2013 Volume 30 Pages 236-243
2013 Volume 30 Pages 236-243
It is demonstrated by means of XRD, IR spectroscopy, electron microscopy, laser light scattering and measurements of the specific surface area that mechanical activation of a mixture of lithium carbonate, niobium pentoxide and copper oxide in the planetary centrifugal activator AGO-2 involves mixing, dispersion and amorphization of the components, as well as the phase transition of the monoclinic form of niobium (V) oxide into its orthorhombic form. Subsequent mechanical treatment results in the partial formation of a lithium-niobate phase. Thermal treatment of the compacts prepared from mechanically activated powders, at a temperature above 400°C, leads to the appearance of the structure-forming trigonal phase based on LiNbO3. The data obtained provide evidence that the mechanochemical method is promising for the synthesis of solid solutions based on lithium niobate.
The majority of ceramics manufactured in the world are based on lead-containing materials. These materials possess unquestionable advantages but they also have essential disadvantages; one of them is the substantial volatility of lead and its compounds, which causes complications of the technological process for the conservation of the stoichiometry of compounds during ceramics sintering. At the same time, lead is a toxic element, a poison of cumulative action, and it is very hazardous for living organisms. At present therefore, much attention is paid to the development and synthesis of lead-free piezomaterials and piezoelements as the basis for manufacturing measuring devices. These materials include niobates of alkaline metals modified with various additives.
According to the classic technology, the synthesis of the niobates of alkaline metals is generally carried out at a temperature above 750°C in two stages (each stage lasts for 6 hours) with intermediate grinding, and sintering is carried out at even higher temperatures (>1000°C) for 5–6 hours, for example, similarly to 1). The duration of synthesis and its labor-intensive character stimulated the development of new methods for the synthesis of piezoceramic materials; one of these methods is a mechanochemical method2,3). Most attention is paid in these works to investigation of the parameters of mechanochemical synthesis affecting the target characteristics of niobates and ceramics based on it. Substantially poorer is the study of the role of mechanical activation in the synthesis. In the present work we study the mechanism of the mechanochemical synthesis of the solid solution based on lithium niobates with copper oxide as the modifying additive. The choice of this system as the subject of investigation is connected with the fact that the materials of this type have a high Curie point (TC >1200°C), high working temperature −up to 950°C, and low permittivity, which is favorable for the use of these materials in high-temperature high-frequency measuring devices4,5).
Initial reagents for the synthesis of the indicated material were Li2CO3 and CuO powders of a quality not less than the pure reagent grade, while the initial Nb2O5 was of extra-pure grade. According to the data of XRD, niobium oxide is the monoclinic modification with the space group P2 (no. 3) and lattice parameters close to a = 21.16 Å, b = 3.822 Å, c = 19.350 Å, β = 119.83° (card no. 72-1121). The components’ ratio was as follows:
A mixture of powdered reagents was placed in provisionally lined cylinders as the weighed portions of 10 g; activation in the planetary-centrifugal mill AGO-2 was carried out6) with ball acceleration 40 g for 10 and 30 minutes. Steel balls 8 mm in diameter were used for activation, the mass of balls was 200 g. After five minutes of activation in each case, the cylinders were opened, the powder was taken out and mixed, then put back into the cylinders for further mechanical activation.
X-ray diffraction of the initial reagents and the activation products before and after thermal treatment was carried out using a DRON-3 diffractometer (CuKα radiation, focusing scheme according to Bragg – Brentano).
The specific surface was determined using the gas adsorption method on the basis of argon desorption7). The milled iron content was determined by means of atomic adsorption with a Varian 280FS spectrophotometer. Photographs of the powders were taken with the scanning electron microscope TM-1000 made by «HITACHI». The samples were weighed with an UW 220H balance made by SHIMATZU with an accuracy of 0.001 g, the geometric size was determined with the help of a micrometer with an accuracy of 0.001 mm.
Samples were pressed in tablets 10 mm in diameter using a hydraulic press with a pressing force up to 10 t/cm2.
