2014 Volume 31 Pages 111-125
2014 Volume 31 Pages 111-125
Graphene (GR) is a flat monolayer of sp2-bonded single carbon atoms densely packed into a honeycomb crystal lattice. Due to its unique characteristics, GR is expected to contribute to enhanced nanoelectronic, bio-electronic devices, etc. in the near future. However, the single-layered GR sheets have a tendency to form irreversible aggregates or even to restack easily due to strong attraction between sheets. Preventing of aggregation and restacking of GR is achieved by the dimensional transition from flat sheets to fractal-dimensional, nearly spherical crumpled balls using aerosol spray pyrolysis. This review first introduces the fabrication of crumpled GR and GR-composite by aerosol spray pyrolysis, and then discusses the material properties of GR: strain-hardening, compression and aggregation-resistant behavior. Finally, we introduce effective applications of hollow crumpled GR balls for oil absorbent and crumpled GR-composites for glucose biosensor, respectively.
Graphene (GR) is a flat monolayer of sp2-bonded single carbon atoms densely packed into a honeycomb crystal lattice1). GR can be synthesized by various methods such as mechanical exfoliation of graphite, chemical vapor deposition from carbon based precursor, and chemical oxidation and reduction of graphite. Fig. 1 shows structural models of GR, GR oxide (GO) and reduced GO (r-GO). r-GO is often mentioned as chemically modified GR. So here, we use GR and r-GO interchangeably for convenience. The structure of GR has been demonstrated to have a high specific surface area, high thermal conductivity, excellent mechanical stiffness, high optical transparency, good biocompatibility, and fast electron transportation2–7). The unique characteristics of GR have attracted tremendous interest for many applications. GR-based polymer nanocomposites exhibit improved mechanical, thermal, and electrical properties8–15). GR is also able to replace metal conductors in electronic and electrical devices due to its excellent electrical conductivity and mechanical flexibility16–18). A few ongoing studies have reported that GR can replace brittle and chemically unstable indium tin oxide in flexible displays and touch screens. Due to its excellent electronic properties, GR can be used in an enzyme immobilization matrix in the construction of a bio-sensor. In addition, GR as an electronic circuit material is considered to be potentially superior to other carbon-based nanofillers19–21). Therefore, GR is expected to contribute to enhancing nanoelectronic and bio-electronic devices in near future22). It is obvious that these excellent properties are relevant at the nanoscale and the manufacture of nanocomposites is highly dependent on the single-layered GR in the matrices.
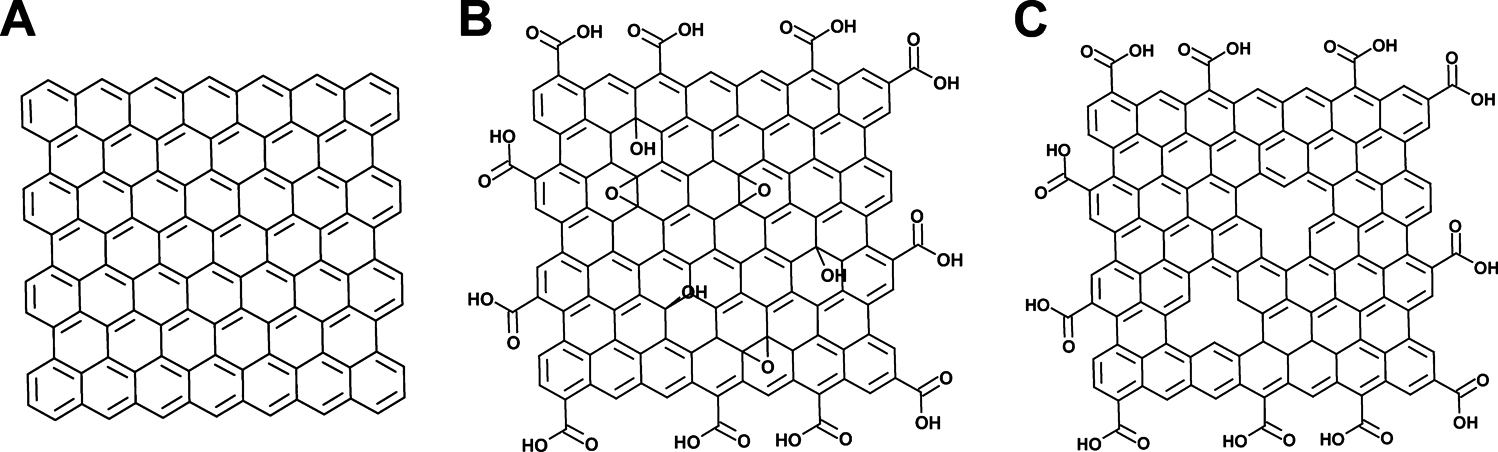
Structural model of (A) graphene, (B) GO and (C) r-GO.
