2014 Volume 31 Pages 156-162
2014 Volume 31 Pages 156-162
Calcium carbonate particles with various shapes and morphologies were prepared via precipitation in an octylamine/water self-assembly bilayer systems. Crystal structure and shape of the CaCO3 particles were determined by the water to octylamine molar ratio R of the bilayer. At R = 16.0, phase pure calcite particles with a “hopper crystal” morphology were formed, the average particle size of the hopper crystal is 10 μm with well-defined edges on the hopper faces. Decrease the R ratio to 7.2 eventually leads to the formation of 3 μm tabular CaCO3 particles which are predominated by vaterite structure. For an intermediate R of 10.8, spherical vaterite aggregates and rhombohedral calcite particles were produced. Thermal decomposition of the CaCO3 particles was observed at around 710°C. The mechanism of particle evolution in the self-assembly bilayer, particularly the formation of “hopper crystal” calcite was discussed.
Calcium carbonate is one of the most abundant minerals on earth and finds its increasing applications in pigments, water treatment, biomineralization and energy storage (Colfen, 2003). It is equally important in scientific research and much attention had been focused on the crystal growth and morphology development under a number of conditions (Adair and Suvaci, 2000; Meldrum, 2003). McCauley and Roy (1974) extensively explored the processing parameters that affected the crystal growth of CaCO3 polymorphs precipitated from aqueous solutions. The growth of CaCO3 crystals was intertwinedly determined by solution pH, reactant concentration, and impurity ions at ambient conditions. This effort was extended by Wang et al. (1999) in the precipitation of Ca ions with urea at 90°C, and by Jung et al. (2000) for calcium carbonate formed in a Couette-Taylor reactor. A theoretical understanding of the crystallization habit of precipitated CaCO3 was carried out by de Leeuw and Parker (1998) using atomistic simulation, which indicated the surface and hydration energy may directly control the formation of favored crystal structures. More recently, the precipitation of calcium carbonate particles was incorporated with polymeric additives (Archibald et al., 1996; Osman and Suter, 2002; Park and Meldrum, 2002; Rock et al., 1997; Rudloff and Colfen, 2004; Wei et al., 2004; Xu et al., 2005) and gold nanoparticle template (Lee et al., 2001) so as to gain adequate control of the crystallization and morphology development.
The crystallization of CaCO3 particles under confined environment was initially studied by Mann et al. (1991), Rajam et al. (1991) and Heywood et al. (1991) using compressed Langmuir monolayers of stearic acid and octadecylamine. The structure and morphology of the CaCO3 particles were significantly dedicated by the type of surfactant molecule as well as Ca2+ concentration (Walker et al., 1991). Analogy to the monolayer-confined synthesis, octylamine/water bilayer systems was employed by Adair et al. (1998) to grow a number of nanoscale platelets including CdS, (Adair and Suvaci, 2000; Adair et al., 1998) Ag (Yener et al., 2002) and SiO2 (Wang et al., 2006) at ambient condition. When mixed with water under appropriate weight ratio, the amines will orient in such a way that allows organic layer and aqueous layer to alternate in the solution, mainly because of the amphiphilic nature of the organic molecules (Adair et al., 1998; Ralston et al., 1942). The thickness of each layer is directly related to the amine to water weight ratio, which provides an effective pathway to manipulate the template thereby the nanoparticle thickness during synthesis. This approach is applicable to numerous materials where chemical reactions such as precipitation, redox, and hydrolysis are feasible in aqueous media.
Previous studies demonstrated that crystal structure and shape of the CaCO3 particles are sensitive to a number of processing parameters (Colfen, 2003; McCauley and Roy, 1974; Walsh et al., 1999), thus preparation of CaCO3 with desired phase and shape may require precise control over the experimental procedures. Therefore, the intent of this work is to report the preliminary study on CaCO3 particles precipitated from octylamine/water bilayer systems, an interesting crystal habit known as hopper crystal was observed. The possible mechanisms responsible for shape and morphology changes were discussed.
All chemicals involved in the synthesis were reagent grade and used as-received without further purification. CaCl2 (99+%, J. T. Baker, NJ) and Na2CO3 (99+%, J. T. Baker, NJ) were used as the precursors for CaCO3. Octylamine (99%), amylamine and polyethylenimine were purchased from Aldrich (Aldrich Chemical Co., Milwaukee, WI), while ethanol (94.4%, 188.8 proof), nitric acid and glacial acetic acid were obtained from J. T. Baker Chemicals. Deionized water was used (specific conductivity = 0.4 × 10−7 S/m) for all experiments.
