2014 Volume 31 Pages 234-246
2014 Volume 31 Pages 234-246
Core-shell nanoparticles and other nanostructured particles have high potential in applications such as heterogeneous catalysis and energy conversion and storage. However, a hurdle in their utilization is that typically, large amounts of such nanostructured materials are required. Gas-phase coating using atomic layer deposition (ALD, a variant of chemical vapour deposition) can be used to provide the surface of a particle with either an ultrathin continuous coating or a decoration of nanoclusters. When carried out in a fluidized bed, ALD is an attractive way of producing nanostructured particles with excellent scale-up potential.
We demonstrate the fabrication of catalysts by deposition of the active phase (Pt) on fluidized nanoparticles (TiO2 P25) at atmospheric pressure. We show that ALD is a technique that 1) guarantees efficient use of the precursor; 2) allows precise control of the size and loading; 3) can be used for low and medium loading of catalysts by adjusting the number of repeated cycles; 4) leads to high-quality (low impurities level) end-products.
In recent years, researchers have proposed a wide range of novel nanostructured materials for applications such as catalysis (Canlas et al., 2012; Li and Somorjai, 2010) and photovoltaic devices (Jancar et al., 2010; Kamat, 2008). Often, such materials use nanostructured particles as building blocks. In this paper, we will use the term nano-structured particles for particles of at least two materials, typically a host particle (not necessarily < 100 nm) onto which a second material is present in some structured way, e.g. as a film or set of clusters with dimensions < 100 nm. For most applications in catalysis and energy conversion and storage, large amounts of such materials are required. Most of the current synthesis routes for nanostructured particles, however, do not take into account the scalability of the process. It is not considered whether the synthesis of small amounts of materials in stirred flasks could be taken to some large-scale reactor to produce commercial amounts of materials. Moreover, the amount of waste produced in small-scale synthesis typically receives little or no attention.
While for research purposes it is not a problem to produce 100 g of waste to obtain 1 mg of a desired product, such a ratio is unacceptable at industrial scale. Sheldon (1992) proposed quantifying the efficiency of chemical processes by the E-factor: the mass ratio of waste to desired product. Table 1 gives the E-factor for some industry segments. It is clear that nanomaterial production has the poorest performance by far. If nanomaterials are going to be widely applied in medicine, energy conversion and storage, their product throughput will increase to approximately the levels of pharmaceuticals and fine chemicals, respectively, which will give unacceptable amounts of waste. If we do not improve the manufacturing routes for nanomaterials drastically, it will lead to a low public acceptance and fewer market introductions of products based on such materials.
| Industry segment | Annual product throughput (log kg) | E-factor | Typical amount of waste (log kg) |
|---|---|---|---|
| Oil refining | 9–11 | ∼0.1 | 9 |
| Bulk chemicals | 7–9 | < 1–5 | 8 |
| Fine chemicals | 5–7 | 5–50 | 7 |
| Pharmaceuticals | 3–6 | 25–100 | 6 |
| Nanomaterials | 2–3 | 100–100,000 | 6 |
One of the reasons why the current synthesis of nano-structured particles is so polluting is that a combination of solvents and surfactants is often used, and the produced concentration of nanoparticles is very low; this yields very high E-factors. Moving to the gas phase eliminates the use of solvents, and is therefore intrinsically cleaner. Moreover, gas-phase processes are typically easier to scale up when nanostructured materials (Wegener et al., 2011) are concerned, although there are exceptions. For example, graphene can be more easily synthesized in large amounts in the liquid phase than in the gas phase (Coleman, 2012). When producing nanostructured particles in the gas phase, two basic approaches are possible:
The first approach has the advantage of minimizing the number of steps in the production process; it can be considered as an example of process intensification. Several illustrative examples of this approach can be found in literature (Knijnenburg et al., 2013; Minnermann et al., 2013; Stepuk et al., 2012). However, it is not always straightforward to control the obtained morphology, and tuning of parameters might be needed when moving to a new combination of materials. The second approach—separating particle production and particle decoration—gives more degrees of freedom. Moreover, it has the advantage that commercially available particles can be used. It will depend on the individual application which of the two approaches is preferred. In this paper, we will focus on the second approach, and especially on its second step: depositing nanostructured material on already available particles.
For the well-controlled deposition of material at the nanoscale, a number of approaches are available. Some of them are line-of-sight methods, and are therefore not easily applicable to particles; examples are e-beam deposition (Laukaitis et al., 2006) and sputtering (Ferguson et al., 2008). Other methods can be applied to particles, but are not so easy to scale-up. An example is electrospraying deposition of nanoparticles (Ellis et al., 2010; Yurteri et al., 2010), which might be useful for pharmaceutics production, but could be cumbersome for applications that require large amounts of materials. Chemical vapour deposition (CVD) and similar techniques can be applied to particles and have a good scale-up potential, but have difficulty in reaching nanoscale precision. Atomic layer deposition (ALD) is a modified version of CVD that can achieve such precision. We will discuss it in more detail in the next section.
This paper will discuss ALD as a method of producing nanostructured particles that is scalable and has a limited production of waste. We will use the deposition of platinum nanoclusters on titania nanoparticles as an example.
Atomic layer deposition (ALD) is a technique that relies on the distinct, consecutive binding of two (or more) compounds in a combined structure under a self-limiting chemisorption/reaction scheme. ALD presents basic self-assembly characteristics (Kim et al., 2009). However, one can argue whether it fully matches the definition of self-assembly as given by Gates et al. (2005): the spontaneous organization of two (or more) components to form larger aggregates by means of covalent and/or non-covalent bonds. Nevertheless, ALD is an inherently rational technique relying on a layer-by-layer (or cluster-by-cluster) assembly of the deposited materials. Similar to the production of layered composite natural or biomimetic materials such as nacre (Finnemore et al., 2012), ALD can be considered as a bio-inspired approach for the fabrication of tailored nanomaterials. ALD proceeds through a sequence of self-terminated gas-solid reactions (R1 and R2) that can be expressed as (King et al., 2007):
Unlike traditional wet-phase or gas-phase deposition (e.g. CVD) schemes, ALD offers digital control of the process since the growth of the materials relies on the number of repeated cycles rather than on the exposure time or concentration of precursors. Moreover, the sub-nm precision (induced by the use of molecular-sized building blocks) enables the exact translation of the material design to an accurately fabricated structure.