However, single-layered GR sheets have a tendency to form irreversible aggregates or even restack easily due to π-π stacking and van der Waals attraction if the sheets are not well separated from each other23). Preventing aggregation and restacking is essential for GR sheets because the unique properties are only associated with individual sheets. Restacking and aggregation of GR sheets not only reduce their effective application but also compromise their properties, such as accessible surface area. Making exfoliated GR sheet materials will help to standardize the materials and their performance for various applications. Many methods for preventing aggregation in solution have been developed and typically include reducing the size of the sheets, tailoring the solvent-GR interactions, employing dispersing agents and attaching functional groups24–29). However, once the dispersions are dried, preventing aggregation of the solid state GR products and making them re-dispersible have been challenging topics23).
It will also be beneficial to develop crumpled paper balls because the dimensional transition technology from flat sheets to near three-dimensional (3D) crumpled balls can resist compression and aggregation properties. Like crumpled paper balls, crumpled GR is capable of tight packing into a near three-dimensional (3D) form without significantly losing surface area30).
In order to make crumpled GR, the free-standing aqueous droplets containing exfoliated graphene oxide (GO) in an inert atmosphere are evaporated. Then, the soft GO sheets can be compressed to form a near-spherical particle just like a crumpled paper ball and are then converted into r-GO by thermal reduction at high temperature31). This concept has been experimentally achieved by an aerosol-assisted capillary compression process called aerosol spray pyrolysis (ASP).
All the previous studies employed a liquid phase reaction that included many time consuming unit operation stages, such as liquid-solid separation, washing, and drying, in order to synthesize the GR32–40). On the other hand, the ASP has many advantages in fabricating of crumpled GR from GO because it is a very fast, simple and continuous process for fabricating self assembled composites as a one-step method41). It also takes a very short time of several seconds for crumpling and reducing of GO together and does not require any post heat treatment, purification or reduction agents23,41–43). The size of the crumpled particles and the degree of crumpling can be tuned with the concentration of GO in the aerosol droplets. The as-prepared crumpled GR by ASP is compression-resistant just like paper balls as compressive stress makes them stiffer and harder, and it has consistently higher and much more stable surface areas after the same processing steps compared to the GR sheets23). In addition, an anode modified by the crumpled GR exhibits enhanced performance in electricity generation over those covered with activated carbon and regular GR sheets, which lead to a significantly high short-circuit current and maximum power density44).
As mentioned above, GR-supported composites have attracted a great deal of attention for their enhanced material properties, such as their high electrical and thermal conductivity, and so on. Many materials such as polymers, metal oxides and noble metals have been used widely to fabricate functional GR composites31,45). When the crumpled GR-based composite is fabricated, improved material properties for many applications are expected.
In this review, we introduce the aerosol processing of GR and demonstrate the superior properties and interesting potential applications for crumpled GR. We first introduce the fabrication of crumpled GR and its composite by ASP. The material properties of crumpled GR, such as strain-hardening, compression and aggregation-resistant behavior, are then discussed. Applications for crumpled GR balls and GR-composite such as oil absorbent and glucose biosensor are also introduced, respectively.
To fabricate crumpled GR by ASP, a colloidal solution of exfoliated graphene oxide (GO) is prepared as an aerosol precursor. The colloidal GO is generally prepared by oxidation of graphite powder using a modified Hummers’ method as follows46–49). In the first step, graphite, K2S2O8, and H2SO4 are stirred together in an 80°C oil bath for the pre-oxidation process. Then, for the oxidation process, KMnO4 is slowly added in 30°C oil bath. Once mixed, the solution is transferred to an ice bath and forms a thick paste. Next, distilled water is carefully added, and the solution is stirred for 30 min while the temperature is maintained below 50°C. Finally, H2O2 is slowly added, turning the color of the solution from dark brown to yellow. The warm colloidal mixture is then filtered, washed with distilled water and dried. The dried filter cake is then dispersed in distilled water under ultra-sonication for the exfoliation process. Low-speed centrifugation is done at 3000 rpm for 15 min. It is repeated until all visible particles are removed (about 5 times) from the precipitates. The supernatant then undergoes two more high-speed centrifugation steps at 8000 rpm for 30 min to remove small GO pieces and water-soluble by-products. The final sediment is re-dispersed in distilled water using an ultrasonic bath, giving a colloidal solution of exfoliated GO47).
Fig. 2 demonstrates how the GO is successfully synthesized from graphite by the modified Hummers’ method. The X-ray photo emission spectroscopy (XPS) spectrum of the as-prepared GO shows that C and O peaks appeared at 285 and 533 eV, respectively (Fig. 2(A)). Further, the C1s spectrum shows two peaks at 284.7 and 286.6 eV, which are attributed to C-C and C-OH/C=O, respectively31). According to the result of the XRD analysis, the peak of GO appears at 10.21° (Fig. 2(B)). The Raman spectrum of the as-prepared GO shows a G band at 1600 cm−1 and a D band at 1337 cm−1 (Fig. 2(C))45). The G band of the GO is broadened compared to graphite, mainly due to the extensive oxidation. The UV-Vis spectrum of the GO dispersion shows a peak at 225 nm (Fig. 2(D)).
