2014 Volume 31 Pages 214-233
2014 Volume 31 Pages 214-233
Nanopowders or nanoparticles can be used as building blocks for the preparation of new materials with a prescribed structure. Monte Carlo simulations have shown that the morphological properties (bulk porosity and surface roughness) of a granular deposit can be tailored by properly adjusting the velocity of the particles approaching the deposit. Based on these theoretical predictions, experiments have been conducted to prepare nanostructured materials from carbon nanoparticles. By electrohydrodynamic atomization of a liquid suspension (carbon nanoparticles dispersed in ethanol), an electrospray of small droplets is generated. The charged droplets are driven by the electric field with the ethanol evaporating along the droplet path, leaving dry nanoparticles that deposit on the collecting surface. The surface roughness of the resulting material has been characterized as a function of the voltage drop. Moreover, catalytic suspensions of platinum supported on carbon nanoparticles (Pt/C) in Nafion®-alcohol solutions have been electrosprayed over carbon paper to prepare electrodes for proton exchange membrane fuel cells (PEMFC). The fuel cell power density was measured as a function of the platinum loading and the range of parameters leading to optimal platinum utilization was obtained.
Powder technologies have increased their application field ranging from environmental issues of social concern (i.e. the filtration and elimination of unwanted powders and the reduction of particulate emissions from industrial activities, car engines or energy production) to the fabrication of new materials with specific properties. Thus to comply with the social needs and the environmental regulations, the control of particle emissions requires the development of clever filtration systems (Konstandopoulos and Papaioannou, 2008). On the other hand, the fabrication of advanced materials from aerosols promotes the development of new powder processing techniques (Milosevic et al., 2009; Naito et al., 2009; Yurteri et al., 2010). In all these cases, a proper knowledge of the dynamics of particle-laden gases is needed to control the particle motion (Garcia-Ybarra et al., 2006; Rosner et al., 1992) and the deposition of particles on the walls confining the gas stream (Castillo and Rosner, 1989; Castillo et al., 1990; Garcia-Ybarra and Castillo, 1997). This dynamic control allows either the delivery of the powdered material to specific locations (as it is required in filtration systems or in some pharmaceutical applications of aerosols) or to avoid the deposition of particles on some valuable surfaces (in applications ranging from large furnaces to the fabrication of microelectronic devices, for instance).
The distinctive large surface/volume (surface/mass) ratio of powders make them especially suitable for applications requiring a large active surface area which is the case for catalyst applications or pharmaceutical products, as well as for different solvent-based fabrication techniques. In this way, powders can be used as building blocks for the preparation of new materials (Luyten et al., 2010) which still retain this large surface/volume ratio as a distinctive feature. This is the central topic of this work: the fabrication of granular materials from powders (Section 3) emphasizing several ways to control the structure of the final product (based on the simulations described in Section 2) and showing in Section 4 a specific application of these granular materials (their performance as fuel cell electrodes).
Granular materials formed by the accumulation and adhesion of incoming aerosol particles acquire a morphological structure controlled by the form of the constitutive particles and by the way in which these particles arrive and attach to the forming material (Rodriguez-Perez et al., 2005, 2007). A proper control of the aerosol particle dynamics may lead to granular deposits with a well-prescribed bulk morphology and surface structure.
This paper joins together three complementary studies. First, in Section 2, a Monte Carlo simulation is used to relate the final structure of a granular material to the arrival dynamics of the constitutive particles. The knowledge gained with the simulations guides the preparation of materials with a given morphology and nanostructure. To achieve this goal, and as the key starting point, a simplified theoretical model is implemented to simulate the growth of a granular material from the deposition of aerosol particles. Monte Carlo simulations of particle deposition provide a correlation of the growing deposit morphology (bulk porosity and surface roughness) with the aerosol dynamics.
These theoretical predictions are then checked by performing some experiments conducted under controlled conditions. Thus, in Section 3, a liquid suspension containing carbon nanoparticles is electrosprayed at a constant flow rate to form a cloud of small liquid droplets. The electrically charged droplets are driven by an externally imposed electrical field and evaporate along their flight toward a collecting plate, leaving dry nanoparticles which deposit on the collector. The parameters controlling the electrospray dynamics are the physical properties of the liquid suspension (surface tension, electrical conductivity …), the flow rate and the voltages applied at the electro-spray needle and at the collector plate. For a given liquid, there is a range of flow rates where a proper combination of needle voltage and collector voltage leads to an electric field at the needle that keeps the electrospray working in a stable cone-jet mode, Martin et al. (2012). These voltages also play a major role in controlling the electrical field at the collector (which affects the dynamics of the dry nanoparticles arriving to the forming deposit). The structure of the final granular deposit is analyzed as a function of some of these controlling parameters (applied voltages and liquid conductivity) and the results are compared with the Monte Carlo simulation predictions.
Finally, in Section 4, the use of these highly porous materials as fuel cell electrodes is analyzed by preparing cathodes made by this electrospraying and deposition technique starting with a liquid suspension with carbon nanoparticles doped with platinum. The performance of such a cathode as compared with the performance of a cathode prepared by a standard (impregnation) technique is studied in a test fuel cell with controlled gas feeding, cell working temperature and outlet pressure. The platinum utilization (maximum fuel cell power per gram of platinum in the cathode) obtained with the electrosprayed cathode is an order of magnitude larger than the platinum utilization achieved with standard cathodes.
The details of this research carried out at three complementary levels (i: theoretical simulations, ii: preparation of granular materials, and iii: demonstration of their performance as electrodes) are presented in the following Section 2, 3, 4. In Section 5, the relations between these three research lines are discussed.
The growth of a granular material from the deposition of nanopowders (aerosol particles in the surrounding gas phase) comprises several steps: particle arrival, particle attachment to the deposit and deposit re-structuring. Each step is governed by some distinctive mechanisms. Thus the particle arrival is affected by the particle dynamics above the deposit. Particle attachment depends on particle arrival velocity and on the intensity of the forces between the deposit and the impinging particle (see for instance Walton, 2008). Moreover, deposit restructuring linked to the coagulation, sintering and compaction of the deposit is controlled by particle coalescence dynamics which is strongly dependent on temperature and on the presence of vapors that may condense between the particles. One may try to model the formation of a granular material including all these phenomena, but it is clear that such a model would require the determination of a large number of parameters measuring the importance of the different physical mechanisms controlling these three steps. Instead of trying to model all these details, one may focus on the mechanisms which play a major role and disregard all the other effects.
Following this approach, a Monte Carlo (MC) method has been implemented to simulate the motion of particles above the deposit and the other two effects have been oversimplified by assuming a complete passive deposit. Thus in this model (Rodriguez-Perez et al., 2005, 2007; Tassopoulos et al., 1989) once a new particle reaches the deposit, this particle remains attached at the touching location and does not move anymore. This assumption (absence of rebounding and re-entrainment of particles in the gas) is valid when the kinetic energy of the impacting particle is low with respect to the potential energy of the interaction between two particles in contact or when a kind of glue coats the particles enhancing their sticking. Moreover, deposit restructuring is disregarded and the particles incorporated into the deposit keep their original shape. This frozen deposit assumption is valid when the working temperature is not elevated (i.e. is much lower than the fusion temperature) and the ambient pressure is not too high, in such a way that particle coalescence within the deposit is precluded.
Under these simplified assumptions, the only remaining factor to control the deposit formation (i.e. the deposit evolution and the final structure of the resulting material) is the dynamics of particle arrival to the collecting surface.
In the MC simulations, time and space are discretized, depicting the particle motion at intervals of time τ and the particle position on a cubic lattice of size a (which coincides with the particle diameter). That is, τ is the characteristic time for a particle displacement equal to the particle diameter a. Therefore, time and space are measured in units of τ and a, respectively. A spatial domain with a square base L × L (i.e., na × na) and periodic boundary condition on the lateral walls is used.
The concentration of particles in the gas phase is assumed to be small and thus the motion of each particle is not affected by the other aerosol particles. In this case, the deposit growth is due to the successive arrival of uncorrelated particles and can be modeled by a sequence of independent events, each due to a single particle. Thus one can introduce a particle at a random position over the topmost height of the deposit and follow its motion until either the particle moves far away from the deposit (escaping from capture and being carried by the gas) or it touches the deposit and attaches there. The MC model simulates the motion of one particle after another, depicting the evolution of the deposit growth evolution.
The motion of an aerosol particle in a gas is split into two distinct contributions:
In this way, the assumption of independent aerosol particles (i.e. that the motion of each particle is not affected by the other aerosol particles) can be relaxed considering that the aerosol cloud generates a mean field (as in the case of electrically charged particles that will be considered in the following section) plus a random force (due to the incessant temporal and spatial changes on the distribution of particles inside the cloud). The mean field affects the deterministic motion of each individual particle (that is, the value of v), whereas the random contribution adds to the particle Brownian motion (or even plays the major role in the random particle motion). These Monte Carlo simulations do not represent the motion of aerosol particles along the whole particle path but the arrival of the particle to form the granular deposit, in this sense v stands for the (ensemble-averaged) arrival velocity which results from the gas-aerosol interactions and by the presence of other fields acting on the particle dynamics.