2.2 Particle nanostructuringDespite the foundation of ALD as a technique to grow thin, uniform layers of materials (hence its alternative name atomic layer epitaxy), the technique has also been used in the deposition of particle clusters during the initial growth stage (Haukka and Suntola, 1997). This observed deviation from the ideal character of the technique gave rise to an interest in applications that essentially rely on the utilization of a support material of particulate matter for the deposition of an active material. There are several factors affecting growth during the initial stages of ALD. These factors can be divided into three groups as depicted in Fig. 1. We will briefly discuss them with respect to the deposition of a (noble) metal on an oxide-terminated surface:
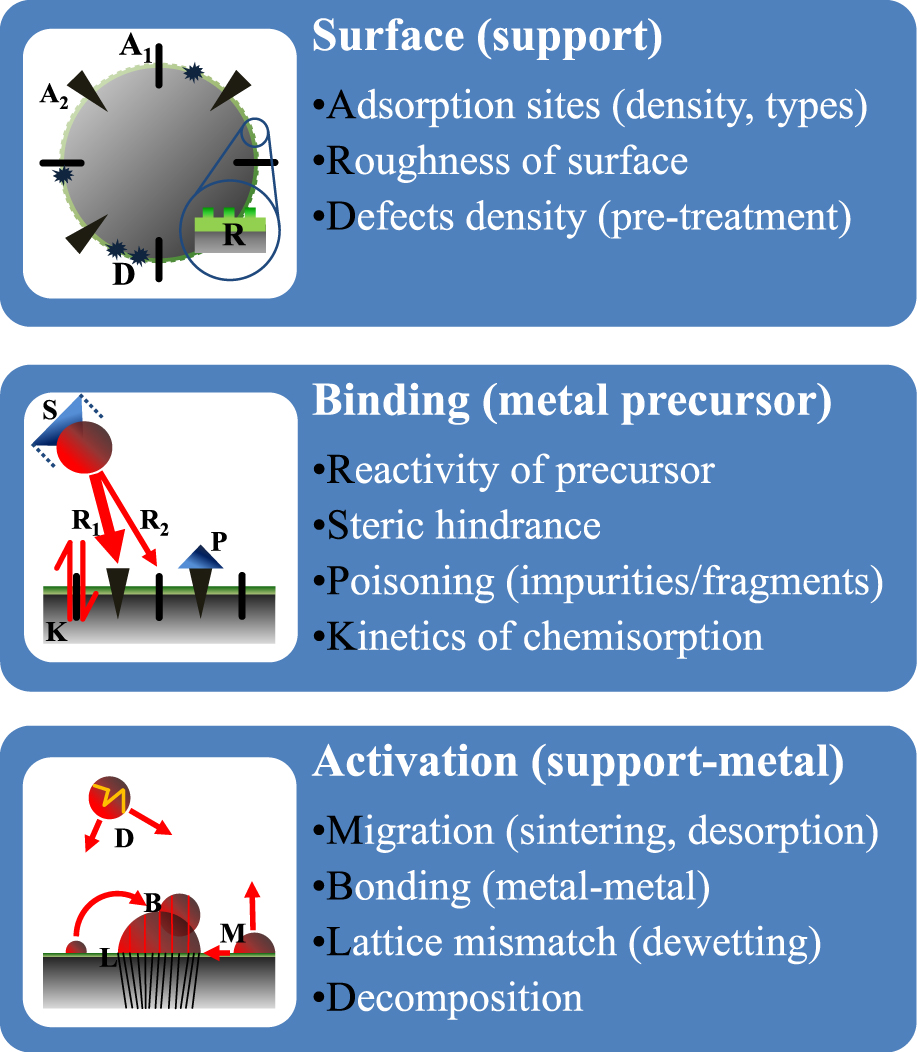
Island growth dependence on surface-, binding- and activation-related parameters during the ALD of noble metals.
ALD line-of-sight independency for the precursor delivery enables the deposition in high-aspect-ratio, complex topologies, and high-surface-area substrates. The limiting condition is the presence of a certain surface functionality to promote material growth by ALD. Ultrathin seeding layers deposited by ALD (usually 0.2–1.1 nm thick) can further enhance the growth in support surfaces that show slow/inhibited growth behaviour.
So far, ALD has been used in the modification of oxide supports via the growth of thin layers of oxides, resulting in the fabrication of particulate support materials that can retain some inherited structural properties of the parent material (e.g. external surface area) while modifying others (e.g. acidity, pore size for mesopores, blocking of micropores, surface functionality, etc.). ALD coverage—especially in the initial growth regime—is just a fraction of a true monolayer value. An effort to study the mono-layer coverage of various oxides on oxide powders shows that the coverage increases in the order of SiO2/TiO2 < TiO2/SiO2 < Al2O3/SiO2 or TiO2 (average values of 10%, 19% and 30–57% as shown in Table 2.
| Support | References | ||||
|---|---|---|---|---|---|
| |
|||||
| SiO2 | TiO2 | Al2O3 | |||
| GPC (Å cycle−1) | SiO2 | – | 0.3 | – | (Kim et al., 2009) |
| TiO2 | 0.6 | – | – | (Lei et al., 2012; Mahurin et al., 2006; Sereda et al., 2012; Williams et al., 2012) | |
| Al2O3 | 1.1 | 2.0 | 1.2 | (Cronauer et al., 2011; Feng et al., 2010; Feng et al., 2011; Hakim et al., 2005; Hakim et al., 2006; King et al., 2007; Lei et al., 2012; Sereda et al., 2012) | |
Looking at the different structuring possibilities, ALD can be used for particle nanostructuring, either to induce or inhibit certain composition and structure functionalities. Depending on the growth type of the deposited material, core/shell or core/seed particles (inactive core/active shell or seeds) can be fabricated in an activation scheme. Noble metals (Pt, Pd, Ru, Ir) have been deposited as core/seed active structures so far, while core/shell particles are usually functionalized with the deposition of transition metal oxide shells (e.g. TiO2 and ZnO). ALD has proven its potential to unlock new application areas for core/seed nanoparticles, since it can reach extremely small sizes (about 1 nm) of deposited particles (clusters) compared to conventional (wet-chemistry-based) techniques. ALD can deposit a variety of different materials (or combinations of them); a comprehensive review of the compounds that can be deposited by ALD was recently presented (Miikkulainen et al., 2013).