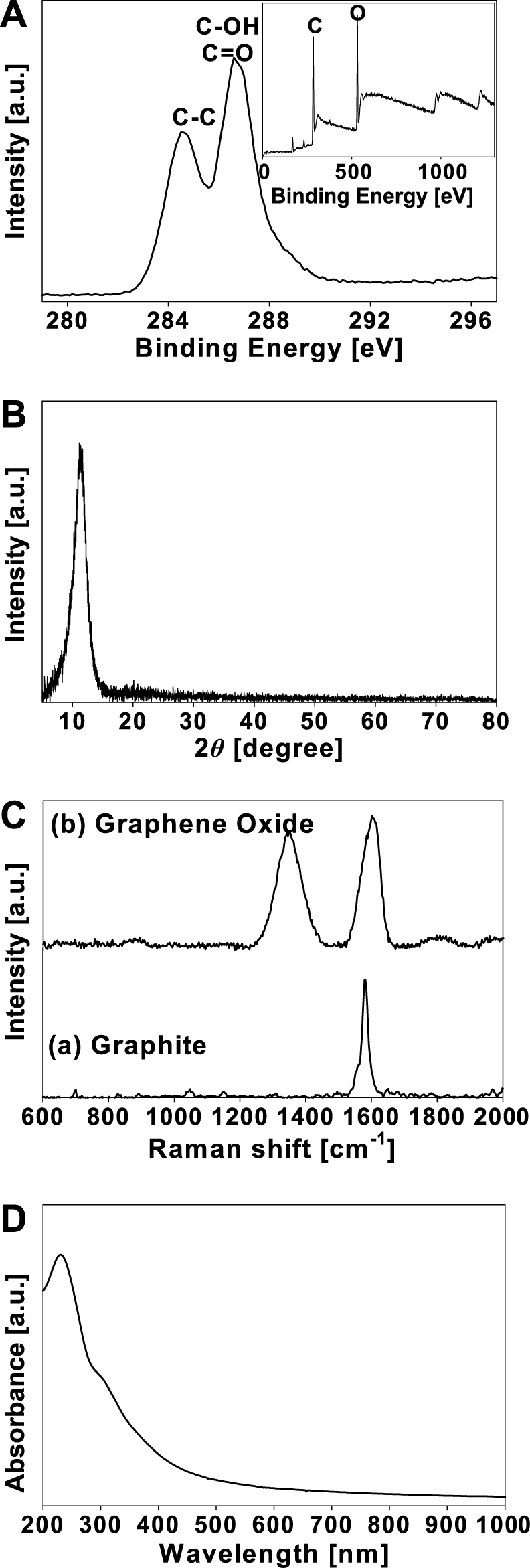
A: X-Ray photoelectron spectroscopy of the GO. Reprinted with permission from ref 31. Copyright 2012 ELSEVIER. B: The x-ray diffraction of patterns of the GO. C: Raman spectra of (a) graphite and (b) GO. Reprinted with permission from ref 45. Copyright 2013 ELSEVIER. D: UV-Vis spectrum of GO.
Fig. 3 shows a schematic illustration of aerosol process for fabricating crumpled GR from a colloidal GO solution. Since exfoliated GO sheets are essentially soft, water-dispersible, and in single atomic layers, crumpled GR can be easily fabricated from the GO colloid using the ASP process. The experimental apparatus for the ASP process consists of an ultrasonic atomizer, an electrical tubular furnace, and a filter sampler. An aqueous colloid of micron size GO sheets is sprayed to generate many aerosol droplets of micron size and flowed through a tube furnace preheated at 800°C by inert carrier gas (nitrogen or argon). Rapid evaporation of the solution causes shrinkage of the droplets, first concentrating the GO sheets and subsequently compressing them into crumpled balls of sub-micrometer scale. By heating, the GO is thermally reduced to GR as indicated by the color change from brown to black. It takes only several seconds to prepare the crumpled GR and there is no need for any post-heat-treatment or purification steps23).
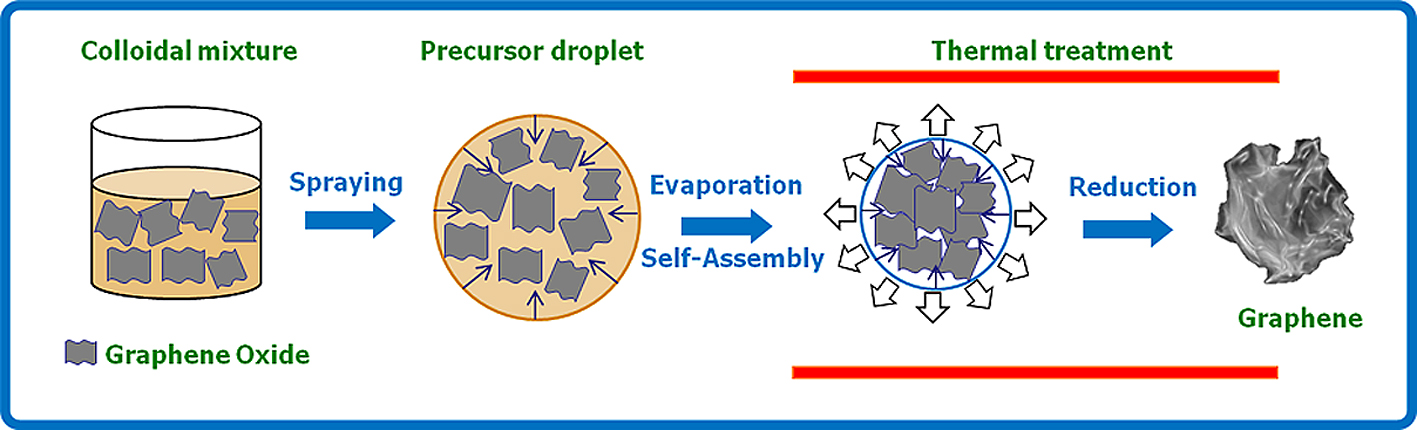
Schematic illustration of the formation of crumpled GR from GO via aerosol spray pyrolysis.