Therefore, the model incorporates three independent variables: a, v, and D. Once the direction of the particle velocity is given (perpendicular to the collector in our simulations), the particle speed, v, together with the characteristic length (the particle diameter, a) and the diffusion coefficient (D) can be combined to form the Peclet dimensionless number
| (1) |
This Peclet number, Pe, measures the relative importance of the mean particle motion with respect to the random particle motion. The limit Pe→∞ corresponds to a ballistic behavior of the aerosol particles moving with constant speed v and vanishing diffusion whereas the opposite case, Pe = 0, accounts for the pure diffusive limit (v→0). Indeed, the Peclet number can be written as the ratio of the characteristic times associated to the two contributions to the particle motion,
| (2) |
With the characteristic convective time given by tconvective = a/v, and the characteristic diffusive time by tdiffusive = a2/D.
On this discretized space, the two contributions to the particle motion are simulated by two stages (Fig. 1):
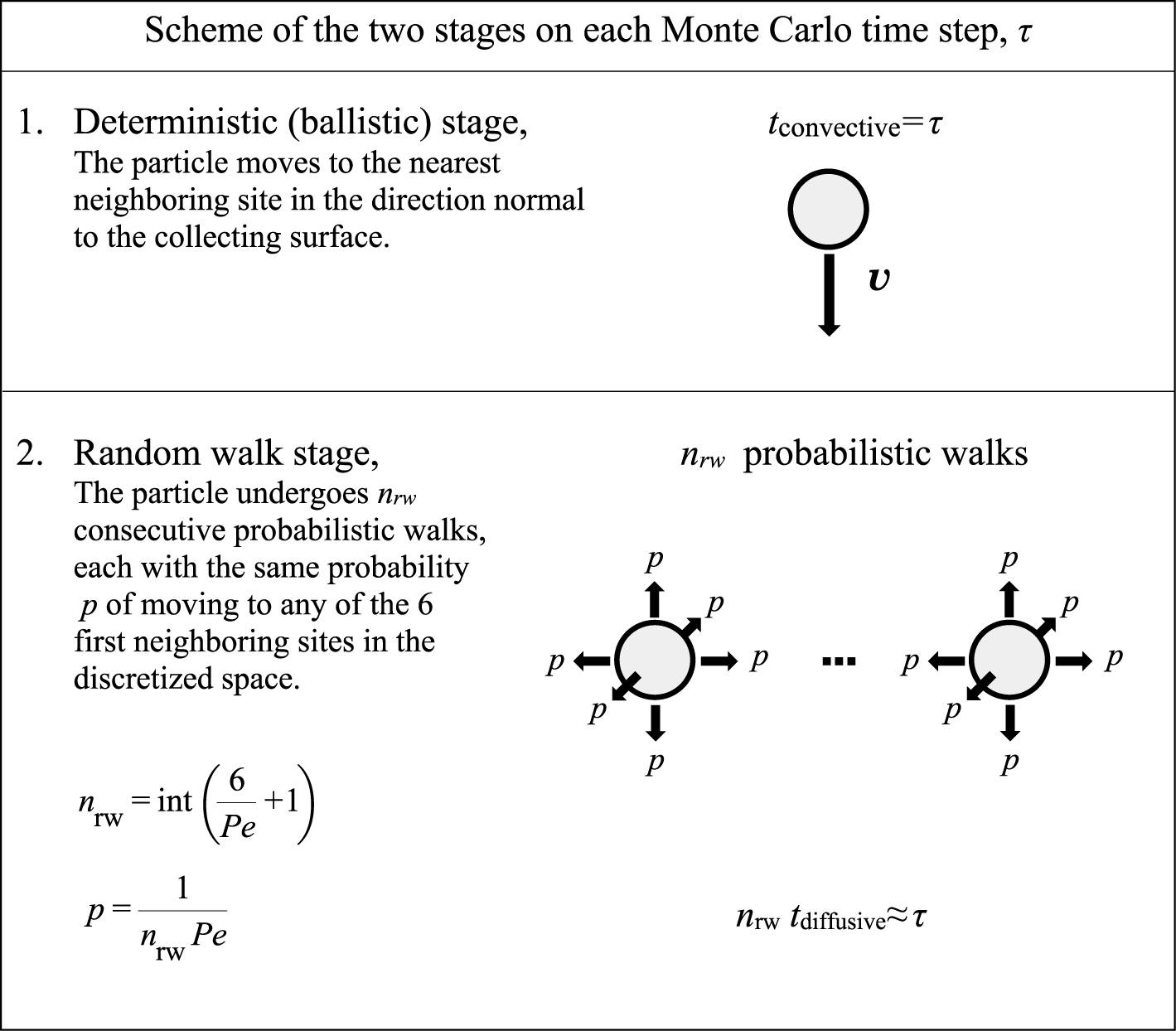
Two-stage Monte Carlo simulation. Deterministic stage and random-walk stage (with nrw consecutive probabilistic walks).
| (3) |
The characteristic diffusion time is of the order of the characteristic time for each single random motion. As in this two-stage scheme, there are nrw single random steps for each time step τ, it results tdiffusive≈ τ/nrw≈ τPe. Thus the value of the probability p is chosen to provide the value of Pe; that is p = 1/(nrwPe).
The MC simulation starts with a flat and clean collecting surface at the lower boundary of the computation domain. The simulation scheme based on these two stages is implemented to trace the motion of one aerosol particle until either it escapes from the computation domain or it touches the deposit (the collecting surface or another particle that has already being fixed to it). In the first case, the particle escapes and in the second case the particle becomes part of the deposit contributing to the deposit growth. After that, a new particle is introduced at a random location on a plane parallel to the collecting plate and located one site above the uppermost location of the deposit.
It can be shown (see for instance Tassopoulos et al., 1989 or Rodriguez-Perez et al., 2005) that this computation scheme corresponds to the discrete description of an ensemble of diluted aerosol particles whose number density (number concentration per volume), np, is governed by a convective diffusive equation (in dimensionless variables, measuring time in tconvective units and distances in units of the characteristic length, a, and therefore, velocities in units of the mean particle velocity)
| (4) |
For an attracting surface, the mean particle velocity v is directed towards the collecting surface and the relative intensity of the surface attraction with respect to particle diffusion is given by the Peclet number Pe. The sequence of particles driven to the attracting surface forms a granular deposit on this surface (Fig. 2) whose main structural features were derived by Rodriguez-Perez et al. (2005, 2007) and Castillo et al. (2010). A computer drawing of the structure of the granular deposits is given by Fig. 3a showing the lateral view of a deposit for a low value of the Peclet number (Pe = 0.1) with the grey intensity decreasing with the depth of the field in the figure (for illustration purposes, only the upper half of the deposit is shown). On the other hand, Fig. 3b shows the upper view of the same deposit characterized by bunches of particles forming a rough surface on the scale of the particle size. Moreover, Figs. 4a & b depict the lateral and upper view, respectively, of a deposit formed by particles whose motion is characterized by Pe = 1. Lastly, Figs. 5a & b correspond to Pe = 100. In all these figures, the MC domain has a square base of 512 × 512 cells and the simulation is stopped when the same maximum deposit height (hmax = 1024 a) is reached. It becomes clear that ballistic-dominated deposition (large Peclet numbers, Figs. 5) leads to compact deposits with a rough patched surface. However, diffusion-controlled deposits (low Peclet numbers, Figs. 3) are tree-like and fluffy, with a very porous bulk structure formed by open structures and a highly branched surface.

Sketch of deposit growth on an attracting surface. Particle arrival by deterministic + diffusive transport.