In certain applications, it can be desirable to modify—or even fully suppress—the activity of particles; a passivation scheme can yield core/shell particles of a reversed composition (active core/inactive shell). In the case of core/seed particles, this technique was developed as a protection scheme, applicable on the seed-nanoparticles. The most commonly used passivation materials are oxides such as Al2O3 and SiO2, with the required thickness ranging from values as low as 0.5 nm up to 50-nm films, depending on the application. Special treatment can tailor the nature of the protective layer to induce a certain accessibility (Canlas et al., 2012; Lu et al., 2012). Moreover, supported active multi-component materials (e.g. multi-metallic catalysts) can also be prepared via ALD either in a consecutive deposition scheme (He et al., 2010; He et al., 2012; Molenbroek et al., 1998) or—recently—a mixed-metal ALD process (Christensen et al., 2010; Lei et al., 2012). A schematic depiction of the different possibilities for ALD modifications of different functionalities is shown in Fig. 2.
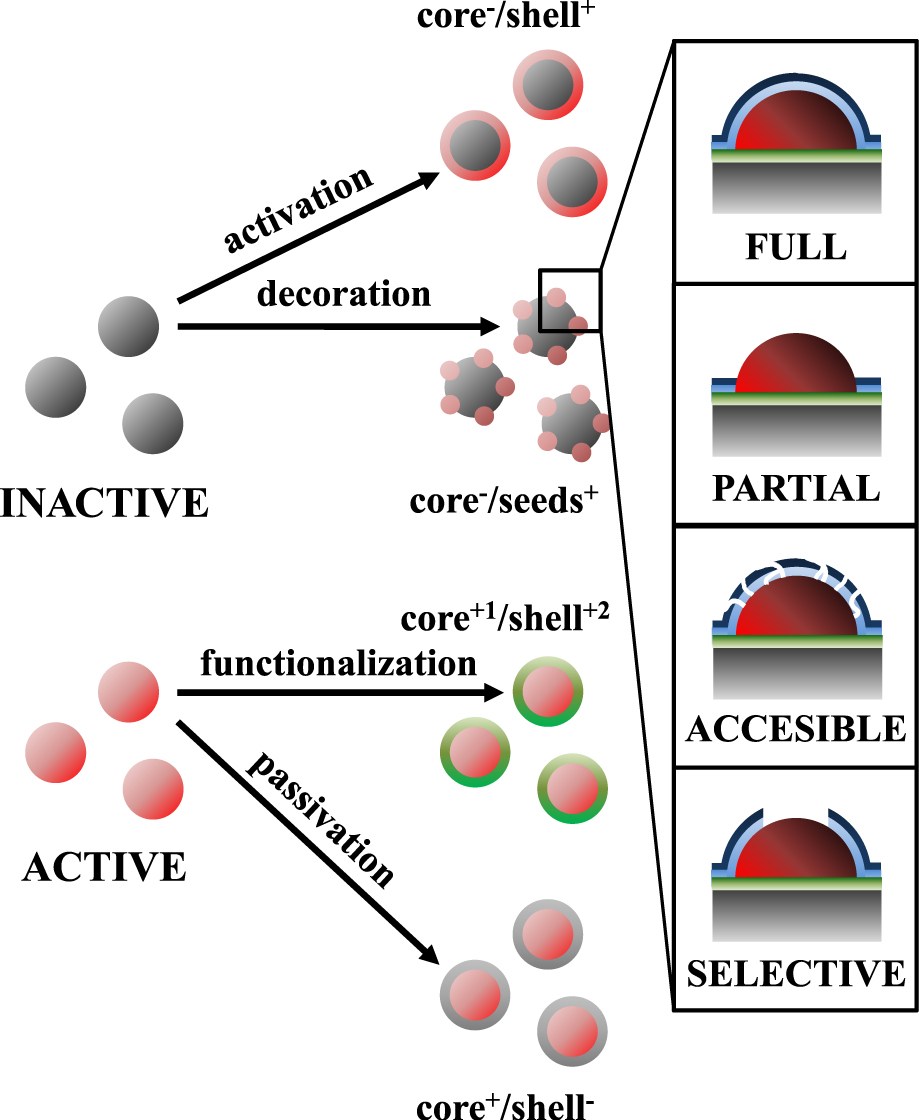
Functionalization possibilities for active and inactive particles by film or cluster deposition via ALD.
Thus, ALD offers a 3-stage rational design approach for the synthesis of nanostructured particles. In the first stage, the active phase can be deposited with high precision, either in the form of thin films or nanoparticles. Protection of the active phase can be achieved either by full overcoats (passivation) or partial coverage (preferential growth on the support). Finally, selectivity is induced by manipulating the accessibility of the active phase (in a passive, e.g. thermal treatment, or active, e.g. blocking agent grafting way).
2.3 Reactor technologyMaterials of a particulate nature often pose challenges during their treatment with ALD due to the high total surface area that needs to be coated and the—often present—requirement of coating high-aspect-ratio structures such as micropores. Flow-type reactors for ALD introduced by Microchemistry Ltd. (primarily designed for the treatment of flat substrates) have been also used for the treatment of powders; the MC-120/F-120 being one of the most commonly used reactor type. In the reaction chamber, the reactant, introduced from the top of the fixed powder bed (up to 10 g), is allowed to chemisorb and react for a sufficient time to reach saturation (Lakomaa et al., 1992). This reactor configuration yielded consistent saturation coverage (±0.2 atoms nm−2) but required reasonably high pulsing times (on the hour-scale) due to the relatively high operating pressure of 1–10 kPa. Recently, a modification of the reactor cell to a semi-fluidized powder cell was reported for particle ALD (Rauwel et al., 2011).
A viscous-flow ALD reactor (Elam et al., 2002) operating at reduced pressure (120–270 Pa) has also been used by researchers in Argonne National Laboratory for ALD treatment of powders. High-surface-area supports (up to 930 m2g−1) have been effectively coated, at faster saturation exposure times (on the min-scale). Only small batches (up to 1 g) of materials have been processed in this viscous-flow reactor type.