Field emission scanning electron microscopy (FE-SEM) images indicate morphology of crumpled GR by the ASP (Fig. 4). The samples reveal the near three-dimensional crumpled ball-like structures with many ridges and vertices23).
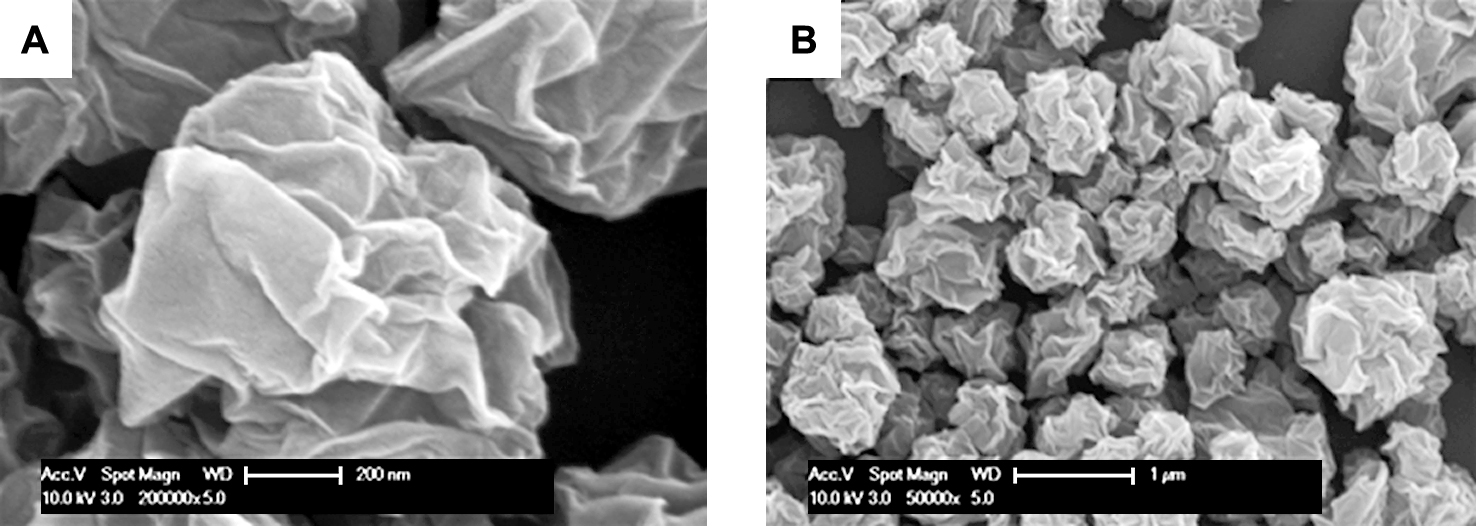
FE-SEM images of GR as shown in the (A) representative high magnification single particle and (B) low magnification overview.
The fabrication of hollow GR has been studied because of their promising properties, such as large specific surface area, low density, and high porosity50–63). Accordingly, various methods such as the sol-gel and hydrothermal methods have been developed to synthesize hollow particles of inorganic materials using templates and a series of many steps for treatment64–69).
However, when the ASP technique is employed, rapid formation of hollow GR balls can be achieved by capillary molding and template removal in one step and a continuous mode of production. The schematic drawings in Fig. 5 illustrate the process for fabricating hollow GR capsules. Aqueous aerosol droplets containing GO sheets and polystyrene (PS) colloids are first generated by an ultrasonic atomizer, and then flowed into a preheated tube furnace by argon carrier gas. As they pass through the heating zone, the aerosol droplets first rapidly evaporate. Then hollow GR balls are eventually shaped by wrapping PS beads by GO, GO reduction and PS evaporation. The low magnification scanning electron microscopy (SEM) image in Fig. 6(A) gives an overview of the sample morphology, which clearly shows their spherical shape and hollow nature. The transmission electron microscopy (TEM) image shows that the diameter of the capsules is around 580 nm, which is similar to that of PS templates. When a second component, such as metal or metal oxide nanoparticles, is added to the colloidal precursor, metal or metal oxide nanoparticles decorated by hollow GR composites can be easily synthesized (Fig. 6(B))43).