After pressing, the samples were placed in a crucible filled with aluminum oxide, and heated within the temperature range 400 to 1040°C. The rate of sample heating to 600°C was 20 degrees per minute; at 600°C and above it was 10 degrees per minute. At all the given temperature points, the samples were heated for 2 hours. Samples were cooled in the furnace after it was switched off.
The IR spectra were recorded with the help of a Tensor 27 spectrometer. The samples for investigation were prepared according to the standard procedure.
According to the XRD data (Fig. 1), after mechanical activation of the mixture for 10 minutes we observe a decrease in the intensity and broadening of the reflections of all the initial components, and the appearance of the reflections of niobium oxide in the orthorhombic modification. The appearance of the reflections in the orthorhombic modification is evidence of the mechanically stimulated transition of the monoclinic modification into the orthorhombic one with the space group Pbam (no.55)8). Mechanochemically stimulated phase transition in niobium oxide was observed previously in 9). It should be stressed that after mechanical activation for 10 min, a mixture with a lower copper oxide content (0.1CuO) exhibits a more substantial phase transition in Nb2O5 (monoclinic – orthorhombic) than the mixture containing 0.2CuO. It may be assumed that an increase in the amount of copper oxide is the deterrent for this transition.

a)–initial powdered mixture containing 0.2 CuO;
b)–mechanical activation for 10 min
c)–mechanical activation for 30 min
♣−Nb2O5 monoclinic;
♥−Nb2O5 orthorhombic;
●−Li2CO3;
♦−doping additive CuO,
○−lithium niobate LiNbO3
A further increase in activation time to 30 minutes causes even more noticeable (almost complete) amorphization of the initial reagents, the disappearance of the reflections characteristic of lithium carbonate and copper oxide, and a further decrease in the intensity of reflections related to niobium pentoxide, both in the initial monoclinic modification and in the orthorhombic one, formed during mechanochemical treatment. In addition, with an increase in activation time to 30 minutes, we observe the appearance of reflections that are close in their positions to the reflections of lithium niobate (012, 104, 110, 113, 116) of orthorhombic modification (S.G. R3c).