The procedure used to prepare CaCO3 particles is similar to that applied to produce platelet-like CdS particles in the octylamine/water bilayer systems (Adair et al., 1998). The general procedure can be described as follows, 25 mL 0.05 M CaCl2 and 0.05 M Na2CO3 aqueous solutions were mixed with 31.97 mL octylamine and 1.33 mL amylamine to form two individual batches of self-assembled bilayers (amylamine is able to stabilize the octylamine/water bilayer and provide a well-defined bilayer structure) (Yener et al., 2002). After shaking for 10 minutes, the two bilayers were mixed together and shaken for 24 hours. Precipitation and condensation of CaCO3 particles takes place in the octylamine/water bilayer during vigorous shaking. The bilayer system was subsequently broken by adding 60 mL 0.5 M HNO3/ethanol solution and 1.25 mL 0.1 wt% PEI/ethanol solution (1 w/w PEI). The washing step was repeated 5 times by simply collecting CaCO3 particles on the bottom of the beaker and removing the supernatant. It is important to control pH of the suspension close to pH 9 in each washing step, which allows the maximum yields of CaCO3 (McCauley and Roy, 1974). The final suspension of CaCO3 particles in ethanol has a concentration of 3.0 mg/mL and a pH close to pH 9. A schematic flow sheet for the entire procedure is shown in Fig. 1.

Flow sheet of CaCO3 particles synthesized from octylamine/water bilayer systems. The pH of the bilayer is controlled at pH 10. The octylamine to amylamine weight ratio is 25 (Yener et al., 2002).
The morphology of the CaCO3 particles was analyzed with scanning electron microscope (SEM, Hitachi S-3000H, Tokyo, Japan). Structural analysis for CaCO3 particles was carried out with powder X-ray diffraction (XRD, Scintag pad V, Cu Kα 1.5418 Å). Raman spectra of the CaCO3 powders were obtained using an ISA microfocus Raman spectrometer (Spectra Physics 164 Ar+ laser, λ = 514 nm), CaCO3 powders were placed on a zero background glass slide for each scan. Thermogravimetric analysis (TGA, TA 2050, TA Instruments, USA) was used to study the thermal stability and decomposition of CaCO3 powders as a function of temperature under constant air flow.
Fig. 2 shows the morphologies of the CaCO3 particles with various processing conditions. At R = 16.0 (water to octylamine molar ratio), CaCO3 particles precipitated in the bilayers are about 10 μm and form the so-called “hopper” crystals (Fig. 2a) (Buckley, 1952), each face of the crystal showing an inverse growth pyramid. The same hopper crystal morphology was observed in CaCO3 precipitated from alcohol/water solution (Dickinson and McGrath, 2003) and in calcium iodate monohydrate (Shitole and Saraf, 2002) grown by silica gel technique. Decrease the R ratio to 10.8, the thickness of the aqueous layer is compressed due to a reduced amount of water, particle size decreases accordingly. It is obvious that the particle shape changes as revealed by the SEM analysis in Fig. 2b, two types of particles, sphere-like and rhombohedral coexist in this condition. Fig. 2c shows that tabular CaCO3 particles can be produced when R ratio is 7.2, corresponding to 70 wt% octylamine in the bilayer. Average face diameter of the tabular CaCO3 particles is about 3 μm. The thickness of the primary tabular particle is unknown because of the agglomerated nature of the particles as well as the limitation of the SEM. A well-defined crystal morphology is formed with R = 16.0, although particles show hopper habit of sharp growth edges. However, at low R values with thinner aqueous layers, the morphologies of the CaCO3 particles are poorly-evolved, resulting agglomerated sphere-like (R = 10.8) and tabular (R = 7.2) shapes. More precisely, the morphology at R = 10.8 is a mixture of near perfect rhombohedra and sphere-like particle aggregates.

SEM micrographs of CaCO3 particles prepared from octylamine/water bilayer systems. (a) R = 16.0, hopper crystals of calcite, (b) R = 10.8, sphere-like and rhombohedral CaCO3 particles, (c) R = 7.2, tabular CaCO3 particle aggregate. R is the molar ratio of water to octylamine.

SEM micrographs of CaCO3 particles prepared from octylamine/water bilayer systems. (a) R = 16.0, hopper crystals of calcite, (b) R = 10.8, sphere-like and rhombohedral CaCO3 particles, (c) R = 7.2, tabular CaCO3 particle aggregate. R is the molar ratio of water to octylamine.

SEM micrographs of CaCO3 particles prepared from octylamine/water bilayer systems. (a) R = 16.0, hopper crystals of calcite, (b) R = 10.8, sphere-like and rhombohedral CaCO3 particles, (c) R = 7.2, tabular CaCO3 particle aggregate. R is the molar ratio of water to octylamine.