Additionally, modifications of conventional ALD reactors aiming at the minimization of gradients (concentration, temperature) during the deposition introduced the use of fluidized bed reactors (Hirva et al., 1994). A fluidized bed ALD reactor operating at low pressure (133–1066 Pa) introduced by Wank et al. (2004) has been extensively used. Large batches of powders (up to 75 g) have been effectively coated in this reactor type, successfully proving the scale-up potential. Since many of the powders used as substrates exhibit cohesive behaviour, fluidization assistance methods such as stirring or vibration are typically required. van Ommen et al. (2012) give an overview of the various assistance methods to fluidize nanopowders. Although it may sound counterintuitive, nanopowders can indeed be fluidized. In contrast to particles of say 200 μm, however, nanoparticles are not fluidized individually but as agglomerates: very dilute structures of around 200 μm consisting of ∼1010 primary particles (van Ommen et al., 2012).
Despite the fact that the fluidized bed reactor provided a good scalability potential in terms of the batch handling capabilities, the high total surface area of the powders—and the subsequent need for large amounts of precursor dosing (due to the residence time of the gaseous precursor in the fluidized bed)—called for research focused on more efficient utilization of the precursor. The use of a rotary reactor operated at very low pressures (less than 133 Pa) was suggested (McCormick et al., 2007), in an effort to obtain a static exposure scheme and proper mixing during deposition. In this reactor, fluidization and agitation of the particles is achieved by rotation, and therefore a constant gas flow is not required. However, it was not demonstrated that this reactor was more efficient than a fluidized bed in terms of precursor utilization. Until today, this reactor has been used to coat batches of up to 2 g. A different rotary reactor configuration was recently proposed (Longrie et al., 2012). They operate this reactor at a very low pressure (< 2 Pa). Their system is capable of applying both thermal and plasma-enhanced ALD.
Recently, the atmospheric pressure operation of a fluidized bed ALD reactor was demonstrated for the growth of thin films (Beetstra et al., 2009) and the deposition of highly-dispersed nanoparticles (Goulas and van Ommen, 2013). Although the pulsing times required are longer than the equivalent in low-pressure operation, the process was proven to be efficient in terms of precursor utilization while excluding the need for expensive vacuum equipment. Fluidization at atmospheric pressure is also more attractive from the perspective of the scale-up potential. To our knowledge, the 120 g-batch of LiMnO4 particles coated via ALD in this fluidized batch is the largest amount reported in open literature. The next step is to move from batch to continuous production, e.g. via the pneumatic transport of particles (van Ommen, 2010); research on such reactors is currently being carried out in our group. A summary of the main types of reactors used is given in Fig. 3.

Categorization of the main research ALD reactors used for particle nanostructuring based on the operating pressure range and maximum batch size reported (logarithmic scales).
Moreover, certain commercial reactors such as the Beneq P400 (Nevalainen et al., 2012), Ultratech/Cambridge NanoTech Savannah 100 (Sun et al., 2013), Picosun SUNALE R-200B (Williams et al., 2012) and a shower-head reactor from Korean Vacuum Tech. Ltd. (Yoon et al., 2011) have been already used for ALD on particles.
2.4 Example: deposition of platinum nanoclusters by ALDThe ability to precisely tailor the structure of particles on a nanoscale gives us a tool to greatly improve metal-supported catalysts, enhancing atom efficiency while minimizing the cost of the expensive and scarce metal of the active phase. Currently, Pt, Ir, Rh are all priced above $32,000 per kg. In an effort to establish well-defined structure-reactivity correlations and unravel the role of size in nanocatalysis, materials with well-controlled dimensions in the size of (sub) nanometres have to be delivered. Apart from achieving better material utilization—a high surface-to-volume ratio yields a high amount of active sites per active material mass—different functionalities can also be assessed, due to the changed local reactivity characteristics of nanoparticles (more corners, edges, kinks) compared to their bulkier counterparts. Considering that in the fcc structure of Pt (atomic diameter of 0.278 nm), 12 shell atoms can cover 1 metal atom to form the single full-shell cluster (n = 1) (Schmid, 1990), the structure has 93% of its atoms on the outer surface (Fig. 4). The Ns/Nt is inversely proportional to the number of shells (n−1), this decrease can be seen in a 2.5 nm nanoparticle, where only 52% of its atoms are directly accessible and thus highlighting the reactivity potential of smaller clusters.

Ratio of surface to total (Ns/Nt) atoms as a function of the Pt nanoparticle size (inline graphic indicates the size and percentage of Pt nanoparticles for 1 to 4 shells in the fcc cluster).
The activity of catalytically promoted reactions is often expressed as the turnover frequency (TOF) which describes the amount of moles converted per mole of the exposed catalyst material. The particle structure (size and shape) can affect the TOF if the reaction is intrinsically dependent on size/shape or is structure-sensitive, while some other reactions are not affected by the size (intrinsically size/shape-independent or structure-insensitive). For structure-sensitive reactions, a positive size effect marks an inversely proportional relationship between particle size and TOF, while a negative size effect shows a proportional interaction (antipathetic particle size-TOF dependence). For structure-insensitive reactions, the main objective during deposition is the maximization of dispersion to achieve optimal use of the catalytic material. Typical Pt-catalysed structure-insensitive reactions are ethylene and cyclohexane hydrogenation, while an example of positively size-dependent reactions are the hydrogenolysis of ethane and the dehydrogenation of cyclohexane (Somorjai and Park, 2008). As seen from Fig. 5, small Pt nanoparticles (≤ 3 nm) can greatly improve the TOF of structure-sensitive reactions. Additionally, the catalyst selectivity can also be improved by controlling the particle size distribution over a narrow range, as demonstrated for hydrogenation reactions (Somorjai and Park, 2008).

TOF values for structure-insensitive Pt-catalysed reactions, marks indicate experimentally determined data-points (C2H4 hydrogenation: blue dotted line and ♦, C6H10 hydrogenation: red dotted line and ▴) and structure-sensitive reactions (C6H10 dehydrogenation: red solid line and ▵, C2H6 hydrogenolysis: blue solid line and ⋄ ; plotted on secondary vertical axis) (based on Somorjai and Park (2008)).
