procrustean use of light scattering methods (PCS and Mie-Fraunhoffer) for particle sizing by the pharmaceutical industry, while understandable in a number of regards, is unfortunate. Particle size distributions calculated from light scattering data are the result of rather complex calculations and numerical approximations. These calculations impose restrictions on the form of the calculated distribution which may not correspond to the form of the real distribution. These restrictions are most severe in the case of PCS. Commercial implementations of Mie-Fraunhoffer assumes that particles are spherical. When particles are spherical, no azimuthal dependence of the scattering pattern is observed. In contrast, if particles are not spherical a marked azimuthal dependence may be observed23–25). Application of a model intended for spherical particles to non-spherical particles may result in the reporting of ghost particles. Ghost particles are not present in the real population and are an artifact of the calculation. Each equipment manufacturer, necessarily, makes numerical assumptions in the calculation of particle size from the scattering intensities. Unfortunately, these assumptions do not appear to be the same between manufacturers. Results from Mie-Fraunhoffer instruments from different manufactures can differ significantly26).(See Fig. 1) Conclusions made using results from such instruments may be in error due to the presence of ghost particle or numerical artifacts. Number counting devices fair much better.

Percentage of Particles greater than 5 μm as determined by Laser Diffraction versus that determined by Light Obscuration for unstable Lipid Emulsions26). Laser diffraction shows non linear response to particle population. Note various laser diffraction instruments give different results. Light Obscuration has been shown to be accurate.
Problems associated with the use of light scattering methods have been discussed by a number of authors4,11,18–21,26–28). The reader is directed to these resources for a more detailed discussion.
3.3 Particle sizing summaryParticle size (and the particle size distribution) is a fundamental characteristic of a powder that influences the behavior of the powder in various applications. The determination of particle size is difficult for both philosophical and technical reasons. The method chosen to assess particle size affects the result as particle size for irregularly shaped particles is represented by the equivalent spherical diameter. As any measure of length, volume (mass) or surface area spherical diameters determined using different measures may differ. Particle size can be represented as a number, volume (mass) or surface area distribution. Not all authors make clear which type of distribution is reported or make clear that the chosen type of distribution is appropriate to the problem at hand. Particle size distributions determined by light scattering methods (PCS and Mie-Fraunhoffer) may at least partially reflect artifacts caused by fitting inappropriate optical models to the scattering data or resulting from numerical artifacts.
In order for QbD to be fully realized it will be necessary to develop and use new particle sizing tools. Limitations of currently available particle sizing equipment and the impact of these limitations on product development must also be recognized. Comparison of particle size distributions obtained using different methods may be the best way, at present, to assess the nature of a powder.
The adhesion between particles is also important to the behavior of powders. Zimon29) has published and extensive and important review concerning the physics of the adhesion of particles and particles to surfaces. The principal forces acting on powders are gravity and interparticle adhesion forces. Frictional forces are also important.
4.1 The role of surface free energy – adhesion fundamentalsThe adhesion between materials is determined by the area of intimate contact and the strength of the interaction between bodies. Factors such a particle shape and size as well as the degree of compression, in the case of tablets, control the quantity of interaction. Surface chemistry and surface free energy control the quality of this interaction.
The thermodynamics of the surface free energy, γ, of solids has been reviewed by Etzler30). The ideal work of adhesion, WA, between materials A and B is defined by the following relation.
| (1) |
| (2) |
Intermolecular forces between molecules result from interactions between their corresponding electron orbitals. The principal non-bonding interactions result from induced dipole–induced dipole (London), dipole–induced dipole (Debye) and dipole–dipole (Keesom) interactions. The intermolecular potential energy function, U, for each of these three types of interactions is of the same form. Here, r is the separation distance between bodies.
| (3) |
| (4) |
| (5) |
The interaction potentials between molecules have been used to determine the interactions between macroscopic bodies. In this instance, the Hamaker constants, A, derived from β, also follow the geometric mean rule30,34–35). Thus,
| (6) |
| (7) |
| (8) |
The Lewis acid-base component of the surface free energy has been modeled in a number of ways. Etzler30) has discussed the various models in detail. Here we highlight the use of two models, one by van Oss, Chaudhury and Good and the other by Fowkes, that are frequently discussed in the literature.