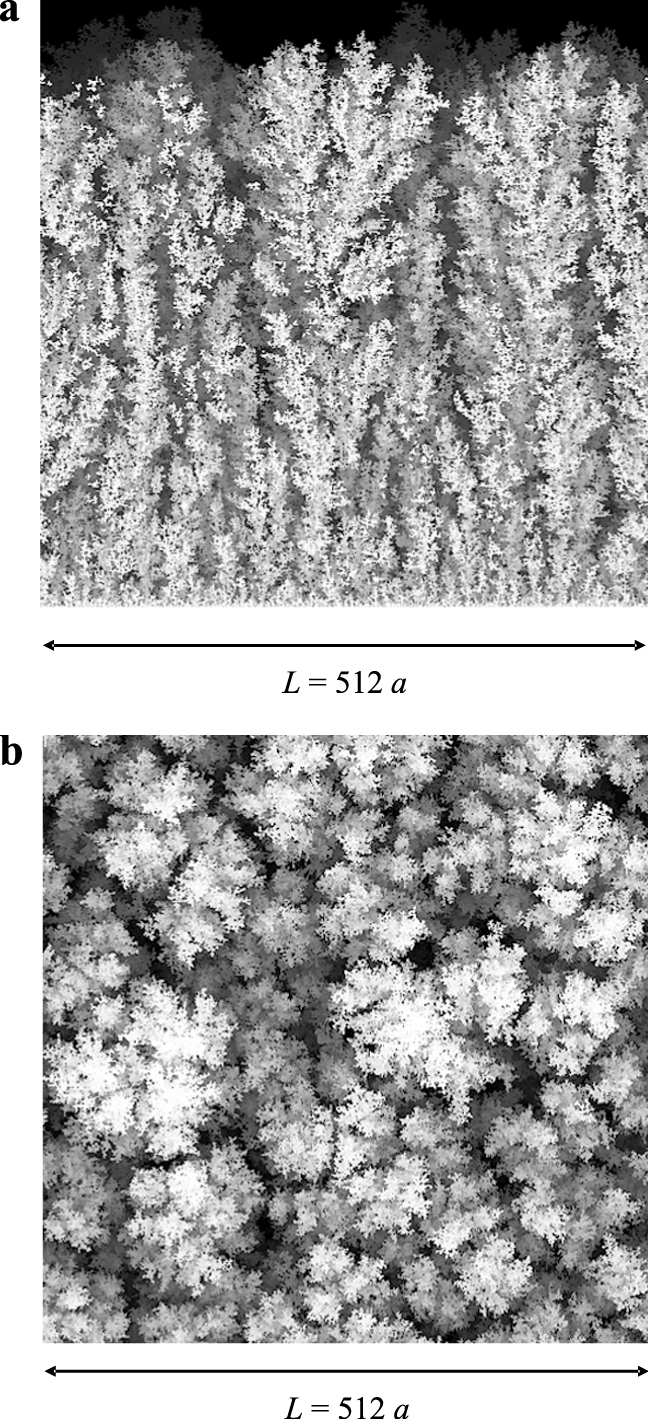
a, Lateral view of the granular deposit with the grey intensity decreasing with the depth of the field. Here Pe = 0.1, L = 512a, hmax = 1024a. b, Upper view of the same deposit shown in Fig. 3a.

a, Lateral view of the granular deposit with the grey intensity decreasing with the depth of the field. Here Pe = 1, L = 512a, hmax = 1024a. b, Upper view of the same deposit shown in Fig. 4a.
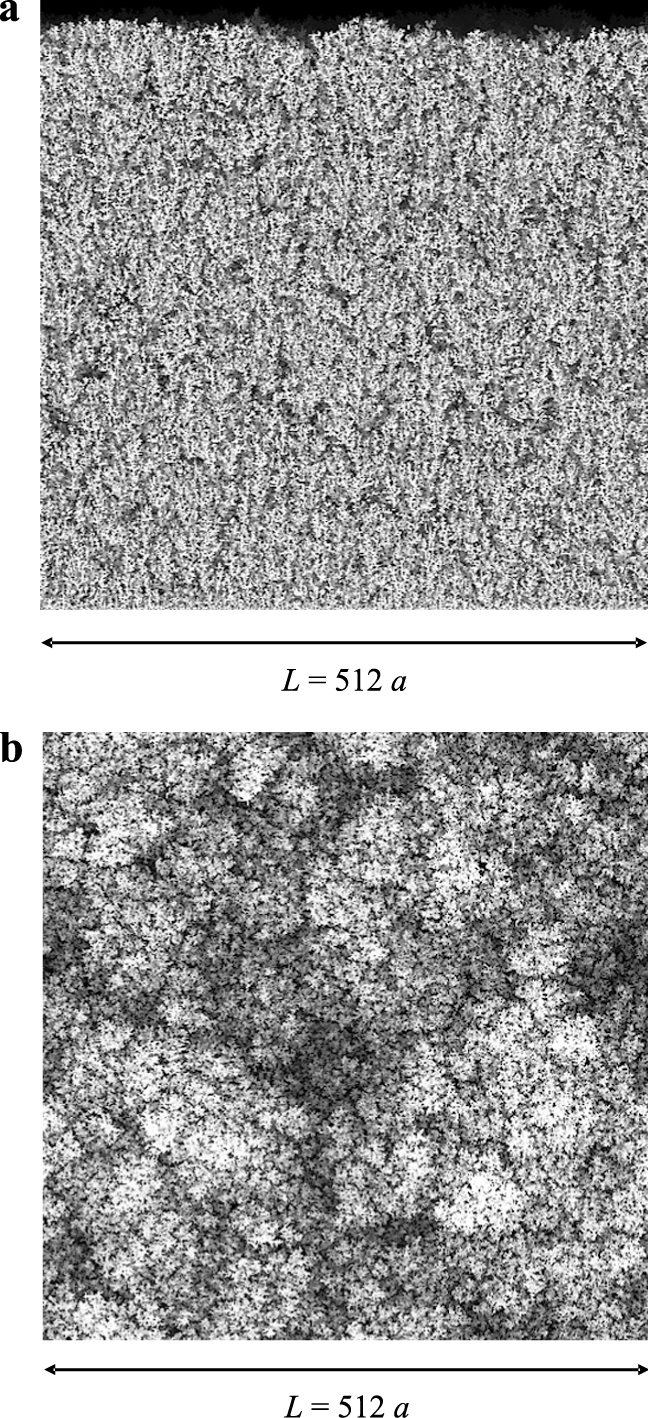
a, Lateral view of the granular deposit with the grey intensity decreasing with the depth of the field. Here Pe = 100, L = 512a, hmax = 1024a. b, Upper view of the same deposit shown in Fig. 5a.
The deposit bulk structure can be analyzed by determining the mean density (the occupation number or relative number of space cells occupied by particles within the deposit) at different heights, ρ(h). To achieve representative values, the density at each height is averaged over 10 deposit simulations performed with the same Peclet number. It turns out that the bulk of the granular deposit becomes layered in three different regions (Fig. 6):
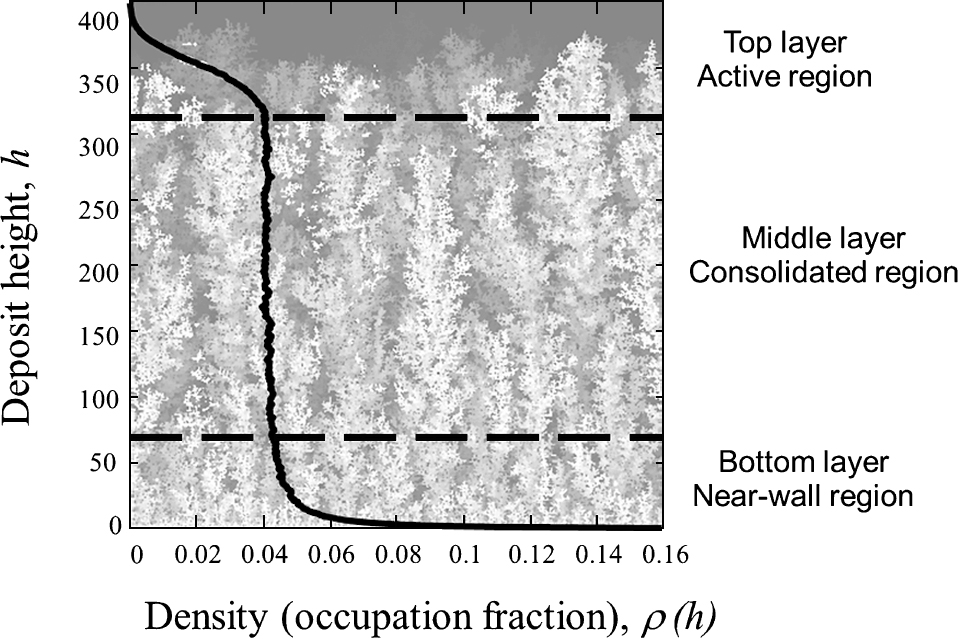
Density profile (averaged over 10 deposit simulations performed with the same Peclet number) showing the three-layer structure of a deposit for Pe = 0.1, the deposit height h measured in units of cell size, a.
The size of the bottom layer depends on the Peclet number, whereas the thickness of the active regions increases slightly with time. Some properties of these two extreme regions were provided by Rodriguez-Perez et al. (2005, 2007). Here we will focus on the features of the consolidated region. The thickness of this central consolidated region depends on the deposition time. In Fig. 7, the density profile (averaged over 10 deposits) for Pe = 0.1 is represented, depending on the maximum deposit height, hmax (measured in units of cell size, a). It becomes clear that the arrival of new particles to the deposit (increasing hmax) enlarges the thickness of the consolidated region where the mean density is almost constant and time-independent, as it was already obtained by Houi and Lenormand (1984).

Density profile (averaged over 10 deposit simulations) for Pe = 0.1 and different maximum heights hmax (measured in units of cell size, a). The arrival of new particles at the deposit (increasing hmax) enlarges the thickness of the consolidated region where the mean density is almost constant and time-independent.
In Fig. 8 the mean density profile is depicted for deposits grown at different Peclet numbers up to the same maximum height, showing that the consolidated region becomes more porous (and the depth of the active region thickness increases) as Pe decreases. When the tendency of the particles to move toward the collecting surface is reduced, a more porous deposit with a larger active surface is obtained.

Density profile for the same maximum height hmax = 400 (measured in units of cell size, a), and different Pe numbers.