The first report of a supported Pt catalyst fabricated by ALD was the Pt/Al2O3 and Pt/SiO2 hydrogenation catalysts prepared by Lashdaf et al. (2004). The cyclopentadienyl precursor (trimethyl) methylcyclopentadienyl-platinum (IV) (MeCpPtMe3) was used as a source of Pt and air was used for the removal of the organic ligand at 350°C. For a single exposure of the precursor, larger islands were observed in SiO2 (3.1 nm) as compared to the Al2O3 support (1.2 nm) in consistency with the available number of active OH groups (proportional to the available surface area and surface density). Additionally, an acetylacetonate Pt precursor (Pt (acac)2) was utilized at lower deposition temperatures of 180–200°C, which had as a drawback the inclusion of carbon-based impurities of a high concentration (∼3.5 wt.%) in the catalyst (Plomp et al., 2008; Vuori et al., 2006).
The importance of Pt as a catalytic material has driven efforts for deposition on strontium titanate, titania, zirconia and alumina high-surface-area supports in the temperature window of 150–300°C (Christensen et al., 2009; Enterkin et al., 2011; Lobo et al., 2012; Setthapun et al., 2010; Xie et al., 2012). Moreover, the technique has also been used for the doping of Co-based Fischer-Tropsch catalysts with small amounts of Pt (0.1 wt.%) that acts as a promoter (Cronauer et al., 2011). Recognizing the technique’s potential for creating the high dispersion characteristics necessary in fuel cell applications, ALD has been used for the decoration of relevant carbon-based supports such as carbon aerogel, tungsten-carbide, multi-walled carbon nanotubes, graphene nanosheets and carbon nano-tube/graphene oxide hybrid materials (Hsieh et al., 2012; Hsu et al., 2012; Hsueh et al., 2012; King et al., 2008; Sun et al., 2013). Additionally, bimetallic Ru-Pt and Pt-Pd catalysts have also been fabricated (Christensen et al., 2010; Lei et al., 2012). All the aforementioned efforts were carried out in fixed bed reactors that treated small batches of the powders (< 1 g).
Zhou et al. (2010) reported the deposition of Pt islands on TiO2 nanoparticles in a fluidized bed reactor operating at reduced pressure. Additionally, highly dispersed Pt clusters have been deposited effectively on micron-sized SiO2 (Li et al., 2010), resulting in an extremely low loading of 3.1·10−6 mg cm−2 (3.1 · 10−8 kg m−2) for 3 ALD cycles. According to a recent publication, thermal decomposition of the Pt precursor along with Pt nanoparticle sintering at a deposition temperature of 320°C are responsible for the disruption of the self-limiting ALD feature (Liang et al., 2011).
Atomic layer deposition in a fluidized bed reactor operating at atmospheric pressure conditions was used for the decoration of TiO2 nanoparticles with Pt nanoclusters. The deposition scheme involves the sequential exposure of the support material to MeCpPtMe3 and ozone (Goulas and van Ommen, 2013) at 250°C. The ALD reaction proceeds via a mechanism proposed by Kessels et al. (2009) adapted to the following generic scheme:
The custom-built fluidized bed ALD reactor consists of a vertical reaction chamber with an inner diameter of 10 mm that was loaded with ∼0.25 g of nanoparticles, supported on a porous metallic gas distributor. The TiO2 nanoparticles used (Aeroxide P25 from Degussa, ≥ 99.5% purity) were sieved prior to deposition. Their surface area was ∼50 m2g−1 and their average primary particle size was 21 nm with a crystal structure of about 85% anatase and 15% rutile. During fluidization, these particles formed agglomerates of around 200 μm.
The precursor (trimethyl) methylcyclopentadienyl-platinum (IV) (Strem Chemicals, 99% purity) was utilized as a Pt source. The precursor was transported in the reaction zone by means of a dry nitrogen carrier gas (N2, 99.999 vol.%) that was diverted through the precursor bubbler (50°C). A dry air stream enriched with ozone (around 1.5 wt.%) was used as an oxidizer agent for the removal of the precursor ligands and repopulation of the support surface with active sites. Ozone was obtained by feeding the dry air flow (0.20 L min−1) through an ozone generator (OAS Topzone). No significant decomposition of ozone was observed during the transportation of the stream to the reactor zone via the reactor distributor. Nitrogen was also used for the intermediate purging pulses that were implemented to evacuate the reactor from the excess of the precursor and the reaction by-products during the ALD half-cycles. During operation, the TiO2 powder was suspended in an N2 flow of 0.20 L min−1 (corresponding superficial gas velocity of 4.2 cm s−1), ensuring that the particles were sufficiently mixed. Fluidization was assisted using a dual vibromotor. The reaction temperature of 250°C was maintained by means of an IR-heater with feedback control. A more elaborate description of the set-up is given by Beetstra et al. (2009). The loaded TiO2 nanoparticles were pretreated (heating under inert atmosphere) to ensure a constant initial number of active OH-groups in the surface. The typical pulsing sequence for Pt-N2-O3-N2 consisted of exposure times of 3–12 min for MeCpPtMe3, and 10 min for ozone separated by N2 purging pulses of 10 min.
Optical emission spectrometry (ICP-OES) using a Perkin Elmer Optima 3000 DV spectrometer was used to determine the amount of Pt deposited. The level of carbon impurities in the samples was monitored by IR absorption spectroscopy in a C/S determinator (LECO CS-225). The structure of the deposited islands was determined from TEM images using a FEI Tecnai TF20 microscope equipped with an EDX detector (Oxford Instruments). XRD measurements were carried out with a Bruker D8 Advance diffractometer. The BET surface area of the coated and uncoated samples was determined via N2 adsorption with a Quantachrome Autosorb-6B surface area analyser.
Two of the important intrinsic properties of ALD are the self-saturating character of the growth for a single cycle and the linear increase of the amount of material deposited by increasing the amount of cycles. Coating powder batches of increased surface area (up to 14 m2 per sample batch) requires much longer precursor exposure, oxidation and purging times. It has been shown that during the prolonged exposure times, the reactor temperature should be properly controlled to avoid thermal decomposition of the precursor (Liang et al., 2011). When utilizing a lower deposition temperature (250°C instead of 320°C), an indication of the self-saturating ALD characteristic was seen (as shown in Fig. 6) with the Pt loading stabilizing at ∼1.6 wt.% after 12 min of precursor exposure.