According to the van Oss, Chaudhury and Good model 36–37) the Lewis acid-base component is modeled as follows
| (9) |
| (10) |
| (11) |
Earlier Owens and Wendt39) described surface free energy in terms of two components which were called dispersive γ d and polar γ p. Thus
| (12) |
Recalling Eqns. (1), (8) and (9) together with the relation
| (13) |
| (14) |
As discussed above, Fowkes31–32) first suggested that surface free energy could be considered as a sum of components resulting from different classes of intermolecular interactions. The van Oss, Chaudhury and Good model draws upon the idea of Fowkes and as discussed above uses the geometric mean approximation to model Lifshitz-van der Waals interactions. Fowkes 31,41–42) however, has suggested a different approach to evaluating the acid base character of surfaces (also see43)). Fowkes has criticized the use of contact angles for measurement of interfacial properties44). Fowkes’ approach is, for experimental reasons, more applicable to powdered samples. As stated previously,
| (15) |
| (16) |
| (17) |
| (18) |
Gutmann46–47) introduced the notion of electron donor numbers (DN) and electron acceptor numbers (AN)48). These parameters are similar to the van Oss, Chaudhury and Good surface free energy parameters as they both describe the same molecular parameters but from different points of view. In 1966 Gutmann46) introduced the donor number based on the interaction with SbCl5. DN has units corresponding to enthalpy (e.g. kJ/mole). In 1975, Mayer et al49). introduced the acceptor number based on the relative 31P shift induced by triethylphosphine oxide. AN has arbitrary units. In 1990 Riddle and Fowkes48) removed the dispersive component from AN. The corrected AN* values have the usual units of enthalpy. According to Gutmann’s theory
| (19) |
Inverse gas chromatography is an excellent method used to determine γLW and ΔHAB. Etzler30) has discussed, in detail, the use of inverse gas chromatography (IGC) for determination of surface free energy components.
4.2 Adhesion and atomic force microscopyThe atomic force microscope has, in recent years, become an important tool to investigate the nature of surfaces. AFM can be used to both acquire fundamental information on particle-particle and particle- surface interactions. It can furthermore be used to investigate surface heterogeneity. Each of these factors can contribute significantly to the understanding of product or process performance. Here, an overview of the AFM and its application to the contamination surfaces and the assessment of particle properties is presented. General reviews of AFM have been published by Leitte and Herrmann50) and by zur Muhlen and Niehus51).
The atomic force microscope52) does not use the transmission of electromagnetic radiation or electron beams to form images. Instead, a probe is scanned over the surface in a raster fashion similar to that employed in television. Typically (but not exclusively) a sharp probe is located at the tip of a diving board shaped or V shaped cantilever composed of silicon. A piezoelectric scanner moves the sample with sub-nanometer displacement with applied voltage. The piezoelectric scanner is capable of three dimensional movements with respect to the probe tip. In order to create an image the probe tip is brought in close proximity to the surface or in contact to the surface. The piezoelectric system is then employed to scan the probe over the sample in raster fashion. Forces between the sample and probe caused either by sample topography or the nature of forces between the sample and probe result in cantilever deflection. An image is thus constructed on a line by line basis from the deflection of the cantilever. A photodiode detector is used to detect the deflection of the cantilever using a laser light reflection of the surface of the cantilever. See Liette and Herrmann for further details regarding the operation of an AFM50). Fig. 2 is a cartoon illustrating the configuration of an AFM.

Schematic drawing of atomic force microscope53). Scanner moves in three directions and deflection of cantilever is detected by a photodetector.