A detailed analysis of the granular deposits for different Pe numbers leads to correlation of the mean density of the consolidated region with the particle Peclet number, Pe (Fig. 9), resulting in
| (5) |
| (6) |

Mean density in the consolidated region of the deposit as a function of Pe.
The fitting expression Eq. (5) has a general application whereas the numerical values of the fitting coefficients given in Eq. (6) indeed depend on the model features. Thus, for on-lattice models like the one used here, the limiting occupation fraction for ballistic deposition is ρ∞ = 0.302. However, for off-lattice models (when the particle location is not restricted to the lattice cells but can be any point in the computational space), a packing density (normalized with respect to the maximum density of fully packed spheres) ρ∞ = 0.17 is achieved (Jullien and Meakin, 1987). Moreover here, ρ∞ accounts for the relative number of occupied cells, but for comparison with the mass density of actual deposits one has to take into account the fraction of the cell occupied by a particle, which in the case of a spherical particle in a cubic lattice corresponds to π/6. Moreover, the value of Pe0 depends on other model features such as the space dimension or the angle between the mean velocity and the collecting surface (taken as perpendicular directions here).
Note that in the case of a material with fractal dimension DF, when looking at a cubic volume V of side 2R built on any point inside the material, the mean occupation fraction would depend on R in the form
| (7) |
Based on Eq. (7), the fitting expression (5) defines a characteristic length
| (8) |
Thus diffusion dominates on the short distances being responsible for the fractal structure of the deposit on the small length scales up to RPe, whereas convection introduces the cut-off distance RPe (which increases as diffusion becomes more and more important) beyond which a constant mean density is achieved and the fractality is swept off.
Therefore, the numerical value of the exponent B in Eq. (5) is related to the fractal dimension induced by diffusion at the short scales. For diffusion-limited deposition, a deposit in the pure diffusive limit (Pe = 0) has a fractal dimension DDLD = 2.5 (Tolman and Meakin, 1989), and it turns out that
| (9) |
Eq. (5) provides the density of a granular deposit as a function of the particle dynamics in the gas over the collecting surface, with the dynamics characterized by the Peclet number. On the base of these simulations, one can prepare materials with a prescribed bulk density or mean porosity and with a tailored structure by controlling the way the particles arrive at the attracting surface. Also, layered materials formed by the superposition of layers with a different mean density can be prepared by modifying the particle dynamics during different timed intervals.
This result has a broad application. For example, Elmøe et al. (2009) carried out a simulation of particle capture by filters and found out that the dusty layer growing over the filter has a mean density which is well fitted by Eq. (5).
Indeed, the MC simulation described here is free from any constraining hypothesis, and thus Eq. (5) is expected to be valid in any dusty layer created by accumulation of fine particles in the absence of erosion or deposit consolidation. The simulation opens doors for the design of controlled experiments dealing with material processing from aerosols. One of these doors is explored in the following section.
The simple model underlying the MC simulations described in the previous section shows the trends for controlling the deposit structure and indicates several ways to prepare granular materials from nanopowders with a tailored morphology depending on their final use. A step forward in nanomaterial research will consist of the performance of experimental studies that could confirm these theoretical predictions. To achieve this goal, an experimental set-up (sketched in Fig. 10) was implemented to generate nanoparticles in a gas that are later driven towards a collecting surface. In this study, a standard catalyst ink commonly used for preparing electrodes that are valuable for proton exchange membrane fuel cells (PEMFC) was used. The inks are constituted by a catalyst supported on carbon nanoparticles (Pt/C: 10 wt% Pt on Vulcan XC-72R) dispersed in a liquid solvent (ethanol 96% v/v, Aldrich) and an ionomer (Nafion® 5 wt% in lower aliphatic alcohols and water). The Nafion® loading was fixed to 30 wt% in the solid fraction (fraction of dry mass of Nafion® on the dry mixture of Nafion® and Pt/C). The electrical conductivity of the liquid suspension was measured as a function of the concentration of carbon nanoparticles (Fig. 11), showing that the electrical conductivity can be well fitted by a linear dependence on the concentration of carbon nanoparticles.

Sketch of the experimental set-up. The liquid suspension in the syringe is pumped through the needle and a potential difference between the metallic needle and the collector substrate is maintained. Evaporation of the droplets formed in the electrospray leaves dry charged nanoparticles which are attracted by the substrate.

Liquid electrical conductivity dependence on the concentration of carbon nanoparticles in the suspension.
The electrospray technique (Barrero and Loscertales, 2007, Jaworeck and Sobczyk, 2008) is used to generate almost monodispersed sprays of small electrically charged droplets from this liquid suspension. The liquid suspension is ultrasonically dispersed for at least 2 hours before the electrospray deposition is started. Then, a syringe pump (KDS 100) drives the liquid suspension through a stainless steel needle (inside diameter of 0.75 mm) at a constant flow rate Q. Moreover, by means of two DC high-voltage power supplies (Bertan 225-30R and Spellman MM15P2.5W, respectively), a high voltage difference is applied between the metallic needle (kept at a constant voltage Vn) and the flat collecting plate (at a voltage Vp) respectively, with this plate located some distance away from the needle tip. The electric charge passing through the needle and the charge collected by the plate are measured by two ammeters (Isotech IDM67) inserted in the circuit lines of the needle and the substrate connections to their respective power supplies.
The electrically conducting liquid in the needle is affected by the electric field there (Fernandez de la Mora, 2007; Higuera, 2010), which leads to an accumulation of charges at the liquid surface formed at the needle exit. Under suitable conditions, the electrical stresses elongate this surface to form a Taylor cone with a very thin jet emerging from the cone tip (Fig. 12). In this cone-jet configuration, the jet is steady and adjusts to the fixed flow rate Q. At some distance away from the cone tip, the jet breaks up in a spray with droplets much smaller than the needle diameter forming a cloud of tiny droplets with a narrow distribution of droplet sizes (Fig. 12). The flow rate and liquid properties (conductivity, surface tension, density) determine the characteristic droplet size (Chen and Pui, 1997; Fernandez de la Mora and Loscertales, 1994; Gañan-Calvo et al., 1997; Yurteri et al., 2010). Due to the dependence of the electrical conductivity on the nanoparticle concentration (Fig. 11), a wide range of droplet sizes can be analyzed. These charged droplets evaporate along their flight in the gas leaving dry charged nanoparticles which are driven by the electric field towards the collecting surface. The arriving nanoparticles accumulate on the collector plate and form a structured granular deposit. The Nafion® covering the nanoparticles facilitates their adhesion to the deposit and reduces particle surface diffusion after adhesion. Fig. 12 is a composed picture representing the whole electrospraying process. The cone-jet at the needle exit forms the electrosprayed droplet cloud and the dry nanoparticles form a nanostructured deposit on the collector plate. For illustration purposes, the deposit image in Fig. 12 is an enlarged SEM image superimposed on the needle-electrospray picture with the scale for the SEM image given by the scale bar at the bottom, whereas the 2-mm bar is the scale for the rest of the picture.

Composed picture of the electrospray depicting the cone-jet and the electrosprayed cloud together with the resulting nanostructured deposit. For illustration purposes, the SEM image of the deposit is enlarged with a different length scale (given by the horizontal bar at the bottom).
The cone-jet mode remains stable for a range of parameters (Cloupeau and Prunet-Foch, 1994; Li, 2007; Martin et al., 2012; Noymer and Garel, 2000; Ragucci et al., 2000; Tang and Gomez, 1996; Yurteri et al., 2010). Roughly, when the electrical field at the needle tip is weak, the surface accumulation of charges is low and the electrical stresses are not able to comply with the flow rate Q. Then, the cone-jet cannot be maintained in a steady way and the system works in a dripping mode with a sparse sequence of large droplets being formed at the needle tip. The droplet grows by the continuous arrival of liquid pushed by the syringe pump until the meniscus cannot support the attached droplet (as large as the needle diameter) which detaches from the needle and a new dripping cycle starts. Moreover, for high enough voltage differences, the electric field at the Taylor cone is quite large, the cone-jet becomes unstable and the electrospray evolves to a more complex configuration (Gu et al., 2010; Kim et al., 2011): oscillatory mode, multiple-jet mode or electrical discharges in the surrounding gas, among others.
The parameter space where the electrospray works in the steady cone-jet mode is one of the issues that must be investigated in advance for determining the range of flow rates and the corresponding applied voltages for which the liquid suspension can be electrosprayed in a regular and continuous manner (Martin et al., 2012). Fig. 13 shows the steady cone-jet domain of a catalyst ink (whose electrical conductivity was 5.77 × 10−4 S m−1 and for a needle-substrate distance fixed to 3.5 cm) when a positive voltage is applied to the needle while the collector plate is connected to ground. In this cone-jet mode the electrospray induces primary droplets and smaller satellite droplets with most of the electrosprayed liquid mass contained in the primary droplets. To obtain the cone-jet mode domain given in Fig. 13, the different electrospraying regimes have been identified by observing the liquid meniscus at the needle tip. The liquid shape at the needle exit was visualized using a CCD camera (Panasonic AW-E600E) coupled with an optical zoom (Optem 70XL). Due to the known hysteresis phenomena linked to the cone-jet stability, the voltage range leading to a stable cone-jet was determined, starting always with a formed cone-jet. Therefore, the resulting range of voltages for a stable cone-jet corresponds to the widest possible range. Starting with a stable cone-jet within the stability island, the applied voltages were either decreased or increased until the cone-jet became unstable, giving rise to the dripping mode or the multijet mode, respectively.