The IR spectra of the reagent mixture (Fig. 2) in the region 550–2000 cm−1 contain the bands related to lithium carbonate (1505, 1443, 1088, 860 cm−1) and niobium oxide (958, 815, 750, 690, 610 cm−1). Preliminary mechanical activation for 10 minutes causes an asymmetric broadening of the doublet in the region 1500 cm−1, which is assigned to the stretching vibrations of C-O bonds of carbonate ions. Broadening is also observed for other bands of carbonate ions that are present in the spectra: 1088 and 860 cm−1. Activation also causes changes of the bands related to niobium oxide. The disappearance of the bands at 750 and 610 cm−1 is observed, as well as broadening of the bands with the maxima at 958, 675 and 820 cm−1.
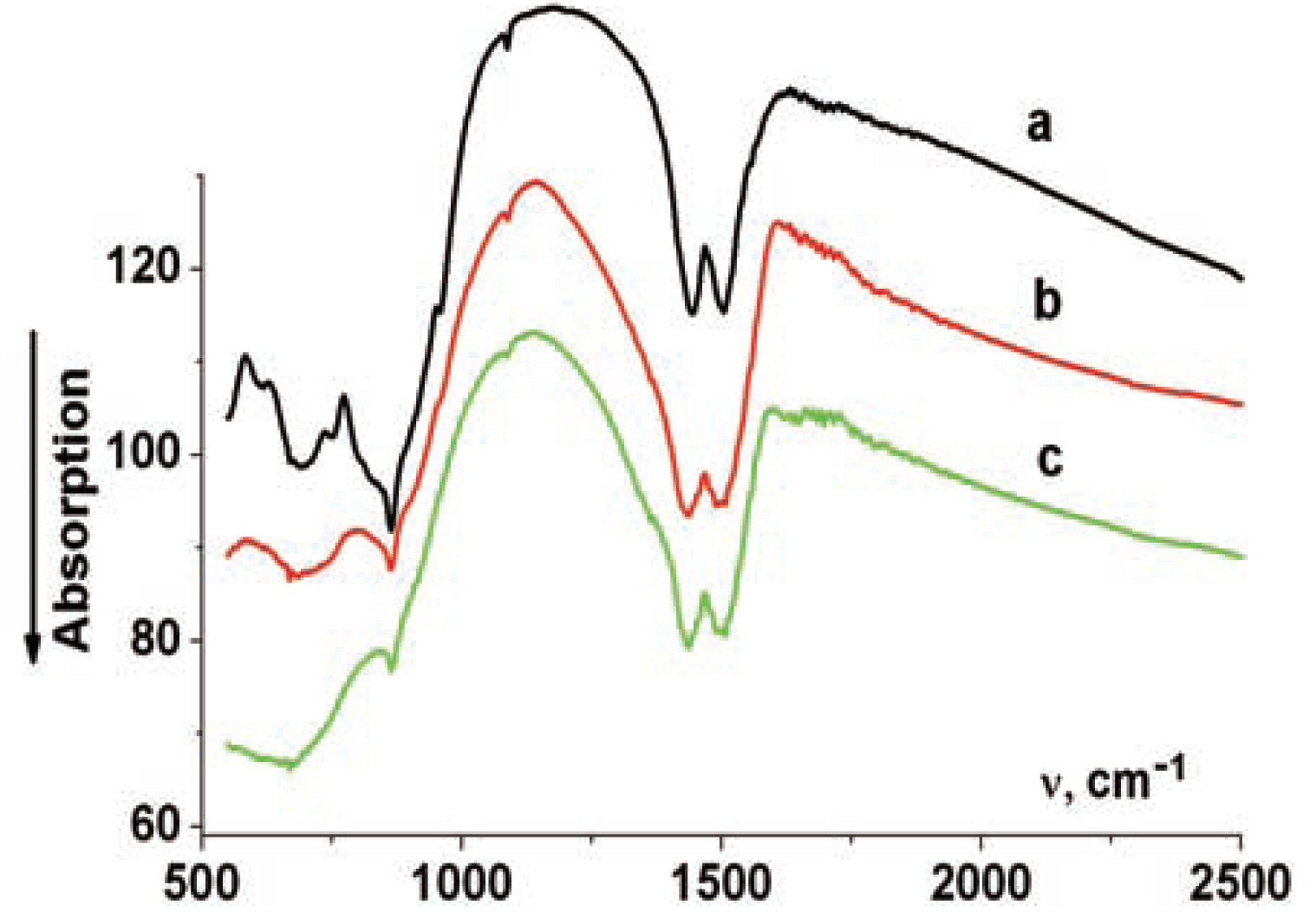
IR absorption spectra of the samples containing 0.2 CuO:
a)–initial composition;
b)–mechanical activation for 10 min;
c)–mechanical activation for 30 min
According to the IR spectroscopic data, an increase in the activation time to 30 minutes leads to further broadening of the doublet at 1500 cm−1; a shoulder is seen to appear on its slope at ∼1340 cm−1. A further decrease in the intensities of other bands related to carbonate ion (1088 and 860 cm−1) is observed. Mechanical activation causes further broadening and a decrease in the intensity of niobium oxide band at 825 cm−1. In addition, a broadened band with the maximum at 700 cm−1 is observed to appear; it is characteristic of lithium niobate10,11).
In other words, the data of IR spectroscopy - similarly to the data of XRD - provide evidence that the processes of lithium niobate phase take place. It is interesting to stress that after mechanical activation for 10 min, the powder becomes gray, whereas after activation for 25 min it becomes greenish, and after activation for 30 min the powder becomes light green. These data allow us to assume that activation involves partial interaction of copper oxide with lithium carbonate resulting in formation of the compounds containing copper cations; their coordination sphere includes carbonate ions.