AFM is a high resolution form of microscopy that can be used to investigate the nature of surfaces. It is able to produce images at much higher resolution than optical microscopy and sometimes can achieve atomic resolution. Gan has recently discussed the resolution of AFM images54). The sample preparation and apparatus is much simpler for AFM than it is for electron microscopy. Samples may be studied in either gaseous or liquid media. The nature interaction of the probe with the surface can, furthermore, be varied; thus several AFM modes are available to the investigator. These different modes allow for the study many aspects of a surface beyond simple topography. In the present context, it will be particularly, important to consider modes that allow measurement of adhesion of particles to other particles and surfaces.
4.3 Measurement of forces using AFMCommercially available cantilevers can be used to measure forces down to about 10pN55). The AFM is thus particularly suitable for making force measurements. The displacement control of the instrument is on the order of 0.01 nm and the sample tip contact area is typically near 10 nm2. Fig. 3 shows a hypothetical force curve for tip sample interaction. Looking at the advancing curve in Fig. 3 beginning with large distance and moving to shorter distances, it is noted that once the attractive force exceeds the cantilever stiffness the cantilever jumps into contact with the surface (distance C in the figure). Moving the cantilever closer to the surface causes repulsive forces into increase. Again, repulsion is caused by the overlapping of electron orbitals. Upon withdrawal, the tip remains in contact with the surface beyond the initial contact point (distance C in the figure). Eventually the force exceeds the adhesion force or pull off force between the sample and the probe causing the cantilever to snap off the surface (point A in the figure). If the receding and advancing curves are not parallel then plastic deformation of the surface has occurred56–57).

Hypothetical force curve for probe surface interaction53). Dashed curve represents probe advancing toward surface. Solid curve represents probe receding from surface. A is the probe snap off force representing the force of adhesion between probe and surface. C is the probe surface contact position with advancing motion. The shaded area has sometimes been used as a method for determining the adhesion strength62).
Displacement of the cantilever is translated into force, F, using Hooke’s Law.
| (20) |
Force-distance curves similar to the example in Fig. 3 can be collected for an array of points on a surface. For each pixel of the surface a force-distance curve has been, thus, collected. Extraction of various quantities from the force-distance curve allows for the construction of various images representing the surface.
The adhesion force may, for example, be extracted from each the force-volume curve to construct an adhesion map that would be capable, for instance, of identifying the presence of surface contamination. Other types of maps constructed from force-distance curves might include measurements of hardness or deformability of surfaces. Any quantity that may be extracted from a force-distance curve may be utilized as basis to form and image62–65).
A particle to be studied can be substituted for the normally sharp tip mounted to the cantilever. (See Fig. 4) The attached particle usually has a diameter between 1–20 μm. In this configuration the AFM may be used to investigate the adhesion of the probe particle with other particles or surfaces. The measured adhesion force depends on the intrinsic adhesion between the materials and the contact topography of the particular site. It is not unusual to average the results of many adhesion measurements to understand the nature of the surface. The colloidal probe method had been used by several authors62,66–71).

Up : AFM cantilever tip. Down : AFM Cantilever with lactose particle mounted as colloidal probe.
The adhesion of particles to surfaces and between particles has been investigated by several authors. A rather comprehensive discussion of particle adhesion has been given by Zimon29). The measured adhesion force between bodies is the summation of forces resulting from different types of interactions. These forces may include van der Waals forces, capillary forces, electrostatic forces and chemically specific forces (acid-base).
The adhesion force between particles and surfaces is usually dominated by London dispersion forces in a dry atmosphere. The adhesion mechanics of spheres to planar surfaces has been described by Johnson, Kendall and Roberts (JKR)72) and by Derjaguin, Muller and Toporov (DMT)73).
According to the DMT theory the adhesion force,
| (21) |
| (22) |
| (23) |
| (24) |
The work of adhesion is determined by the surface chemistry of the materials in contact is given models such as that suggested by van Oss, Chaudhury and Good. (See above discussion concerning measurement of surface free energies.) For real particles with rough surfaces and irregular geometry proper estimation of the correct contact area is difficult or impossible. The correct contact area is necessary for the calculation of the proper adhesion force. The ability of predicting the adhesion force does not, however, preclude the constriction of useful adhesion maps based of observed adhesion forces or cantilever deflections.