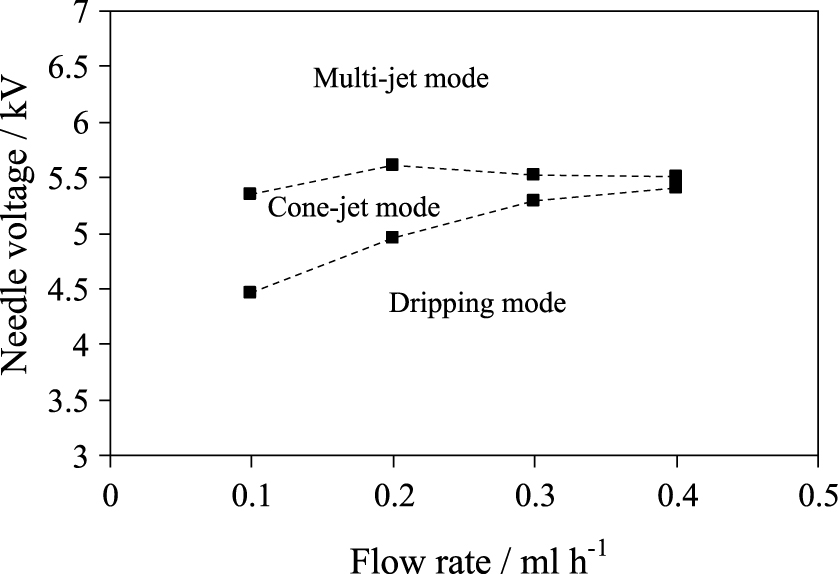
Stability domain for electrospraying a catalytic ink in the cone-jet regime when a voltage is applied to the needle but the substrate is connected to ground. The electrical conductivity of the catalytic ink was 5.77 × 10−4 S m−1 and the needle-substrate distance was fixed at 3.5 cm.
In a recent work (Martin et al., 2012), the stable cone-jet domain was extended by keeping the collector under a negative voltage (and the surroundings to ground). Larger flow rates can be achieved with this double polarization configuration and the electrospray remaining in a stable cone-jet mode.
Outside of this cone-jet domain, the nanoparticles arriving at the deposit would have a broader distribution of sizes (due to the uneven atomization process and the subsequent coagulation of the nanoparticles within the original liquid droplets when drying), and each nanoparticle will have a different dynamics when approaching the collector (due to the dependence of the electrical mobility on size and charge of the particle). Therefore, to compare the morphology of the nanostructured deposits with the results obtained with the MC simulations described in Section 2, the electrospray should work in the steady cone-jet mode.
The main objective of the electrospraying process is to collect all the charged nanoparticles emitted by the needle on the substrate and to form, after the solvent evaporation, a porous deposit consisting of the catalyst particles. The efficiency in the capture of the electrosprayed particles may be substantially improved if an opposite voltage with respect to the needle voltage is applied to the collector substrate. In the double polarization scheme depicted in Fig. 10, the charged particles are forced by the electric field to deposit preferentially over the substrate, thus reducing the leakage of flying nanoparticles and improving the performance of the deposition process. Moreover, the electrospray cone-jet domain may be substantially enlarged by proper selection of the voltage at the collecting plate, and a noticeable increase in the flow rate range for electrospraying in the cone-jet mode can be achieved (Martin et al., 2012). Thus, in summary, the double polarization can be used to control the electric field at the needle tip (which indeed is the key factor for achieving a stable cone-jet electrospray) as well as the electric field at the collecting plate (which determines the particle motion over the deposited material and thus the corresponding particle Peclet number). The Peclet given by Eq. (1) becomes
| (10) |
As expected from Eq. (10) and from the results of the MC simulations given in Section 2.2, the nanostructure of the granular deposit depends on the suspension properties, on the mass flow rate Q, and on the voltages (Vn and Vp) applied.
In all the cases presented in this section, the electro-sprayed deposits were carried out at a needle-substrate distance of 3.5 cm and a voltage drop of 7 kV between the needle and the collector plate (+5.5 kV voltage applied at the needle with the collector kept at −1.5 kV). This double polarization configuration has two main advantages:
Fig. 14 provides some SEM images of the deposited catalyst layers with the same deposit accumulative mass (same final ultra-low Pt loading, 0.01 mgPtcm−2) obtained for several flow rates and different electrical conductivities of the catalyst ink (note that the liquid electrical conductivity increases with the concentration of nanoparticles in the original liquid suspension as shown in Fig. 11). The micron bar in Figs. 14a–d and f is 20 μm in length, whereas in Fig. 14e, it corresponds to 500 nm. Moreover, Fig. 15 shows a diagram of the flow rates and conductivities of the catalyst ink that give rise to the different morphologies depicted in Fig. 14.

SEM micrographs of the morphologies of the electrosprayed deposits classified as: a) fractal-like, b) patched clusters, c) and d) compact structures. Moreover, e) shows a closer view of the building blocks forming the fractal-like deposits, whereas f) shows the carbon paper substrate used as deposition substrate. All the electrosprayed deposits were carried out at a needle-substrate distance of 3.5 cm, a voltage drop of 7 kV (+5.5 kV voltage applied at the needle and the collector kept at −1.5 kV) and the Pt loading was 0.01 mgPtcm−2. The micron bar in figures a)–d) and f) is 20 μm in length, in e) the bar corresponds to 500 nm.
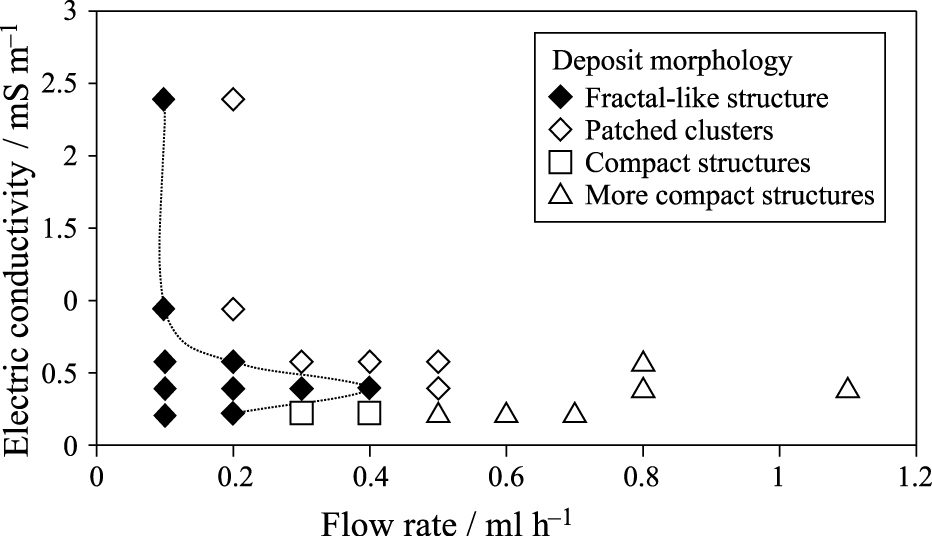
Two-parameter diagram with the different types of deposit structures shown in Fig.14, depending on the electrical conductivity of the catalytic ink and the flow rate. Symbol ♦ corresponds to type a) fractal-like, ⋄ is for b), □ for c) and ▵ for type d) deposits.
Each SEM image in Fig. 14 is representative of a type of deposit. The morphology of the catalyst layer can be classified as a fractal-like structure (a), accumulation of patched clusters (b), compact deposits based on a fractal structure (c) and compact deposits formed from patched clusters (d).
Thus Fig. 14a shows a fractal-like deposit characteristic of low Peclet numbers which are obtained for low-conductivity suspensions electrosprayed at moderate flow rates (♦ points in Fig. 15). Moreover, Fig. 14e shows a closer view of the same deposit.
The fractality of the granular deposit is better seen in the general views a) to d) than in the closer views such as e). In Fig. 14a there is no evidence of any dominant macroscopic length because the corresponding characteristic length RPe (see Eq. 8) is larger than the picture size (compare with the simulation shown in Fig. 3b, corresponding to Pe = 0.1, which leads to RPe ≅ 50). Figs. 14b to 14d show large and rather homogeneous clusters where fractality is confined to much smaller sizes (see Figs. 4b and 5b, corresponding to Pe = 1 and Pe = 100, respectively, leading to RPe ≅ 5 and RPe ≅ 1, respectively).