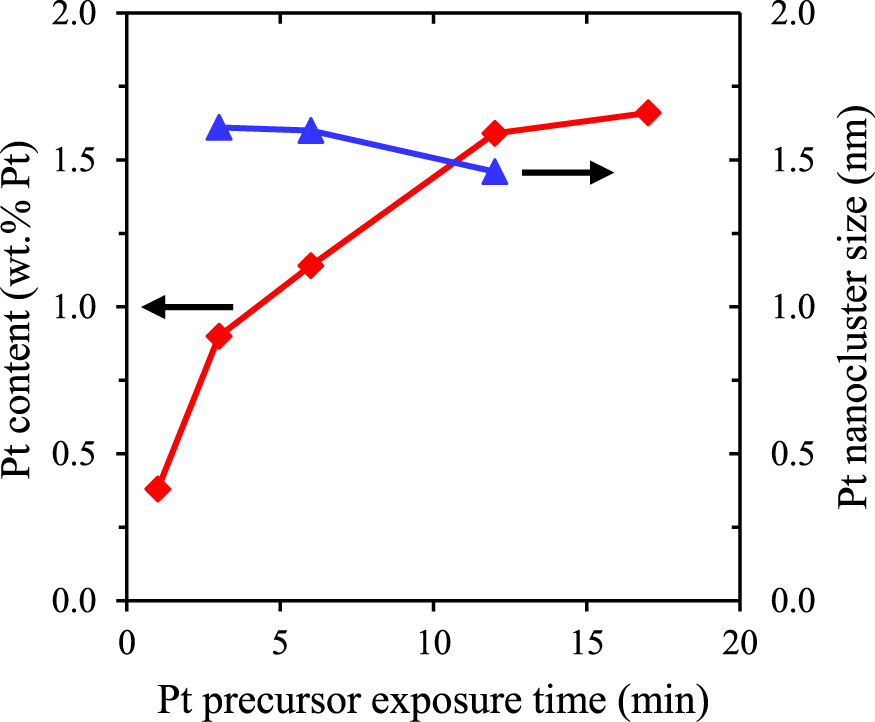
Estimated Pt content (red line and ♦) and nanocluster size (blue line and ▴; on secondary vertical axis) for varied Pt precursor exposure times and 1 ALD cycle.
The average Pt particle size from TEM image processing was estimated to be about 1.5 nm for a Pt pulse of 3 min and appeared to be stabilized for exposures up to 12 min. Uniform decoration of the support material with Pt nano-clusters was obtained as shown in Fig 7. The particle size distributions presented in Fig. 8 verify that the process was well controlled and resulted in very similar, narrowly distributed particles. Moreover, the TEM pictures indicated that the Pt nanoclusters were nicely distributed over the titania particles. This can be explained by the very open structure of the agglomerates, as well as by their dynamic nature: they constantly break-up and reform into new agglomerates (van Ommen et al., 2012).
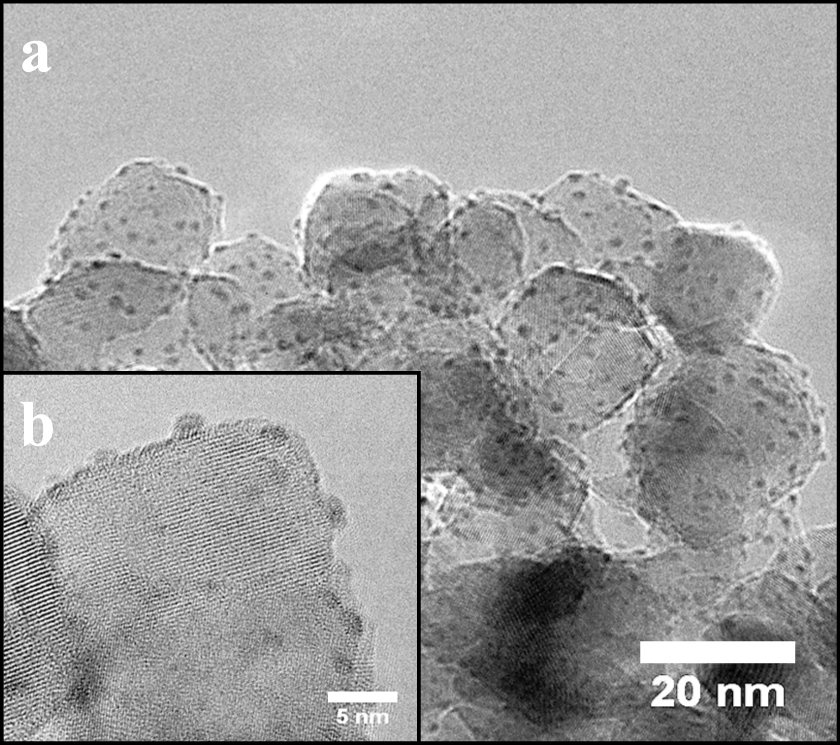
TEM image of the Pt nanoparticles decorating the surface of the TiO2 support after 1 ALD cycle of 3 min Pt precursor exposure. The inline image depicts the decoration at the edge of the TiO2 crystal.
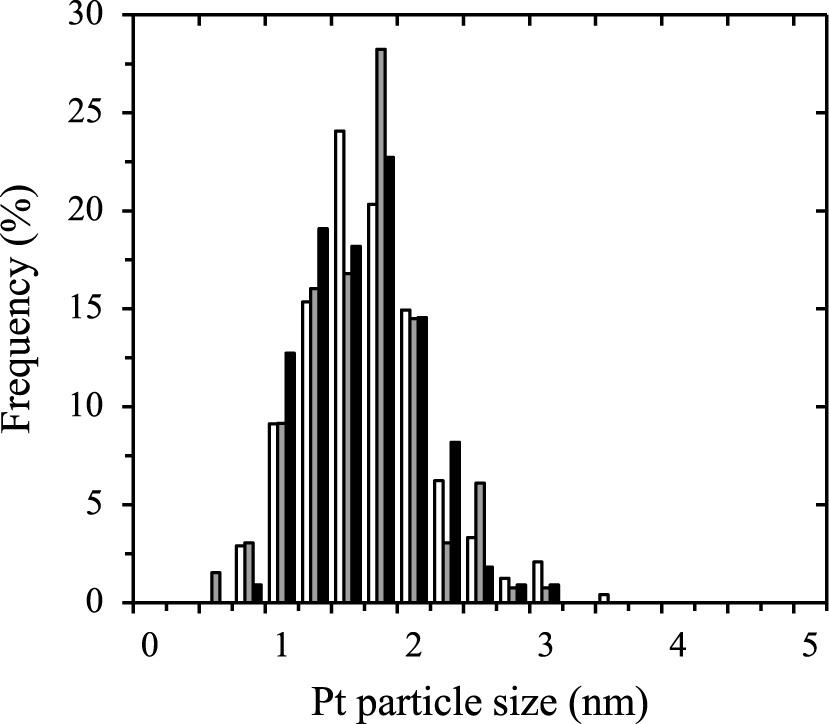
Pt particle size distributions derived from TEM image processing for samples obtained after 3 min (white bars), 6 min (grey bars) and 12 min (black bars).