4.4.1 Friction force modeIn addition to measuring the normal force exerted on the probe the lateral force may be measured from the torsion of the cantilever. The behavior of the cantilever has been found to be consistent with the classical theories of friction but is additionally influenced by adsorbed layers of water when surfaces are studied in air74–75).
4.4.2 Tapping or intermittent contact mode76–78)In tapping mode the cantilever is excited into oscillations near the resonance frequency of the cantilever with amplitude of up to 100 nm. These oscillations cause the cantilever to tap along the surface in a manner not to dissimilar to a blind man’s cane. The tapping mode image is constructed from the contacts of the tip with the surface. Tapping mode is more gentle to the surface and is thus more suitable for use on soft materials such as those used in, for instance, the pharmaceutical industry.
In tapping mode, the phase of the driver oscillation can be compared to that of the cantilever oscillation. Interaction of the probe with the surface will cause a phase difference between the two oscillations. The observed phase shift appears to reflect various properties of the surface including the viscoelastic properties of the surfaces. Tapping mode height images and phase images are collected at the same time. The phase image is particularly useful for identifying the presence of surface contaminants. The phase image is often able to identify surface features that cannot be identified in the height or topographic image.
4.5 Particle adhesion and particle sizeThe adhesion between particles within powders is a complex topic. Zimon, in particular, has reviewed the subject of adhesion of dusts and powders29). In the absence of triboelectric charging or capillary condensation (which is the usual case at moderate humidity) van der Waals and Lewis acid-base interactions contribute to the adhesion between particles. It would be typical to find that van der Waals interactions dominate.
Both the JKR and DMT models indicate that the force of adhesion, Fad, proportional to the particle radius or diameter. The drag force, Fdr, on the particle resulting from fluid flow would be proportional to the radius squared and the mass of the particle proportional to radius cubed.
For small particle diameters
| (25) |
For large particles the gravitational force presents the greatest obstacle for removal of particles. In this instance
| (26) |
From the above discussion it can be seen that both particle size and particle adhesion are interrelated. The relation between particle size and particle adhesion is not often well considered when thinking about pharmaceutical development problems.
4.6 Particle adhesion and crystalline stateParticle adhesion depends, in part, on the surface free energy of particles. The surface free energy depends on the precise chemical details of the surface. Heng, Williams and co-workers79–81) have investigated the surfaces of crystalline materials used in pharmaceuticals. Table 1 and 2 show advancing contact angles of water on individual crystal faces a few pharmaceutical substances and the surface free energy components (Owens and Wendt model) for Paracetamol polymorphs. These crystals were of macroscopic size. From the tables, it is clear that each crystal face has its own surface chemical characteristics. The area fraction of each crystal face is affected by the composition of the solvent from which the crystals are grown and the subsequent treatment of the crystals. The 010 face of paracetamol form I has the highest water contact angle and lowest acid-base contribution surface free energy. Crystal planes such as this are likely fracture planes and are less water wettable. The wettability and adhesion of materials thus can be affected by milling by altering the area fraction of each crystal plane.
| Facet | Paracetamol Form I | Paracetamol Form II | Aspirin | Racemic Ibuprofen | S(+)Ibuprofen |
| (201) | 38.1 (4.6) | ||||
| (001) | 15.9(3.1) | 64.5(3.5) | 60.7(3.5) | 68.5(4.8) | 64.5(3.9) |
| (011) | 29.8(5.7) | 42.9(4.8) | 46.9 (5.5) | ||
| (110) | 50.8(4.9) | 16.6(1.4) | 48.4(4.0) | ||
| (010) | 67.7 (2.5) | 17.9 (2.5) | |||
| (100) | 52.9(2.5) | 77.2(4.0) | 70.7(3.1) |
| Form I | Form II | |||||
| Facet | γ LW | γ AB | γ AB/γ | γ LW | γ AB | γ AB/γ |
| (201) | 34.9 | 27.5 | 0.44 | |||
| (001) | 34.4 | 38.0 | 0.53 | 42.3 | 9.5 | 0.18 |
| (011) | 33.9 | 32.7 | 0.49 | 33.4 | 38.5 | 0.55 |
| (110) | 34.2 | 20.2 | 0.37 | |||
| (010) | 45.1 | 7.0 | 0.13 | 32.2 | 38.9 | 0.54 |
As the surface chemistry of particles affects the adhesion between particles, the studies by Heng and co-workers suggest that the choice of crystallization solvent and milling may affect interparticle adhesion by altering the exposed fraction of individual crystal planes. At present, it is not usual to consider surface variability in the formulation of pharmaceuticals despite the fact that surface chemical variations may cause product and process variations.