These fractal-like structures (Figs. 14a and e) are composed of clusters (of a few catalyst particles each) with a characteristic size of approximately 100 nm and arranged in a dendritic way. This is the most appropriate type of catalytic deposit for PEMFC electrode preparation (due to the large active surface area). The small size of the catalyst clusters causes most of the catalyst to be exposed to the surface and available for the reactant gas. Besides this, the dendritic arrangement of the catalyst clusters results in a highly porous deposit with an enhanced permeability and increased active surface. One can expect that all these morphological properties of the granular material lead to high catalyst utilization when used as a catalyst layer.
On the other hand, Fig. 14b shows a deposit formed by patched clusters of thousands of single particles (⋄ points in Fig. 15). These patched clustered deposits (composed of large aggregates of catalyst clusters with sizes in the micrometer range) appear for moderate Peclet numbers (larger flow rates than those for fractals deposits). Here, the granular material is more compact, leaving void spaces between the substrate fibers which diminish the effective surface of the catalytic layer. There is a significant amount of the catalytic material located in the inner regions of these large clusters that may lead to poorer catalyst utilization. Furthermore, Fig. 14c & d are compact deposits (with the arriving particles compacting on the initial carbon paper which is shown in Fig. 14f) representative of large Peclet numbers (achieved when the flow rate is large enough, see □ and ▵ points in Fig. 15). These compact deposits result from an incomplete evolution of the droplets during their flight toward the substrate. The droplets do not have enough time to evaporate and Coulomb fissions (Maxwell explosions due to the accumulation of electrical charges on the liquid droplet whose repulsion overcomes the surface tension) are not in effect to reduce the droplet size. Therefore, the deposit is formed from wet suspensions with the ethanol evaporating later on. Note that the Monte Carlo simulation described in Section 2 considers that any particle reaching the deposit attaches to the touching position. This condition is not fulfilled in the case of collected droplets carrying a dry residue. Therefore, the results derived from these simulations cannot be directly used when complete drying does not occur during the droplet flight but the droplet evaporates after arrival.
When the Peclet number associated to the arriving droplets corresponds to a fractal-like deposit, the resulting dry compact deposit is as the one depicted in Fig. 14c, with fractal patches formed on the original fibers. Lastly, when the droplet Peclet number corresponds to a clustered deposit, the structure of the dry deposit is depicted by Fig. 14d, with denser deposits on the fibers leaving larger voids between them.
The diagram depicted in Fig. 15 represents the region of the parameter space (electrical conductivity versus flow rate, for a constant voltage drop and a fixed needle-plate distance) leading to fractal-like structures (with the limits of this fractal-like region depicted by the dashed line). The deposit structure is different on each side of the dashed line. For a given electrical conductivity of the catalytic suspension (see Fig. 11), there is a threshold flow rate below which fractal-like structures are formed. Flow rates higher than this threshold value give rise to patched cluster structures or compact structures. The diagram shows that the range of flow rates leading to fractal-like structures expands as the electrical conductivity of the suspension decreases until a critical electrical conductivity is reached. For even lower electrical conductivities, the range of fractal-like deposits is substantially reduced. Moreover, beyond the fractal thresholds and above the critical electrical conductivity, the deposit structure is based on patched clusters. Finally, for electrical conductivities lower that the critical value, the fractal deposit becomes first compact based on fractals and later on compact based on patched clusters as the flow rate increases.
When the needle-substrate distance is increased sufficiently, the ethanol has enough time to evaporate along the droplet flight and only dried base deposits (type a or b) are achieved. Work to obtain a complete diagram of the deposit microstructure depending on several controlling parameters is currently underway.
On the base of the knowledge gained with these experiments, the suspension with an electrical conductivity of 5.77 × 10−4 S m−1 was electrosprayed under the same conditions (3.5 cm distance between needle and collector, and a voltage drop of 7 kV) for longer time periods on a carbon paper collector with two different flow rates: a flow rate Q = 0.2 ml h−1 lying in the range of fractal deposits, and a larger flow rate Q = 0.5 ml h−1 beyond that domain and leading to patched cluster deposits (see Fig. 15). A square carbon paper with a dimension of 5 cm2 was used as a substrate. The mass of deposited nanoparticles was the same in both cases (leading to the same Pt loading of 0.1 mgPtcm−2 in the catalytic layer). Fig. 16 shows SEM lateral views of the respective catalytic layers deposited over the carbon paper substrate.

Cross-sectional SEM micrographs of catalytic deposits electrosprayed over a carbon paper substrate (a) fractal-like structures for a flow rate Q = 0.2 ml h−1 and (b) patched clustered deposit obtained for Q = 0.5 ml h−1. Same platinum loading (0.1 mgPtcm−2) and same micron bar (300 μm) in both images.
The SEM image of the deposit made at a flow rate of 0.2 ml h−1 (Fig. 16a) reveals its fractal character as it is formed by dendritic trees approximately 300 μm in height, and resembling very much the MC simulated deposits for low Peclet numbers (see Fig. 3a). However, the deposit made at the higher flow rate of 0.5 ml h−1 (beyond the fractal threshold in Fig. 15) exhibits a more compact structure as it is based on the growth of patched clusters that lead to globular structures after a long deposition time (Fig. 16b). The same amount of material was deposited in both cases (Figs. 16a and b). Therefore, at the higher flow rate (Fig. 16b) and as consequence of the compactness, the thickness of the deposit was reduced to about one-half with respect to the deposit shown in Fig. 16a.
The porosity for these deposits has been estimated as the proportion of the void volume to the total volume of the catalytic layer (Baturina and Wnek, 2005; Gasteiger et al., 2003). Despite the difference in the thickness of the catalyst layer, the porosity is very similar in both cases. For the fractal deposit made at the lower flow rate (Q = 0.2 ml h−1, Fig. 16a), a porosity of 98% was measured, whereas the more compact deposit (Q = 0.5 ml h−1, Fig. 16b) has a 95% porosity (thus, the occupation fraction or mean density was doubled in the second deposit as it becomes clear from the reduction in the deposit height). In any case given their high porosity, both types of deposits will present similar mass transport resistances in the gas phase although, as was indicated before, the catalyst utilization will be lower in the compact deposit because a significant amount of the catalyst is incrusted inside the patched cluster and is thus unavailable for the reactants in the gas.
It should be mentioned that these values of material porosity are significantly higher than those reported in the literature for catalyst layers prepared by other conventional techniques. Thus, for the decal method (Gasteiger et al., 2003) and the air-brush spray technique (Ihonen et al., 2002), porosity values in the range of 60% and 30%, respectively, have been obtained for catalyst layers with loadings close to 0.1 mgPtcm−2. Using the electrospray technique, Baturina and Wnek (2005) reported a porosity of 84% for a similar catalyst loading (0.09 mgPtcm−2).
To complete the analysis, the surface distribution of the catalyst and the ionomer was determined by energy-dispersive X-ray spectrometry (EDX) for the fractal-like catalytic layer presented in Fig. 16a. Fig. 17 shows the EDX spectrum with the elements detected in the sample surface corresponding to the catalyst (Pt/C) and the Nafion® ionomer (sulfonated tetrafluoroethylene). Table 1 provides the results from a local quantitative analysis of the catalytic layer shown in Fig. 16a, measured at different locations on the top surface of the material. No appreciable composition differences on the surface of the catalytic layer were observed, indicating that the electrospray process occurs in a spatially uniform manner (same radial distribution). This means that in the electrospray phase, the constituents of the catalyst ink are homogenously distributed and there is no accumulation of a component in any particular region within the electrospray. Therefore, the atomization-deposition process maintains the homogeneous distribution of the original liquid suspension. Note that the double polarization with the collector at an opposite voltage with respect to the needle is used to focus the charged particles to the collecting area. Moreover, the main contribution to the deposit comes from the larger primary droplets as the tiny satellite droplets carry a small fraction of the nanoparticles in the liquid suspension.

EDX spectrum of the fractal-like deposit shown in Fig. 16a.
| EDX Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element composition (wt. %) |  |
 |
 |
 |
 |
 |
 |
 |
 |
| C | 19.70 | 19.87 | 19.46 | 19.76 | 18.68 | 18.12 | 19.60 | 19.54 | 19.40 |
| Pt | 5.24 | 4.93 | 4.81 | 5.64 | 5.50 | 4.76 | 4.91 | 5.14 | 5.41 |
| F | 63.93 | 64.23 | 64.70 | 63.60 | 64.67 | 66.16 | 64.22 | 64.06 | 63.90 |
| S | 1.61 | 1.64 | 1.68 | 1.70 | 1.80 | 1.85 | 1.79 | 1.76 | 1.72 |
| O | 9.51 | 9.33 | 9.34 | 9.51 | 9.33 | 9.11 | 9.47 | 9.50 | 9.57 |
The morphological properties of the deposits discussed in this section confirm the MC simulations results of Section 2.2. Fractal and open deposits are obtained when diffusion dominates the deposition process (low Peclet number corresponding to a weak electrical field at the collecting surface in the case of electrosprayed suspensions), whereas the deposit porosity decreases as the relative importance of diffusion decreases (the Peclet number increases). These results provide a way to prepare materials from powders that have a given consolidated structure. The main features of this analysis are not restricted to any deposition mechanism (the electrical drift of charged particles in the experiments shown here) but have a broader application. Indeed, it applies to any other particle-dominated transport combined with particle diffusion.