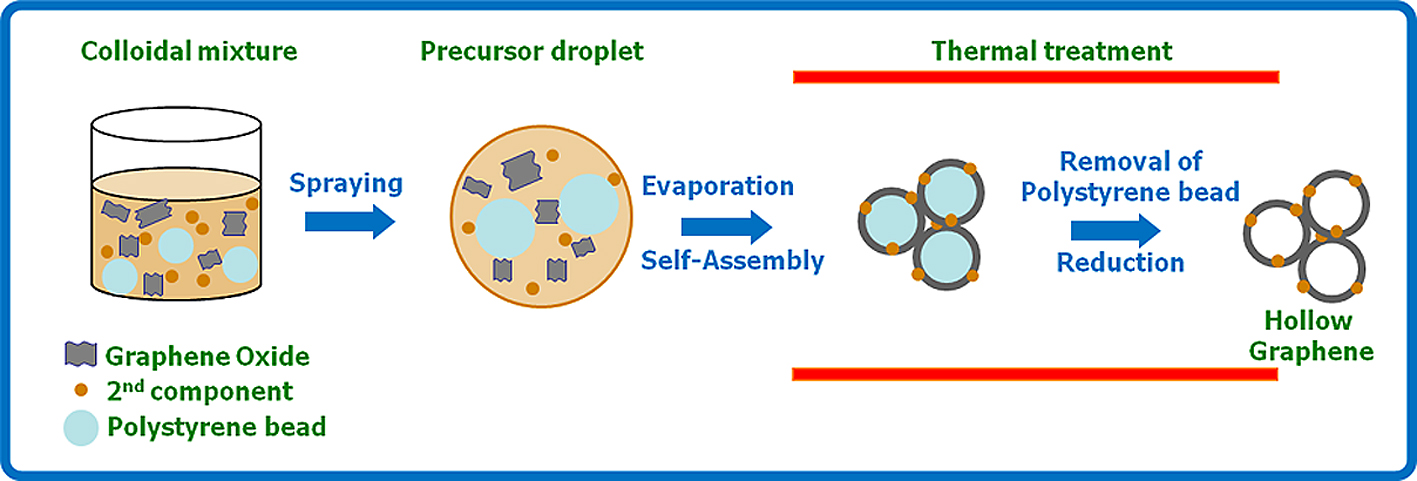
Schematic illustration showing the preparation of hollow GR capsules.
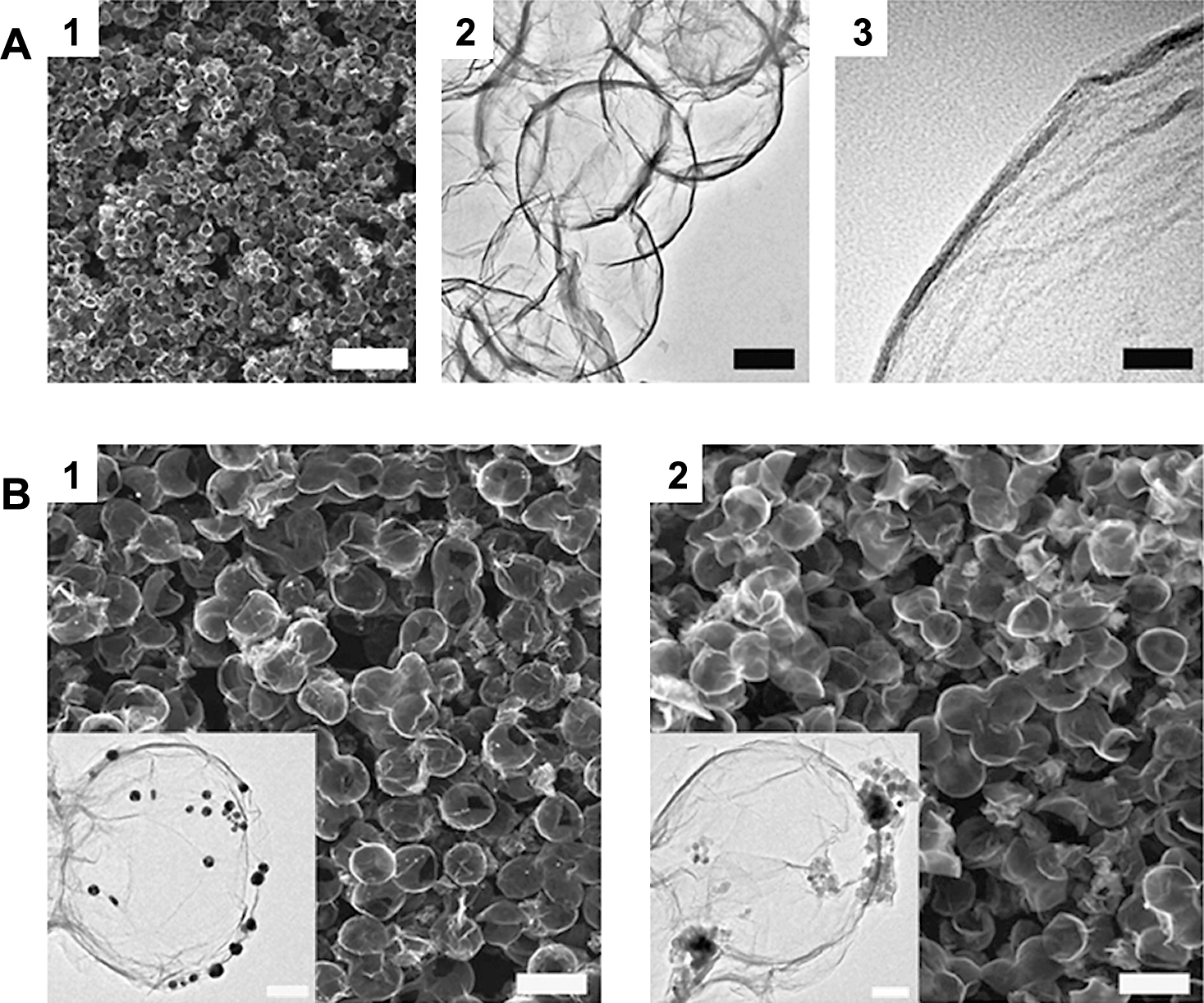
A: (1) SEM image showing an overview of the hollow GR capsules. (2) The hollow nature of the spheres can be clearly seen in the TEM image. (3) High resolution TEM image taken at the edge of a capsule. Scale bars: (1) 4 mm; (2) 200 nm; (3) 20 nm. B: SEM (1 and 2) and TEM (inset) images showing hollow GR capsules decorated by (1) Au and (2) Fe3O4 nanoparticles, respectively. Scale bars: (1 and 2) 1 mm; (inset) 100 nm. Reprinted with permission from ref 43. Copyright 2012 The Royal Society of Chemistry.