4.7 Particle adhesion and surface contaminationIn most discussions of material characteristics of formulation components it is usual to assume that the surfaces of materials reflect the inherent nature of the studied substance. Above it is has been shown that the surface characteristics of a material can be altered by exposing different crystal planes. Alternatively, contamination of surfaces with foreign materials or intentional surface modification may alter the surface characteristics. One of us (Etzler) has investigated the surface contamination of materials used in the formulation of dry powder inhalers. A general discussion concerning dry powder inhalers has be given by Alagusundaram and co-workers82). Particle size of the component materials as well as interparticle adhesion and adhesion of particles to the delivery device affect the performance of such products.
Water is a surface contaminant the affects adhesion between particles. Beach and Drelich83) have investigated the adhesion of insulin particles to acrylonitrile-butadiene-styrene (ABS) and polypropylene (PP) surfaces as a function of relative humidity. These polymers could be used as a lining for a blister to be used in association with a dry powder inhalation device. The results are summarized in Fig. 5. From this figure it can be seen that the pull off force of insulin particles on PP is greater than that of ABS. Estimation of the pull-off forces using the JKR model (Eqn. (4)) combined with estimates of the works of adhesion are unable account for the magnitude of the observed adhesion forces. The RMS roughness determined from height images for the ABS and PP surface are 57.0 ± 6.3 nm and 386 ± 21.4 nm respectively. Presumably, the surface roughness contributes to the magnitude of the observed adhesion force and accounts at least in part for the greater adhesion to PP. Fig. 5 also shows that adhesion is a dependent on humidity and that this dependence occurs is step like manner so that three distinct adhesion ranges can be observed. It appears that in the 1st region (low humidity) the adhesion force is dominated by van der Waals forces, in the 2nd region the force results from a combination of capillary forces and van der Waals forces and in the 3rd (high humidity) region capillary forces dominate.

Lactose surface chemistry has also been has also been investigated and related to adhesion to gelatin capsule surfaces84). Fig. 6 shows the variation of surface free energy parameters for various lots of lactose. The figure suggests that both the Lifshitz-van der Waals and acid-base components vary between lots. The variation of surface acidity has been correlated with retention in (adhesion to) capsules used for dry powder inhalation (Fig. 7). Furthermore, evidence for the surface contamination, of lactose, can be directly observed using AFM. (Fig. 8). It appears that lactose surfaces are variably contaminated with protein and or lipid materials from milk. Lactose is isolated from milk.

Surface free energy parameters for various lots of Lactose as determined using IGC84). γ LW is the Lifshitz –van der Waals component of the surface free energy. Ka and Kd are the acid and base parameters describing the lactose surface using Gutmann’s acid-base model (See Etzler30) for an explanation of acid-base models). The figure suggests that the surface chemistry of lots of lactose is variable.

Surface acidity (Ka) for various lots of lactose and retention in capsule for various lots of lactose versus ratio of OCO carbons to aliphatic (CH) carbons on lactose surfaces84). CH carbons represent surface contamination. The oxidation states of surface carbons were determined using XPS (ESCA). Adhesion to capsule walls is affected by surface chemistry.