Precursor utilization for exposure times in the saturation regime was relatively low: only ∼50% of the Pt precursor provided was eventually loaded in the support. Using ALD for the fabrication of ultra-low loading catalysts, sub-saturated pulses can be implemented to achieve maximum utilization of the precursor. An exposure time of ¼ the saturation time resulted in a deposition amount of about half of the equivalent amount deposited for operation in the saturation regime. The corresponding precursor utilization was estimated to be 95% in that case. An estimation of the E-factor value was made, considering Pt/TiO2 as the final product (∼10–20% of the loaded TiO2 is lost as waste during handling). The outlet waste stream contains the gas products of the organic ligand decomposition and the excess amounts of oxidizer gas used (O3/O2, without taking into account N2). The equivalent calculated E-factor of the deposition process is ∼6–7, fitting in the range of 5–50 that describes processes of the fine chemicals industry (Sheldon, 2007).
Additionally, the loading of the catalyst can be precisely controlled by the number of ALD cycles performed. Fig. 9 presents the measured content of Pt (wt.% of Pt) along with the theoretically predicted value for ideal ALD growth, together with the change in value of the specific surface area of the support. Increasing the number of cycles from 1 to 4 shows a monotonic increase of the Pt loading, verifying the proportional relation between the deposited amount and the number of ALD cycles. The surface area showed a small increase of 5%, in agreement with deposition of small Pt nanoclusters on non-porous supports. The loadings of the catalysts obtained here are higher than the ones obtained in a fluidized bed reactor operating at reduced pressures (Zhou et al., 2010). The use of ozone—a more powerful oxidizer—is one possible cause, but the effect of pressure should also be carefully considered in future studies.
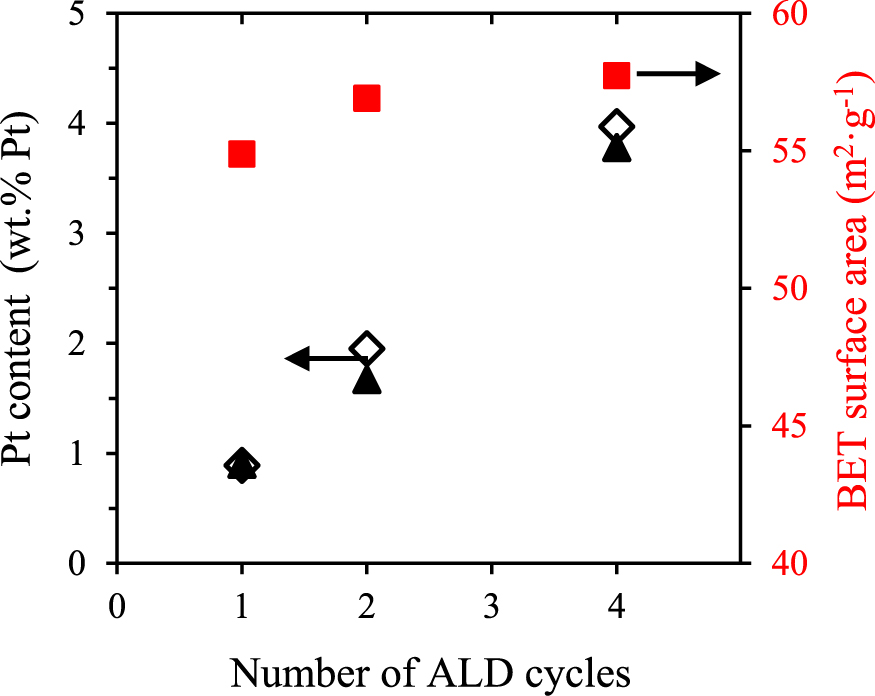
Pt content measured by ICP (▴) and predicted loading value for ideal ALD (⋄) along with specific surface area (▪) for 1–4 ALD cycles (Pt content values corrected for changes in surface area after deposition).
The use of ozone allowed the process temperature to be lowered during the oxidizing step. This can have a beneficial effect on the dispersion of the noble metal nanoparticles, as it prevents their sintering to a certain extent. To further probe the dispersion of the Pt nanoclusters, we examined several TEM images, applying image analysis (ImageJ). A sample of 1 ALD cycle deposited for 12 min exposure time (1.6 wt.%) and 5 ALD cycles deposited for 5 min exposure time (5.8 wt.%) are shown in Fig. 10. As the number of cycles increases, the particle nanoclusters increase in size. All the particle size distributions were calculated based on the processing of 100–200 Pt nanoparticles from different images. The presence of Pt (small clusters of darker contrast on the lighter crystalline support material) was further verified by qualitative EDX analysis. As discussed above, the average particle size increased from 1.5 nm to about 2.3 nm while the particle size distribution was—for both cases—very narrow (Fig. 11). The possibility of ALD to deposit small nanoparticles, even for high loadings of noble metals, makes it a promising fabrication technique for low-and medium-loading noble metal catalysts.

Overview TEM images of Pt nanoclusters deposited on the TiO2 support for 1 ALD cycle (a) and 5 ALD cycles (b).