For the synthesis of the crumpled GR-metal oxide or crumpled GR-noble metal composite by ASP, a colloidal mixture as a precursor must be prepared with the as-prepared GO colloid and sub materials (metal oxide particles (TiO2) or noble metal precursor (H2PtCl6·6H2O and HAuCl4·3H2O)31,45). The experimental apparatus for crumpled GR can be employed to synthesize the crumpled GR composite. An ultrasonic atomizer is used to generate micron-sized droplets of the metal oxide-GO or noble metal-GO colloidal precursor. The droplets are then carried into a tubular furnace by argon gas. Crumpled GR composites are formed in the furnace and then collected by a Teflon membrane filter.
Figs. 7 and 8 show the schematic drawing of the formation of a crumpled GR-TiO2 composite and a crumpled GR-noble metal composite by the ASP process. For the synthesis of the crumpled GR-TiO2 composite, rapid evaporation of water, self-assembly between GO and TiO2, and thermal reduction of GO are carried out in series in the furnace (Fig. 7). In case of the crumpled GR-noble metal composite, the evaporation of water, formation of crumpled GO, thermal reduction of crumpled GO and the noble metal precursor, and the self-assembly between crumpled GR and noble metal nanoparticles are carried out (Fig. 8)45).
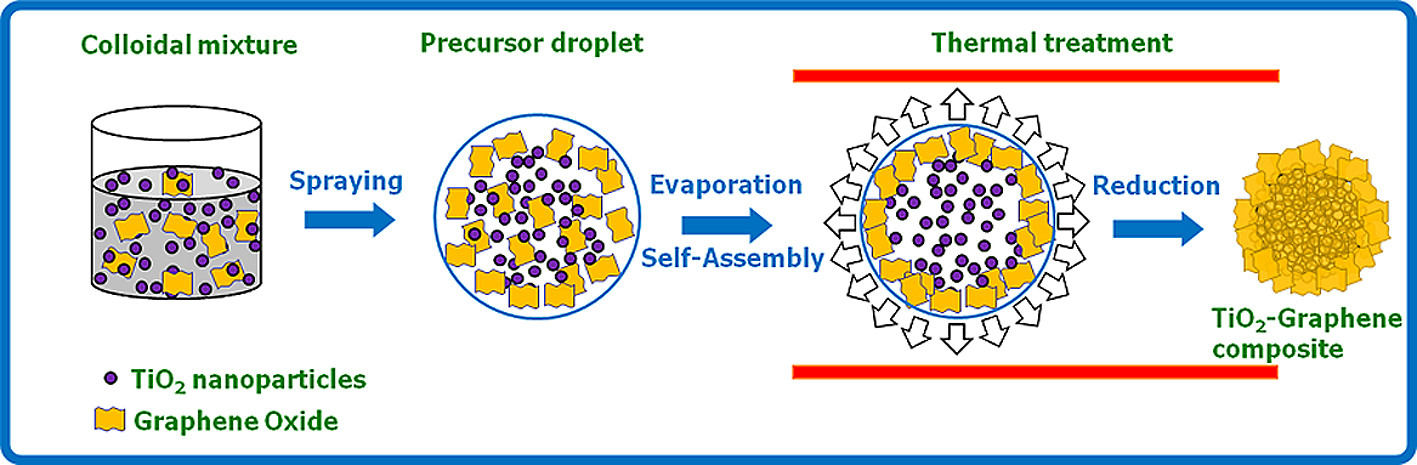
Schematic illustration of the formation of TiO2-GR composite from colloidal mixture of TiO2 nanoparticles and GO via aerosol spray pyrolysis.

Schematic illustration of the formation of noble metal-GR composite from colloidal mixture of noble metal precursor and GO via aerosol spray pyrolysis. Reprinted with permission from ref 45. Copyright 2013 ELSEVIER.