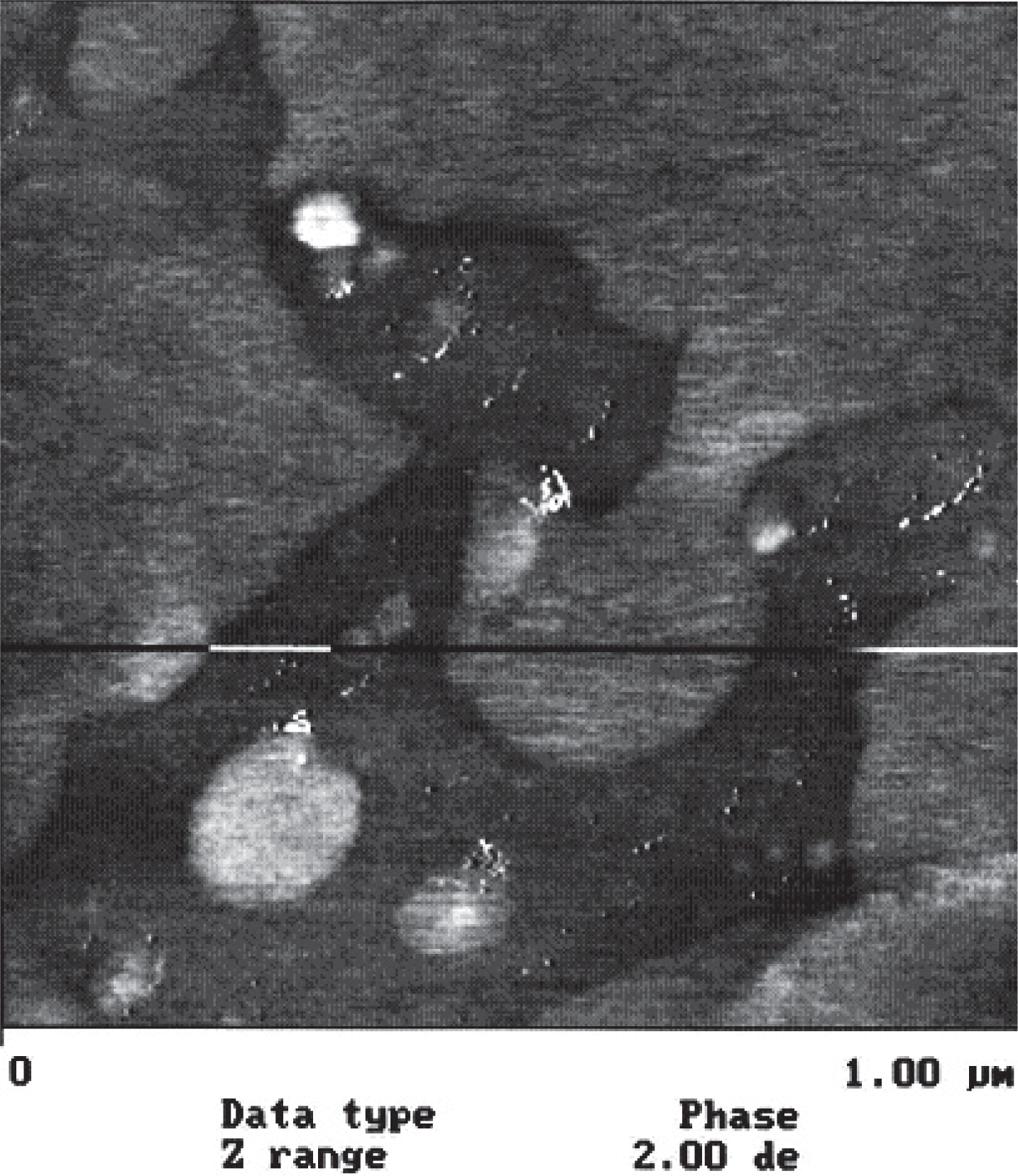
SPM phase image obtained using Tapping Mode (1×1 μm) of a Pharmatose 200M surface. Dark areas and white specks represent surface contaminates. XPS analysis suggests contamination with lipids and proteins from milk84).