Changes of the disperse state and specific surface of the solid phase during activationTable 1 shows the results of specific surface measurements and determination of the milled iron content as a result of mechanical activation of the samples. It follows from the data presented in the table that the specific surface of a powder mixture increases to 8.95 m2/g under the effect of activation.
| Activation time, min. | 0 | 10 | 30 |
| Specific surface, m2/g | 1.67 | 4.71 | 8.95 |
| Fe content, mass % | 0.006 | 0.013 | 0.019 |
The results of electron microscope studies of the samples are shown in Fig. 3. One can see in photograph (Fig. 3a) that the initial powder contains the particles possessing the anisotropic shape. Activation of this powder for 10 minutes (Fig. 3b), along with the formation of smaller particles, causes the formation of agglomerates having the isotropic shape. A further increase in activation time to 30 minutes (Fig. 3c) leads to the formation of smaller particles, although at the same time we observe the formation of rather large aggregates. The average size of particles in the mixture, D, determined from the data on the specific surface according to equation D = 6/ρS (where ρ is the average density of the mixture in g/cm3, which is close to 3.6; S - is the specific surface in cm2/g), is 0.3–0.4μm.

Electron microscopic images of the samples containing 0.2 CuO:
a)–Initial powdered mixture; visible are the particles possessing the anisotropic shape. Magnification - 500
b)–Mechanical activation for 10 min; the formation of smaller particles. Magnification - 1000
c)–Mechanical activation for 30 min; the formation of rather large aggregates. Magnification - 1000
Mechanical activation of the mixture therefore causes the formation of aggregates with the size varying from several micrometers to several tens of micrometers, including submicron particles of lithium carbonate, niobium oxide, and copper oxide.
Thermal treatment of mechanically activated samples Samples after Activation for 10 minutes, with 0.1 CuO ContentCompact samples in the form of tablets 10 mm in diameter and about 1 mm thick were formed without a plasticizer using a hydraulic press under a pressure force of 10 t/cm2.
After heating at 400°C and 500°C, the samples still contain substantial amounts of unreacted niobium oxide phases (Fig. 4b, c). After heating at 600°C (Fig. 4d), a two-phase structure is formed; one of the phases is rhombohedral lithium niobate, the second one has tetragonal modification and corresponds to the compound Cu2LiNb5O15. This phase is conserved for all the studied heating temperature points. Its amount on the surface of the tablets is noticeably larger than inside the tablets. This is confirmed by the fact that after polishing the tablets to a depth of 0.05 mm, this phase is barely pronounced in the X-ray diffraction patterns. For the samples with the high CuO content, and for all the samples with longer activation times, this phase is not formed.