In Fig. 9, the FE-SEM image shows the morphology of a crumpled GR-TiO2 composite according to the concentration of the GO colloid. The TiO2 nanoparticles are encapsulated by the GR. The GO accumulates at the water surface of a sprayed droplet during evaporation and finally forms a GR ball. In contrast, hydrophilic TiO2 nanoparticles are agglomerated and positioned inside of the GR ball during the evaporation process. Further, as the GO concentration decreases from high to low concentration, a decrease in the degree of coverage of GR to TiO2 is clearly observed. Fig. 10(A) shows the X-ray photoemission spectroscopy (XPS) spectra of TiO2-GR composites. The C, Ti and O peaks are measured at 285, 480 and 533 eV, respectively. As the concentration of GO decreases, the intensity of C becomes lower whereas that of Ti and O become higher. This occurs because TiO2 is exposed at a lower concentration of GR. These results prove that the as-prepared crumpled GR composites can be harmoniously made from every piece of precursor by the ASP process. Additionally, according to the XRD analysis, the diffraction patterns of the crumpled GR-TiO2 composite show the anatase and rutile phase of TiO2 only. Although the GR content in the composite varies, the intensity of the TiO2 phases does not change because the intensity of the GR phase is much lower than that of TiO2 (Fig. 10(B))31).
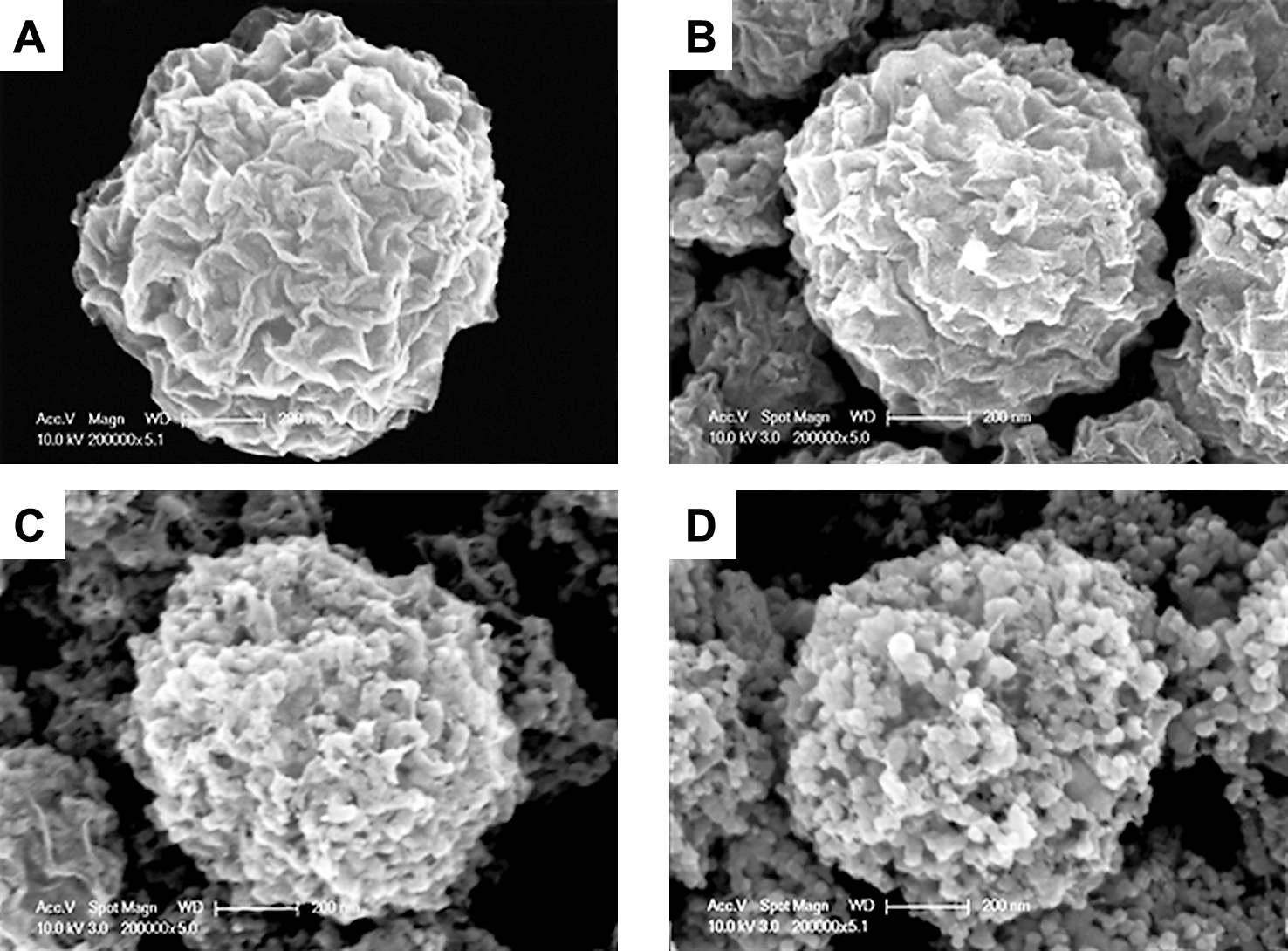
FE-SEM images of TiO2-GR composite at different weight ratios of GO/TiO2 (A) 2, (B) 0.5, (C) 0.1, (D) 0.05 at TiO2: 0.1 wt%. Reprinted with permission from ref 31. Copyright 2012 ELSEVIER.