Given the morphology of the materials formed by this technique (high porosity and large effective surface area), they can be used as catalytic layers with wide applications. Their use as electrodes for PEM fuel cells is discussed in the next section.
The morphological properties (structural stability, large porosity, low weight and high active surface) of granular material growth from nanopowders as those shown in Fig. 16 make them suitable for use as catalytic materials. A small amount of catalyst on the nanopowders composing the material will be enough to achieve a high catalytic performance, as the catalyzers on the original powders will still remain mostly at the active surface of the generated material. These days, the proper manufacture of fuel cell electrodes is one of the areas requiring a high utilization of catalyzers, as Pt requirements strongly affect the development of these technologies. Indeed, DOE 2007 pointed out that one of the main challenges that have to be overcome for introducing fuel cells as competitive power supply devices is to reduce the catalyst loading at the electrodes without compromising the fuel cell performance by means of increasing the catalyst utilization (maximum power output per gram of Pt at the electrodes).
In a Proton Exchange Membrane Fuel Cell (PEMFC) working with hydrogen and oxygen as reactants, both gases (in some cases the oxygen diluted in an inert gas such as nitrogen) enter through different inlet ports (Fig. 18) and are distributed by the gas diffusion layers (carbon fiber paper or carbon fiber cloth). These gas currents are kept separate by a Membrane Electrode Assembly (MEA). The MEA is formed by two porous catalytic materials (acting as electrodes) with a thin membrane (the polymeric electrolyte) between them. Hydrogen is dissociated by adsorption on the Pt at the surface of the anode; the electron in the hydrogen atom generates the fuel cell electrical current, whereas the proton diffuses through the anode and the electrolyte to reach the cathode surface. On the cathode side, oxygen molecules are also adsorbed on the platinum dots and the oxygen atoms react with the incoming protons (receiving an electron from the electrode) to form water. Water management is quite important in these fuel cells as a certain degree of humidity in the MEA is required to favor the proton transport, whereas flooding of the cathode would inhibit the adsorption of oxygen and stop the reactive process. To achieve an effective process, the electrodes should be:
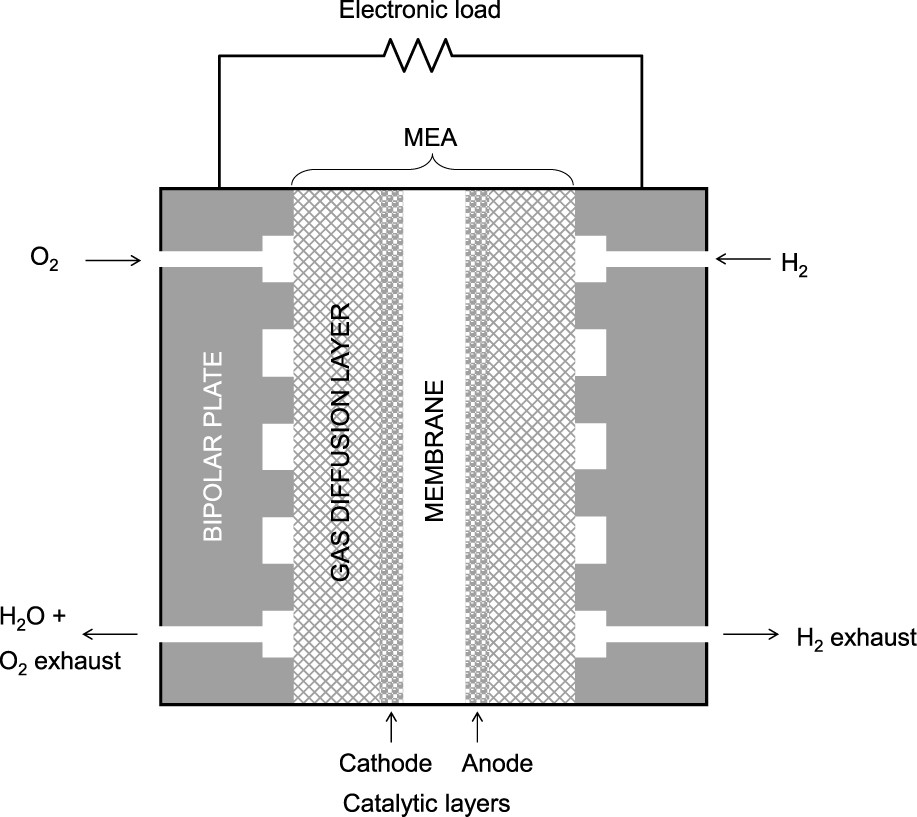
Schematic of a hydrogen-oxygen PEM fuel cell.
The materials described in the previous section which were generated by electrospraying a liquid suspension containing Pt-doped carbon nanoparticles fulfill all these requirements. They have over a 90% porosity (see Fig. 16) and are good electrical conductors due to the combination of the carbon nanoparticles (electron conductors) with Nafion® (proton conductor). Moreover, the Pt dots on the carbon nanoparticles form a large catalytically active area not just restricted to the topmost region of the material but well distributed on the entire surface inside the material pores. Based on these properties, we used them as the cathode in an MEA and tested their efficiency in a fuel cell monitored in our laboratory (Martin et al., 2010a, 2010b).
In these MEAs, the electrolyte was a Nafion® 212 membrane compressed between the two electrodes. The ensemble was bonded by hot pressing at a pressure of 5 MPa and a constant temperature of 120°C applied for 2 min. The electrochemical performance of these MEAs was tested in a commercial fuel cell hardware accommodating a 5-cm2 single cell geometry (FC05-01SP Electrochem, Inc.) connected to an external electronic load (Hocher & Hackl ZS-506). The fuel cell hardware employs machined graphite flow field plates with serpentine flow patterns and copper gold-plated current collectors. The feed gases were dry oxygen and dry hydrogen supplied by mass flow controllers (Bronkhorst Hi-Tec). Thus the fuel worked in a self-humidifying regime.
A reference MEA was prepared with the two electrodes made using a standard impregnation method with a Pt loading of 0.3 mgPtcm−2 at the cathode and 1 mgPtcm−2 at the anode. In a second MEA with the same electrode at the anode, the cathode was prepared by the described electro-spray method with an ultra-low Pt loading of only 0.012 mgPtcm−2; that is, a reduction of 1/25 in the amount of Pt with respect to the reference cathode. The electro-sprayed electrode was prepared under the conditions to obtain the fractal material corresponding to the SEM image shown in Fig. 16a (i.e. a flow rate Q = 0.2 ml h−1, a catalytic suspension with an electrical conductivity of 5.77 × 10−4 S m−1, a needle-to-collector-plate distance of 3.5 cm, and a voltage drop of 7 kV).
The performances of both MEAs are compared in Fig. 19. Fig. 19a shows the fuel cell characteristic polarization curve, voltage provided by the device (left vertical axis) and the resulting power density (right vertical axis) versus the current density (Martin et al., 2010a, 2010b). Solid triangles correspond to the reference MEA whereas squares are for the MEA with the electrosprayed cathode. Although the reference MEA seems to offer a higher performance, it should be emphasized that the Pt content is 25 times larger in the reference cathode. Therefore, the electrosprayed MEA is able to attain 59% of the maximum power density supplied by the reference MEA but with only 4% of the catalyst loading on the cathode.

a) Polarization curve (left axis) and power density (right axis), and b) specific power curve. (▴) reference MEA with a cathode prepared by an impregnation technique and a Pt loading of 0.3 mgPtcm−2, (▪) MEA with an electrosprayed cathode and a Pt loading of 0.012 mgPtcm−2. All measurements were carried out with dry (non-humidified) H2/O2 at a cell temperature of 70°C and atmospheric pressure on cathode and anode exits.
The potential benefits of the electrosprayed catalytic layer become evident when the catalyst utilization is evaluated. The specific power (fuel cell power per gram of Pt in the cathode) is represented in Fig. 19b. The maximum catalyst utilization (referred to the cathode) attained for the MEA with the electrosprayed cathode was 11.5 kW g−1, whereas a significantly lower value of 0.78 kW g−1 was obtained by the MEA with electrodes prepared by a conventional impregnation method. Thus, the MEA with the electrosprayed cathode achieved a maximum catalyst utilization almost 15 times larger than the utilization reached by the reference MEA under the same working conditions. This factor of 15 is quite promising for future applications.
Note that the catalyst utilization of the electrosprayed MEA may even be increased if more suitable operating conditions of the cell are selected. Thus Fig. 20 shows the increase of the cell performance when the back-pressure at the cathode and the anode exit lines is increased from atmospheric pressure up to 1.7 bar overpressure. For the electrosprayed MEA, a maximum cathode specific power of 19.1 kW g−1 was attained (although not shown in this figure, the performance of the reference MEA also increases with the fuel cell working pressure, but this improvement is rather moderate with respect to the electrosprayed MEA). The resulting platinum utilization is comparable to that achieved by cathodes prepared by sputtering, a well-established method for depositing ultra-low Pt loadings. Using these sputtered electrodes, specific power values between 14 kW g−1 and 20 kW g−1 have been reported (Caillard et al., 2008; Cavarroc et al., 2009; Gruber et al., 2005). However, the sputtering technique requires a strict atmospheric control and vacuum conditions that make it quite expensive and difficult to scale-up. In contrast, the electrospraying method described in this paper is carried out with a simple and inexpensive experimental set-up (only a pump-needle system and two high-voltage power supplies), the technique has no vacuum requirements and can be easily scaled-up, making it an attractive option for the mass-production of electrodes for the fuel cell industry.