Diffraction patterns of mechanically activated mixture
a)−10 minutes, 0.1 CuO and the products of its thermal treatment at
b)−400°C;
c)−500°C;
d)−600°C.
○−lithium niobate LiNbO3
∗−Cu2LiNb5O15;
♣−Nb2O5 monoclinic;
♥− Nb2O5 orthorhombic
One can see in Fig. 5 that after activation for 30 min (for any CuO content), the samples exhibit formation of the phase based on lithium niobate, although it is still far from perfect.
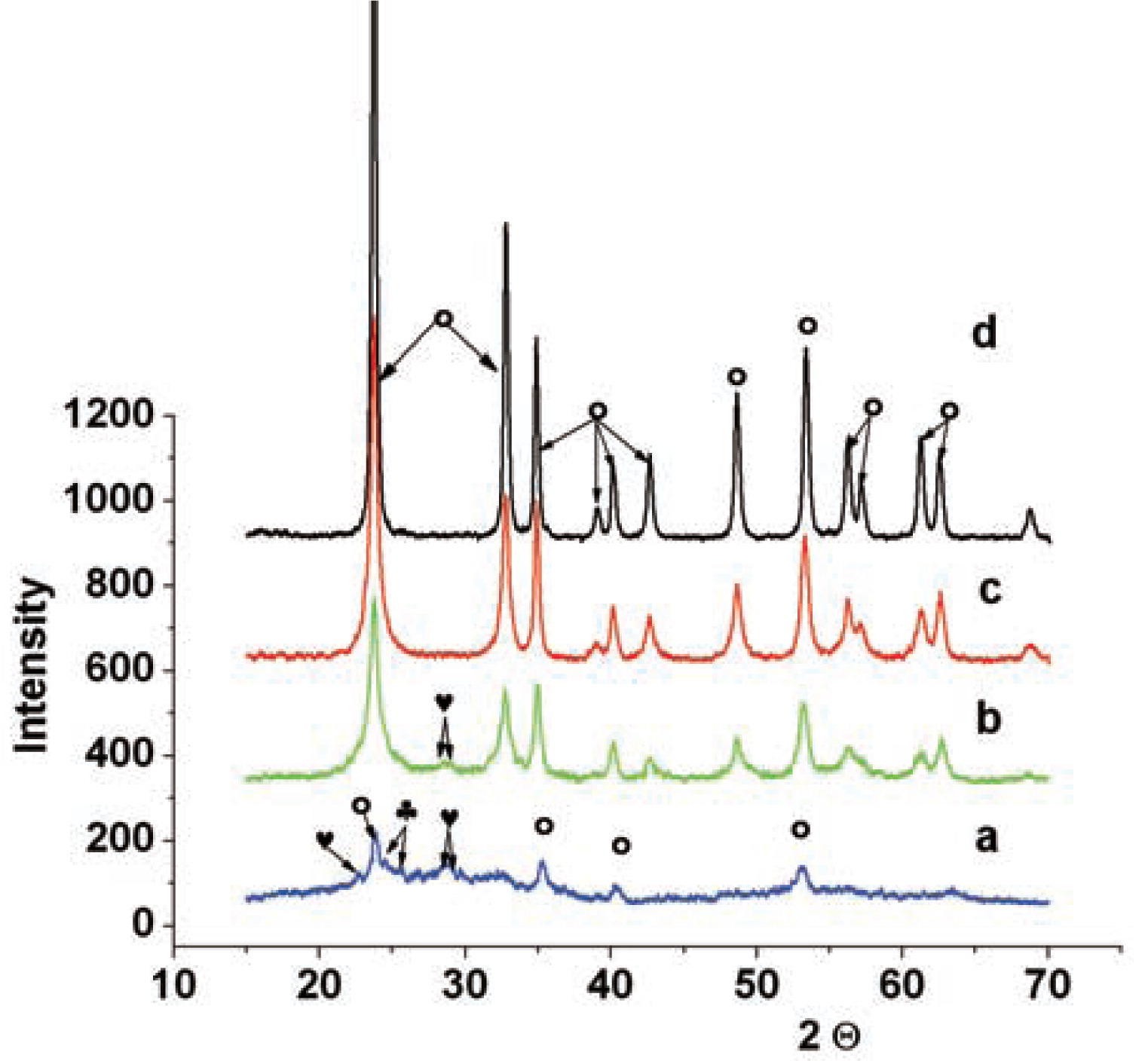
Diffraction patterns of mechanically activated mixture:
a)−30 minutes, 0.1 CuO and the products of its thermal treatment at
b)−400°C;
c)−500°C;
d)−600°C
♣− Nb2O5 monoclinic;
♥−Nb2O5 orthorhombic
○−solid solution based on lithium niobate Li0.97NbO3.
The diffraction patterns of the sample obtained after heating at 400°C contain intense reflections of the new compound along with the weak reflections of unreacted initial components. These reflections coincide with the reflections of lithium niobate having the composition LiNbO3 (Fig. 5b).
After heating at 500°C (Fig. 5c), the structure based on the solid solution of lithium niobate having the rhombohedral modification with the space group R3c (no.161) is formed. Its reflections coincide in their positions and intensities with the deflections of compound Li0.97NbO3 with higher accuracy than they do with the composition LiNbO3. The formation of this structure is characteristic of the samples with any copper oxide content. The concentrations of impurities and unreacted phases are comparable with the background level.
The diffraction patterns of the samples annealed at a temperature of 600°C and above exhibit almost no differences from each other, conserving an almost identical distribution of reflection intensities. Even the traces of initial components are absent from the patterns; the ratios of reflection intensities now coincide better with the data for the solid solution based on lithium niobate having the composition Li0.75Cu0.2Nb1.01O3, rhombohedral, S.G. R3c (no. 161).
It may be concluded from analysis of the diffraction patterns that chemical reactions in the samples stop mainly at the heating temperature in the range of 500°C to 600°C. Lithium carbonate decomposes into lithium oxide, while carbon dioxide evolves into the atmosphere, which is also confirmed by the mass loss of the samples. For example, the mass loss is about 13% after sintering at 500°C, while the maximal mass change is about 14% (1040°C).