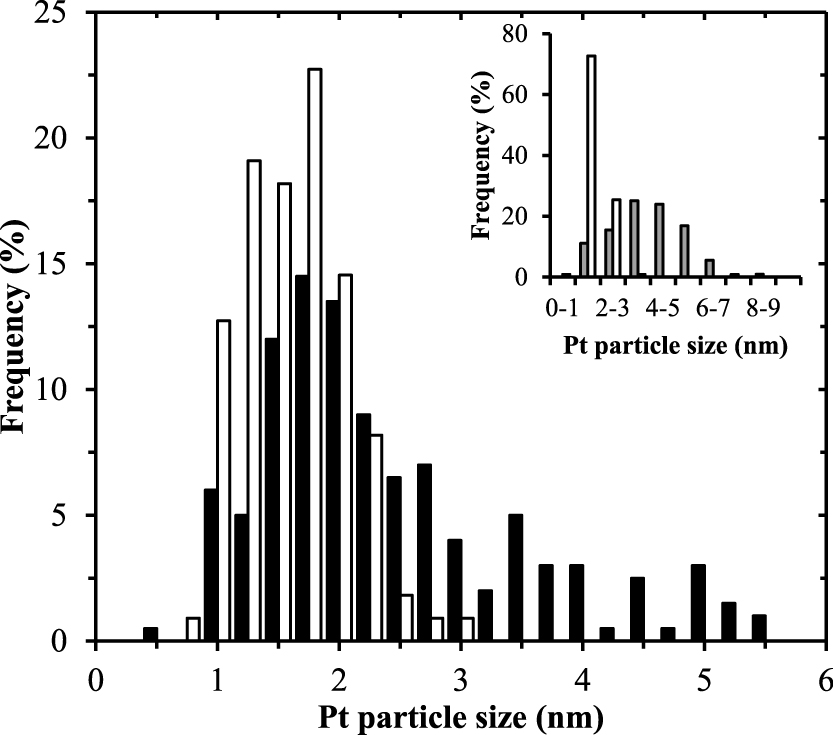
Particle size distribution derived from TEM image processing for Pt nanoclusters deposited onto the TiO2 support after 1 ALD cycle (white bars) and 5 ALD cycles (black bars). The inline graph shows the particle size distribution of the 1 ALD cycle Pt/TiO2 (1.6 wt.%, white bars) and of a Pt/TiO2 catalyst prepared via photodeposition (1.5 wt.%, grey bars) (Colón et al., 2010).
ALD offers a simple way to control the particle size of Pt, which is illustrated in the inline graph of Fig. 11. The ALD catalyst is compared to a Pt/TiO2 catalyst of similar loading (1.5 wt.%) prepared via the photodeposition technique (Colón et al., 2010). The low average particle size obtained by ALD (1.5 nm versus 3.9 nm) ensures a higher utilization of the expensive active material. The increase in the noble metal specific surface area (186 m2g−1 versus 72 m2g−1) is expected to contribute positively to the performance of similar catalytic systems. This thus demonstrates that atomic layer deposition using a fluidized bed reactor at atmospheric pressure is an attractive method that combines scalability and high precision for the fabrication of catalysts and other nanostructured materials.
In the past decades, ALD has firmly established its position as a high-precision thin-film manufacturing technique for the microelectronics industry. Realizing the potential of ALD as an integral nanofabrication toolbox for the design, fabrication and modification of advanced materials is opening a new range of possibilities. The application of ALD to particles enables the production of a whole range of novel nanostructured particles for, among others, catalysis, energy conversion and storage, and medical applications.
In this paper, we focused on the fabrication of catalysts by deposition of the active phase (Pt) on a particulate substrate (TiO2 P25 nanoparticles). We demonstrated that ALD is a technique that 1) guarantees efficient use of the precursor; 2) allows precise control of the size and loading; 3) can be used for low and medium loading of catalysts by adjusting the number of repeated cycles; 4) leads to high-quality (low impurities level) end products.
The future demands for the high-volume production of tailored nanostructured materials require innovation in reactor design for ALD processes. Currently, fluidized bed reactors are the first choice for particle ALD, enabling tonne-scale production in the near future. Operating these reactors at low pressures favours the removal of gaseous compounds in the purging steps; however, operation at atmospheric conditions is beneficial for the scale-up potential.
Compared to liquid-based synthesis routes, ALD improves the fabrication compliance with the principles of Green Chemistry: absence of solvents/residues, minimization and easy handling of the reaction by-products (waste) and overall simplification of the production route (all process steps rely on robust gas-solid-based unit operations). The low value of the E-factor (comparable to the fine chemicals industry) clearly shows the way to more sustainable production schemes. The high degree of deposition control (size, composition, distribution) highlights the potential for high-end-value products such as noble metal-supported catalysts. Mild temperature and pressure conditions make the technique compatible with temperature-sensitive materials.
As ALD matures further, certain research challenges will have to be overcome: gaps such as the need for research on novel precursors (e.g. for silver and gold) and the requirement of clear understanding of process parameters (effect of pressure, growth mechanisms, and overexposure role on the deviation from ideal behaviour). Many challenging areas related to materials properties and nano-structuring can be addressed in a scalable production scheme offered by ALD. Bringing fundamental science and engineering principles together, ALD is effectively driving surface chemistry at an atomic resolution, while enabling industrial-scale production.
Financial support from the European Research Council (AggloNanoCoat and CONiA projects) is gratefully acknowledged. The authors would like to thank Ms. Andrea Fabre for her careful proofreading of the manuscript.
Aristeidis Goulas
Aristeidis Goulas received his Dipl. Eng. in chemical engineering from Aristotle University of Thessaloniki in 2008 and his MSc degree in sustainable energy technology from Delft University of Technology in 2011. He then joined the Product and Process Engineering group (Delft University of Technology) as a researcher. His work focuses on gas-phase nanostructuring techniques for the fabrication of novel nanostructured materials.
J. Ruud van Ommen
J. Ruud van Ommen received his MSc degree in chemical engineering in 1997, followed by a Ph.D. in 2001, both from Delft University of Technology (the Netherlands). He has been a faculty member at Delft since, with stints at Chalmers University of Technology (Sweden, 2004/2005) and the University of Colorado Boulder (USA, 2009). In 2011, he received an ERC starting grant to investigate the interplay between the agglomeration and coating of nanoparticles in the gas phase. Currently, he is an associate professor at Delft University, leading a research team with the focus on scalable processes to make nano-structured materials.