Polarization and specific power curves for the MEA with the electrosprayed cathode when the back-pressure on the cathode and the anode exits is increased from atmospheric (▪) up to 1.7 bar (•). All measurements were carried out with dry (non-humidified) H2/O2 at a cell temperature of 70°C.
The results presented here open a new line of research for the preparation of granular materials from nanopowders. Nanopowders or nanoparticles can be used as building blocks for making new materials with a prescribed structure. For deposits formed by monodisperse particles, a way of controlling the final deposit morphology by adjusting the dynamical behavior of the particles when approaching the collecting surface has been shown.
Thus a simple model of particle motion has been used to simulate the deposit evolution, considering two contributions to the particle motion: A means (ensemble-average) determinist arrival velocity v normal to the collecting surface plus a superimposed random motion characterized by a diffusion coefficient D. The resulting control parameter is the Peclet number, Pe = va/D, where a is the particle diameter. This Peclet number measures the relative importance of the deterministic motion to the random contribution. Thus, the limit of infinite Peclet numbers corresponds to ballistic deposition when particles follow straight trajectories normal to the collector. Whereas the opposite limit of vanishing Peclet number accounts for a pure Brownian motion. A Monte Carlo simulation has been performed to track the particle motion and their attachment to the evolving deposit. These simulations have shown that deposits formed by particles with a large value of the Peclet number (near the ballistic limit) are more compact (see Fig. 5a) but have quite a rough surface (Fig. 5b). However, for low Peclet numbers, the deposit presents a tree-like structure with large voids (Fig. 3a) and its surface is quite rough with large pores entering deep into the deposit bulk (Fig. 3b). In any case, an analysis of the deposit mean density allows definition of three different regions in the deposit (Fig. 6). Thus there is a denser region close to the initially flat collecting surface where the density changes rapidly from a maximum value at the deposit bottom until reaching a middle plateau region where the mean deposit density remains constant and independent of the deposit height. These two regions form the frozen or consolidated deposit where new particles can no longer be attached. On top of the plateau regions, there is an active region where the density decreases from the constant plateau value down to a vanishing value at the uppermost layer of the deposit. On this active region, new particles may be incorporated to consolidate the deposit. A detailed analysis of the plateau region shows that the mean density there depends only on the Peclet number associated with the motion of the particles which arrive to form the deposit. This mean bulk density is given by Eq. (5). This simple relation is one of the main results of the Monte Carlo simulations because (when the features of the particle motion are given) it allows the deposit density to be known in advance. In summary, these Monte Carlo simulations have shown that the morphological properties (bulk porosity and surface roughness) of a granular deposit can be tuned by properly adjusting the velocity of the particles approaching the deposit.
On the basis of these theoretical predictions, experiments have been conducted to prepare nanostructured materials from carbon nanoparticles. These nanoparticles enter the gas from the atomization of a liquid suspension using the electrospraying technique (Fig. 10). Thus the liquid suspension is pumped at a fixed flow rate through a syringe where a high voltage is applied. The charges accumulate on the liquid surface and a stable cone-jet can be achieved (Fig. 12) by proper selection of the applied voltage and liquid flow rate (see Fig. 12). When the electro-spray works in a steady cone-jet mode, the jet breaks up forming small liquid droplets. These droplets are charged and become affected by the externally imposed electrical field that drifts them towards a collector plate located in front of the ejecting needle and perpendicular to it. The evaporation of the liquid along the droplet path leaves dry nanoparticles which deposit on the collector. The intensity of the electrical field over this collector determines the mean particle velocity there, and then the corresponding Peclet number. Using this technique, nanostructured deposits with a tree-like structure have been grown. The scanning electron microscope (SEM) images of the deposits are quite similar to the images of the deposits formed by Monte Carlo simulations (compare the SEM image of the real deposits shown in Fig. 16 with the simulated deposit represented in Fig. 3). In general terms, the experiments confirm the theoretical predictions that denser deposits (or more porous deposits depending on the requirements) can be made when the control parameters are properly adjusted.
The accordance of the theoretical predictions with the experimental results facilitates the preparation of porous granular materials made from powders. These materials have a rough surface with large pores that penetrate into the bulk resulting in a bulk structure of highly interconnected pores. This open structure is especially suitable for applications requiring a large active surface area as in the case of catalysis or any surface-activated heterogeneous reaction. To check the validity of these materials for these kinds of applications, a granular deposit prepared by the indicated electrospraying technique was used as a cathode in a PEM fuel cell. The resulting material surface can be tailored to render a high-quality catalytic electrode for use in a proton exchange membrane fuel cell (PEMFC). Catalytic suspensions of platinum supported on carbon nanoparticles (Pt/C) in Nafion®-alcohol solutions electro-sprayed over carbon paper were used in an experimental fuel cell set-up. In previous works, the fuel cell power density was measured as a function of the platinum loading and the range of parameters was obtained, leading to optimal platinum utilization for a given fuel cell efficiency (Martin et al., 2010a, 2010b). The tests on a controlled fuel cell have shown that the electrosprayed deposits were able to reach a fuel cell performance which overcomes the maximum catalyst utilization achieved with cathodes prepared by state-of-the-art techniques (Martin et al., 2013).
The results for the fuel cell cathode show the validity of the method in a particular case. But the same methodology can be used for other applications. The structure of the material can be tailored according to the application needs to have a given porosity and surface composition. Moreover, layered or composed materials (with a different porosity or different composition on each layer) can be prepared by adequate changes in the control parameters (liquid flow rate and applied voltages in the electro-spraying technique) which determine the dynamics of the particles approaching the deposit.
This work was supported by research funding agencies in Spain: Ministerio de Ciencia e Innovación (grant ENE2008-06683-C03-01, and program Consolider-Ingenio 2010 grant CSD2010-00011), and also by Comunidad de Madrid (project HYSYCOMB, S2009ENE-1597). A collaborative agreement with YFLOW Company (Malaga, Spain) is also acknowledged. The advice from YFLOW team, Prof. Ignacio G. Loscertales (University of Malaga) and Prof. Antonio Barrero† (University of Seville) was essential for the electrospray set-up and operation. Moreover, Jose L. Castillo and Pedro L. Garcia-Ybarra thanks Prof. Daniel E. Rosner (Yale University, New Haven, CT, USA) and Dr. Athanasios G. Konstandopoulos (CERTH/CPERI, Thessaloniki, Greece) for many years of fruitful discussions on these topics and Prof. Sotiris E. Pratsinis (ETH Zurich) for encouraging us to write this paper.
Jose L. Castillo
Jose L. Castillo received an M.Sc. in Physics from Universidad Autonoma de Madrid and a Ph.D. in Physics from UNED. He has been an associate research scientist at Yale University (Chemical Engineering Department), collaborator at Los Alamos National Laboratory (Center for Nonlinear Studies, Los Alamos, NM) and sabbatical visitor at University of Miami (Department of Physics). He is a professor at UNED, member of the editorial board of the Journal of Aerosol Science, and representative of the Spanish Aerosol Society at the European Aerosol Assembly. He was president of the Experimental Science Committee for the assessment of non-tenure university positions at ANECA (National Agency for Quality Evaluation and Accreditation, Spain), and is now an ANECA deputy director for academic staff evaluation.
Santiago Martin
Santiago Martin received a B.Sc. in physics and an M.Sc. in applied physics (fuel cell research) from UNED. He received his Ph.D. thesis at UNED in the applications of electrohydrodynamic spraying for the generation of nanostructured catalyst layers for PEM fuel cell use.
Daniel Rodríguez-Perez
Daniel Rodríguez-Perez received an M.Sc. and a Ph.D. in physics from UNED. He is a senior lecturer at UNED working, among other areas, in discrete Monte Carlo computer simulation of growing structures.
Alvaro Perea
Alvaro Perea received an M.Sc. in physics from Universidad Complutense de Madrid and a Ph.D. in physics from UNED. He is a senior lecturer at UNED, working in the area of particle transport phenomena.
Pedro L. Garcia-Ybarra
Pedro L. Garcia-Ybarra received an M.Sc. in physics from Universidad Autonoma de Madrid and a Ph.D. in physics from UNED, after a predoctoral stay at Université de Provence (Aix-Marseille I, France). He has been a Chercheur Associé at Université de Rouen (France), visiting research scientist at Yale University (Chemical Engineering Department), Universidad Nacional Autonoma de Mexico (UNAM) and University of California at San Diego (Center for Energy and Combustion Research). He is currently a professor at UNED, after being head of the Fossil Fuels Department at CIEMAT (Madrid). He is the secretary of the Spanish Section of the Combustion Institute.