Change in the density of compacted samples during heatingThermal treatment causes no change to the shape of tablets obtained by pressing mechanically activated mixtures. This allowed us to determine the average density of initial pressed samples and the samples subjected to thermal treatment based on tablet size and mass.
The changes in the average density of samples as a function of the activation time, composition and heating temperature are plotted in Fig. 6. The initial density of samples after pressing was averaged over a series of 10 samples; it is 3.29 and 3.35 g/cm3 for an activation time of 10 and 30 minutes, respectively. One can see that these values differ from each other insignificantly. With an increase in heating temperature, value scattering increases; at 500–600°C scattering is maximal, then decreases. Within this temperature range, the density of the samples at first decreases, due to a decrease in their mass and due to an increase in sample volume. Then, starting from the temperature of 600°C, sample sintering starts; it is accompanied by shrinkage and an increase in average density. Within the temperature range 900–1020°C rather strong samples are obtained. One can see in Fig. 6 that after heating at a temperature above 900°C, the density of samples with different copper content changes depending on the heating temperature. With an increase in heating temperature to above 1040°C, the density of samples decreases and their strength decreases.

Dependence of the change to the mean density of samples on the temperature of thermal treatment: A-activation time 10 minutes, B-activation time 30 minutes. Copper oxide content: a)−0.1 CuO; b)−0.2 CuO.
The presented results are related to agglomeration temperatures of 960, 980 and 1000°C. The size of the blocks of coherent scattering (size, nanometer) and their distortion (strain, %), as well as the lattice parameters a and c for the rhombohedral modification (angstroms) were calculated from the XRD data using PowderCell 2.4 software.
One can see in Table 2 that an increase in the time of activation of the component mixture from 10 to 30 minutes causes a substantial (by approximately 20–30%) change of the mass loss of samples during subsequent annealing. This character of mass change is independent of the annealing temperature and copper content. These data provide evidence that after longterm mechanical activation, about 30% of initial carbonate ions undergo mechanochemical interaction with the evolution of carbon dioxide in accordance with the equation:
| Sintering ℃ | CuO | Time activ., min | Size, nm | Strain, % | a angstrom | c angstrom | Density, g/cm3 | Mass loss, % |
|---|---|---|---|---|---|---|---|---|
| 960 | 0.1 | 10 | 44 | 0.1 | 5.149 | 13.824 | 4.15 | 12.2 |
| 0.1 | 30 | 37 | 0.12 | 5.139 | 13.809 | 4.20 | 8.2 | |
| 0.15 | 10 | 32 | 0.14 | 5.139 | 13.814 | 4.30 | 13.5 | |
| 0.15 | 30 | 44 | 0.03 | 5.128 | 13.791 | 4.37 | 10 | |
| 0.2 | 10 | 40 | 0.09 | 5.138 | 13.809 | 4.33 | 13.6 | |
| 0.2 | 30 | 38 | 0.1 | 5.138 | 13.811 | 4.49 | 10.7 | |
| 980 | 0.1 | 10 | 41 | 0.1 | 5.151 | 13.823 | 4.28 | 13.1 |
| 0.1 | 30 | 41 | 0.09 | 5.137 | 13.815 | 4.15 | 11.7 | |
| 0.15 | 10 | 38 | 0.11 | 5.132 | 13.829 | 4.34 | 14.5 | |
| 0.15 | 30 | 61 | 0.2 | 5.134 | 13.812 | 4.21 | 12.2 | |
| 0.2 | 10 | 41 | 0.1 | 5.140 | 13.820 | 4.40 | 13.1 | |
| 0.2 | 30 | 38 | 0.1 | 5.139 | 13.812 | 4.47 | 11.6 | |
| 1000 | 0.1 | 10 | 45 | 0.05 | 5.146 | 13.809 | 4.33 | 13.4 |
| 0.1 | 30 | 42 | 0.1 | 5.151 | 13.856 | 4.19 | 10.9 | |
| 0.15 | 10 | 36 | 0.13 | 5.142 | 13.811 | 4.31 | 13.1 | |
| 0.15 | 30 | 61 | 0.24 | 5.136 | 13.830 | 4.25 | 11 | |
| 0.2 | 10 | 44 | 0.08 | 5.136 | 13.817 | 4.34 | 13.3 | |
| 0.2 | 30 | 36 | 0.2 | 5.134 | 13.810 | 4.44 | 10.5 |
Analysis of the data allows us to state, that during the mechanochemical activation of the mixture with copper oxide addition and following thermal treatment, a solid solution of lithium niobate-copper oxide is formed. The lattice parameters and the parameters of microstructure (coherent length and microstrain values) depend on the conditions of the mechanical activation, the temperature of thermal treatment and the copper oxide content.