From the above discussion, it can be seen that the properties of a powder can be influenced by particle size and by the nature of the interaction between particles. While many processes and products performance are influenced by power properties, this paper will briefly address two aspects – tableting and the performance of dry powder inhalers.
5.1 TabletingTablets are formed by placing a powder under pressure. In order to achieve sufficient tablet tensile strength particles must have sufficient deformability under pressure (quantity of interaction) to increase the area of intimate contact. Fracture of particles may also increase the area of contact. Tablet tensile strength is sometimes referred to as tablet hardness but this type of hardness is not hardness in the traditional sense. (It is not indentation hardness.)
Cao85) and co-workers have measured the indentation hardness of several pharmaceutical materials including ibuprofen, phenacetin, sucrose, etc. using AFM nanoindentation86–88) to determine the hardness of single particles. AFM nanoindentation is similar to more usual indentation hardness tests except for the fact that an AFM tip is used to make the indentation. The use of an AFM tip allows for hardness to be determined on single particles rather than macroscopic surfaces.
The measurements of particle hardness by Cao and co-workers indicates that materials with either high or low hardness show poor tableting characteristics. Recall that adhesion requires both quality of interaction (surface chemistry) and quantity of interaction (surface area of intimate contact). Hard particles may not sufficiently deform under compaction pressure and thus not allow for intimate contact between surfaces. The lack of intimate contact reduces adhesion strength. Soft particles, in contrast, may have low cohesive strength and low surface free energies reducing adhesion strength as a result of poor quality of interaction.
Etzler and co- workers89) have recently investigated tablet tensile strength from an adhesion science perspective. Earlier, Ryshkewitch90) and Duckworth91) proposed an empirical equation to relate tablet tensile strength, τ, to porosity of the tablet, ε. Thus,
| (27) |
The importance of the Ryshkewitch-Duckworth equation is that it suggests that porosity is a measure of the outcome of the tableting process. Tablets of the same porosity have the same mechanical properties even though they might have been made on vastly different tablet presses operating at different speeds.
In an earlier paper, Wu et al.96) proposed a model to calculate the radial tensile strength of tablets from the Ryshkewitch-Duckworth parameters of the individual components of the powder being compressed. Tye et al.95) and Sun97) have also commented further on this approach. In particular, Tye et al.95) have extended the application to quaternary mixtures. These studies establish the importance of the Ryshkewitch-Duckworth equation for understanding tensile strength of tablets composed of multiple components. Furthermore, they reiterate the importance of porosity as a measure of the outcome of the tableting process. Tablets even though produced by vastly different tablet presses operating at different speeds can be expected to have the same mechanical properties if they have the same porosity. The compaction force or compaction pressure may, however, not be the same if different tablet presses are employed.
Etzler and co-workers89) have proposed a model which is similar to that proposed by Wu et al.96) but which differs from that of the earlier authors by drawing more strongly from the principles of adhesion science. The form of the final equation also differs. According to this model the following relation relating tablet sensile strength to the Ryshkewitch-Duckworth parameters of the component materials is proposed.
| (28) |
| (29) |
| (30) |
Further investigations have shown that wet granulation of individual components has no effect on the observed Ryshkewitch-Duckworth parameters. The wet granulation of mixtures containing sodium dodecyl sulfate, in contrast, are found to have lower than expected tensile strength when wet granulated. This result supports the argument that tablet tensile strength results from the adhesion between particles. Recall, that adhesion depends on the properties of the surfaces. Contamination of these surfaces by, for instance, sodium dodecyl sulfate would have the effect of reducing tensile strength. The role of surface chemistry in determining the values of the Ryshke-witch-Duckworth parameters deserves further consideration.

Ryshkewitch-Duckworth parameters for dicalcium phosphate and sodium dodecyl sulfate mixtures. Results conform to the proposed model and Eqns. (29) and (30). The 90% confidence interval of the fit is indicated the dashed lines. The solid is a least squares fit to the data points.