The various crystal morphologies of CaCO3 shown in Fig. 2 actually belong to different crystal structures as illustrated by XRD analysis in Fig. 3. The hopper crystal of CaCO3 particles takes on calcite at R = 16.0, and the dominant peak at around 30 degrees is assigned to the calcite (104) face. At R = 10.8, the XRD result of those particles shows a mixed pattern of calcite and vaterite structures, which is consistent with the SEM results in Fig. 2b. There are two kinds of particles produced in the bilayer under this condition, sphere-like and rhombohedral. The rhombohedral particles can be assigned to calcite, whose space group is R3c. Obviously, the crystal structure of the sphere-like particles is vaterite. A supportive evident for this assignment is achieved at R = 7.2, where tabular CaCO3 particle aggregates are the dominant morphology. The XRD pattern indicates that the crystal structure for the tabular particles remains the same, but the intensity of calcite (104) peak decreases significantly, which suggests the ratio of calcite to vaterite decreases. Based on the SEM and XRD measurements, combined with the crystallographic information available for CaCO3 polymorphs, the sphere-like particles in Fig. 2b and the tabular aggregates in Fig. 2c are vaterite (Dickinson et al., 2002). The other polymorph of CaCO3, aragonite, is not observed for all the synthetic powders (Sunagawa, 1987).

X-ray diffraction patterns of CaCO3 particles prepared from octylamine/water bilayer systems. Note the intensity decrease from calcite to vaterite. Miller indices from JCPDS ICDD 5-0586 (calcite) and JCPDS ICDD 33-268 (vaterite).
The laser Raman scattering results of CaCO3 particles are shown in Fig. 4. The Raman spectrum of CaCO3 is sharp for hopper crystals prepared at R = 16.0 than that of tabular CaCO3 particles at R = 7.2, which is primarily due to different crystal structures. Five normal modes for calcite are observed in the hopper crystal CaCO3, with the most intense A1g mode at 1086 cm−1, the rest vibration frequencies at 155, 281, 712 and 1435 cm−1 are associated with the Eg modes, their frequencies are well consistent with those reported by Rutt and Nicola (1974). The tabular CaCO3 particles show only two weak over-lapped bands at 1084 and 1092 cm−1 because of the low crystallinity of the particles. The spectral profile at 1084 and 1092 cm−1 is the characteristics of vaterite structure (Behrens et al., 1995; Boughriet et al., 2000; Lee et al., 2001), which verifies the crystal structure of the tabular CaCO3 particles determined by SEM and XRD analyses.
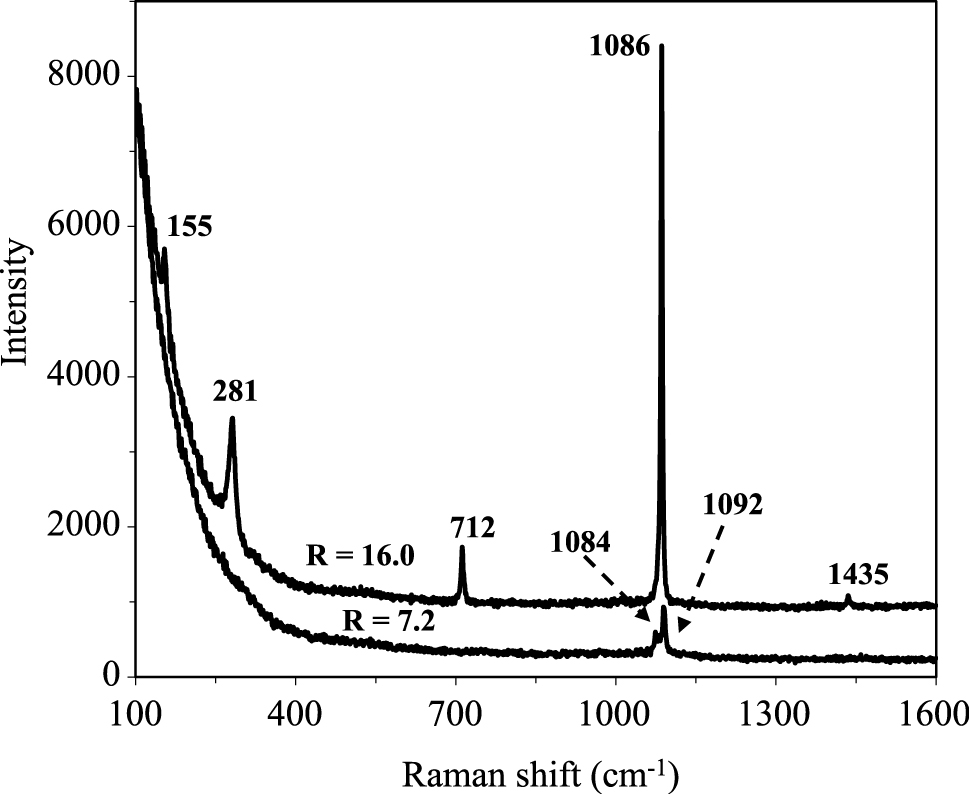
Raman Scattering of CaCO3 particles prepared from octylamine/water bilayer systems. The excitation line wavelength is 514 nm (Ar+ laser). Only the two most intense bands were observed in tabular CaCO3 vaterite due to poor crystallinity.