Thus, for an agglomeration temperature of 1000°C and an activation time of 30 minutes, the parameter a decreases from 5.151 Å to 5.134 Å with an increase in copper content of the solid solution from 0.1% to 0.2%. Similarly, parameter c decreases from 13.856 to 13.810 Å. A decrease in parameters a and c with an increase in copper content of the solid solution can be connected to the fact that copper inclusion in the solid phase causes the substitution of lithium cations by copper with the formation of cation vacancies in the lithium positions according to the reaction:
The presence of cation vacancies is likely to cause a decrease in lattice parameters of the solid solution of lithium niobate with an increase in copper content.
After 30 min activation of the initial components, the coherent length in the solid solution for all agglomeration temperatures depends on the copper content: the largest coherent length is observed for a copper content of 0.15%. A similar dependence on the copper content is also observed for microstrain values 9 at temperatures 980–1000°C.
Discussion of the obtained crystal chemical data and microstructural parameters exceeds the scope of the present work and requires additional investigation.
Mechanical activation of a mixture of lithium carbonate, niobium oxide and copper oxide is thus accompanied not only by the changes of the dispersity of the initial reagents and their amorphization, but also results in the case of longterm mechanical activation in partial interaction of lithium carbonate with niobium oxide; a lithium niobate matrix is formed. The composites formed during mechanical activation are composed of isometric aggregates of micrometer size containing submicrometer-sized solid particles. The formation of isometric aggregations of particles simplifies the formation of compacts when pressing mechanically activated powders. The large area of contact between the particles of reagents in the compact and the increased reactivity of the reagent are the reasons for the substantial formation of solid solution based on lithium niobate during subsequent heating even at 400°C. An increase in the temperature of thermal treatment causes an increase in the extent of the formation of solid solution and the degree of its crystallization.
Gusev Aleksey Alekseevich
Research fellow, Laboratory of Intercalation and Mechanochemical Reactions, Institute of Solid State Chemistry and Mechanochemistry, Siberian Branch of the Russian Academy of Sciences. Candidate of Science in Chemistry. Research area: ceramic materials science. Author of 94 publications including 5 monographs and 2 patents for inventions.
Contact information: gusev@solid.nsc.ru, phone 383-170548.
Isupov Vitaliy Petrovich
Head of Laboratory of Intercalation and Mechanochemical Reactions, Institute of Solid State Chemistry and Mechanochemistry, Siberian Branch of the Russian Academy of Sciences. Doctor of Science in Chemistry.
Research areas: mechanochemical synthesis of solids, chemistry and technology of lithium compounds, chemistry of intercalation compounds, mechanical activation. Author of 200 publications including 30 patents for inventions.
Contact information: isupov@solid.nsc.ru, phone 383-3363837.
Avvakumov Evgeniy Grigorievich
Chief researcher, Laboratory of Intercalation and Mechanochemical Reactions, Institute of Solid State Chemistry and Mechanochemistry, Siberian Branch of the Russian Academy of Sciences. Doctor of Science in Chemistry, Professor, Laureate of the State Award of Russian Federation in Science and Technology, Full Member of the International Institute for Sintering, Honored Researcher in RF.
Research areas: mechanical activation, mechanochemical synthesis of solids. Author of 5 monographs, 272 research papers, 42 inventions.
Contact information: avvakumov@solid.nsc.ru, phone 383-3363843.