A brief overview concerning dry powder inhalers has been given by Alagusundaram82) and Xu and coworkers98). Dry powder inhalers must deliver drugs of respirable size (2–5 μm) to the lung. The adhesion between particles is extremely important to the performance of such inhalers as particle must be seperated and aerosolized. While the design of dry powder inhalers varies, it is typical to have a formulation containing lactose and drug contained in a foil blister or gelatin capsule. Adhesion of particles to the blister walls or variable adhesion between particles is significantly responsible for performance variations in these products. From the data presented above we have learned that the surface chemistry of lactose varies due to the presence of different levels of surface contaminants. The presence of different levels of surface contaminants, furthermore, affects the adhesion of lactose to capsule walls which in turn alters the performance of product. The role of surface contamination in dry powder inhaler performance has been discussed in more detail by Etzler84). Fig. 7 illustrates the role of surface chemistry to adhesion of powder to gelatin capsule walls. Adhesion to capsule walls is a major factor that affects the performance of dry power inhalers.
Both the FDA (U.S. Food and Drug Administration) and ICH (International Conference on Harmonisation) have urged the incorporation of Quality by Design (QbD)1) into the manufacture of pharmaceutical products2).
The performance of many pharmaceutical products and processes used for manufacturing pharmaceuticals depends on knowledge of powder properties. The behavior of powders, in turn, depends on the particle shape and size as well as the adhesion and friction between particles. Full implementation of QbD thus requires knowledge of, at least, the above discussed properties and methods to assess these relevant properties.
The relevant properties addressed in this work include:
Particle sizing is difficult for both philosophical and technical reasons. The pharmaceutical industry employs many methods for particle size analysis but light scattering methods (Mie-Fraunhoffer and PCS) are common. The reported particle size distributions determined by these methods often contain instrumental artifacts. It is important to consider alternate methods, that are less prone to artifacts for particle size analysis. In the pharmaceutical industry it is not common to find rigorous powder sampling protocols in place. Both poor sampling and instrumental artifacts (or limitations) may lead an investigator to improperly assign development or manufacturing problems to particle size.
The adhesion between particles also affects powder properties and the performance of processes and products. The adhesion between particles is affected by both the mechanical and surface chemical properties. The surface chemistry of particles is affected by the area fractions of individual crystal planes. The area fraction of a particular crystal plane can be affected by the choice of solvent used for crystallization or milling. Weakest planes are the most likely to fracture. In addition to the area fraction of various crystal planes, surface contamination affects interparticle adhesion. One of us (Etzler) has found that variable surface contamination of formulation components significantly affects the performance of dry powder inhalers.
It is clear that full implementation of QbD will require further understanding of the role of interparticle interactions in the performance of powders. It will furthermore be necessary to routinely employ appropriate analytical methods to that establish particle size and particle surface chemistry. Routine use of good sampling methods will also required. A considerable amount of work will be required to achieve the level of understanding required for implementation of QbD.
Frank M. Etzler
Dr. Etzler earned a Ph.D. in physical chemistry from the University of Miami. He is currently an Associate Professor of Pharmaceutics in the School of Pharmacy at the Lake Erie College of Osteopathic Medicine (LECOM). Prior to coming to LECOM in 2011, he was the leader of the Material Science Laboratory at Boehringer-Ingelheim Pharmaceuticals for nearly 20 years. This laboratory was responsible for the physical characterization of powders.
Prior to this, Dr. Etzler was a faculty member in pharmaceutics at the University of Kentucky and a faculty member in surface and colloid science at the former Institute of Paper Chemistry.
Mohammad Nasir Uddin
Mohammad Nasir Uddin is an Assistant Professor in School of Pharmacy at Lake Erie College of Osteopathic Medicine, LECOM. He received his M. Sc. in Chemistry from Emory University in 2006 and his Ph.D in Pharmaceutics from Mercer University in 2011. Both institutions are located in Atlanta, GA. He also worked on nanoparticle based vaccine development for Human Papillomavirus (HPV) as scientist at CDC in 2010 and 2011. His research interests include particles used in formulation development of vaccines. He is also interested in adhesion of nanoparticulates. His research has lead to 12 peer reviewed papers and 26 scientific presentations.