Thermal stability of tabular CaCO3 particles (R = 7.2) is measured by TGA and illustrated in Fig. 5. The as-synthesized particles are quite stable below 600°C, a total of about 3.4% weight loss is found which indicates the removal of the bilayer template during washing. When temperature goes above 600°C, a distinct weight loss of about 44% takes place from 600°C to 750°C. The maximum weight loss occurs at 710°C as determined by the derivative of the TGA curve, which is related to the decomposition of CaCO3 that yields CO2. The TGA measurements on hopper crystal and sphere-like CaCO3 particles follow a similar trend.

Thermal stability of CaCO3 particles measured by TGA (R = 10.8). The maximum weight loss occurs at 710°C as shown in the dTGA curve. The total weight loss is less than 4% at temperatures lower than 600°C.
The formation of CaCO3 with various morphologies under variable R ratio is quite understandable because of the nature of the self-assembly bilayer template as well as the particle growth mechanisms in confined environment (Adair and Suvaci, 2000; Mann et al., 1991; Walsh et al., 1999; Yener et al., 2002). However, the presence of hopper crystal calcite with square-like hopper faces is something beyond the expectation. There are two possible mechanisms responsible for the growth of hopper crystal calcite (Buckley, 1952; Dickinson and McGrath, 2003), thermodynamic control based on a screw dislocation and kinetic control linked to the diffusivity of constitute ions. Kinetic control may be a favorable process in this case according to Dickinson and McGrath (2003). Under kinetic model, the growth rate is different in various directions during crystallization, rapid growth appears to accentuate the differences between the preferred and other direction (Buckley, 1952). In the octylamine/water bilayer of pH 10, rapid growth of CaCO3 crystals is feasible because of the high supersaturation of Ca2+. The growth rate along body diagonal direction is faster than any other crystal directions, which explains the face-centered cavity formation as observed in SEM analysis. The apparent contradiction is that none of the three polymorphs of CaCO3 belongs to the cubic system, specifically, XRD shows the hopper crystal of CaCO3 is calcite, which means the cavity should be triangular instead of square since the crystal is rhombohedral with a space group R3c. Furthermore, Dickinson and McGrath (2003) claimed that higher viscosity (>1.6 mPa.s) alcohol water solution would promote the formation of hopper crystal calcite, unfortunately, an opposite trend is observed in the octylamine/water bilayer systems. Current results are far away from determining the dominant mechanism including viscosity effect as well as other contributions from solution pH, Ca2+ concentration and interaction of octylamine and Ca2+. However, the hopper crystal calcite may be used as a host material for inclusion chemistry where large cavity is required (Stucky and Macdougall, 1990).
Hopper crystal of calcite particles with square-like face-centered cavity are prepared in the octylamine/water self-assembly bilayer systems at R = 16.0. The morphology and crystal structure of the CaCO3 particles demonstrate significant dependence on the R ratio, with vaterite tabular aggregate achieved at R = 7.2. The mechanism of particle formation in alkaline self-assembly bilayer system is discussed with an emphasis on the observation of unique hopper crystal particles in rhombohedral calcite.
Jun Wang
Jun Wang started his professional career with Saint-Gobain Northboro R&D center in 2004 prior to joining A123 Systems in 2011, where he is currently senior development engineer in the innovations solutions group. Dr. Wang holds a BS degree from University of Science and Technology of China, a MS from Shanghai Institute of Ceramics and a Ph.D. in material science from Penn State University. His research interests include novel nanoparticle synthesis and dispersion, nanoabrasives for chemical mechanical planarization and nanomaterials for energy storage. Dr. Wang has published over 30 research papers and 4 patents.
William B. White
William B. White is professor emeritus of geochemistry in the Department of Geosciences and the Materials Research Institute at the Pennsylvania State University. He holds a BS degree in chemistry from Juniata College (Huntingdon, PA, USA) and a Ph.D. in geochemistry from Penn State. He joined the Penn State faculty in 1962 and remained there until his retirement in 2002. Dr. White has wide-ranging research interests in materials synthesis, crystal chemistry, glass science, phosphor materials, mineralogy, and hydrogeology. He has published more than 400 research papers and 13 books.
James H. Adair
James H. Adair is a Professor in Materials Science and Engineering, Bioengineering and Pharmacology at Penn State University. His research and teaching interests include biological-nanoscale composite particulates for nanomedical applications, colloid and interfacial chemistry, material chemistry, particle synthesis and characterization, and ceramic and metal particulate processing. Dr. Adair is the author or co-author of over 240 publications, thirteen patents, several copyrights on computer software and co-editor of ten books.