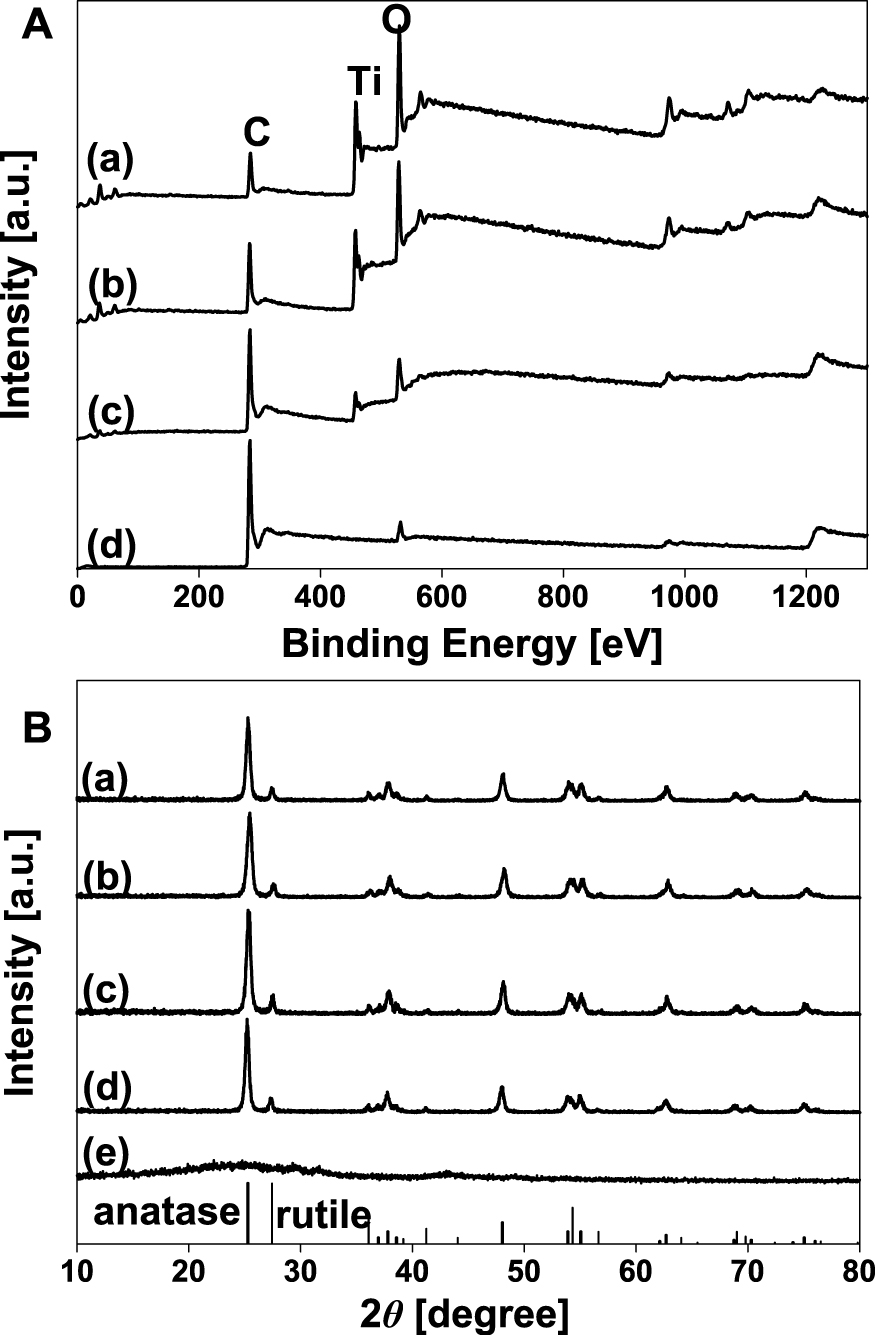
A: X-ray photoelectron spectra of TiO2-GR composite prepared at different GO/TiO2 weight ratio (a) 0.5, (b) 0.1, (c) 0.5, and (d) pure GO at TiO2: 0.1 wt%. B: XRD patterns of TiO2-GR composite with different GO/TiO2 weight ratio (a) 0.05, (b) 0.1, (c) 0.5, (d) 2, and (e) pure GO at TiO2: 0.1 wt%. Reprinted with permission from ref 31. Copyright 2012 ELSEVIER.
Fig. 11(A, B) reveals that the morphology of the crumpled GR-noble metal composites is generally the shape of a crumpled paper ball and that noble metal nanoparticles are deposited on the surface of the GR crumples. TEM analysis shows that Pt and Au nanoparticles 3 and 5 nm in diameter, respectively, are uniformly deposited on GR sheets. Fig. 11(C) shows the diffraction patterns of the noble metal-GR composites according to the XRD analysis. The crystallinity of the as-prepared composites synthesized at 800°C indicates the same peaks as those of the noble metal reference. The peak detected at around 25 degrees is GR. The crystallite sizes that are calculated by the Scherrer equation are 3.3 and 5.5 nm in Pt and Au, respectively45).
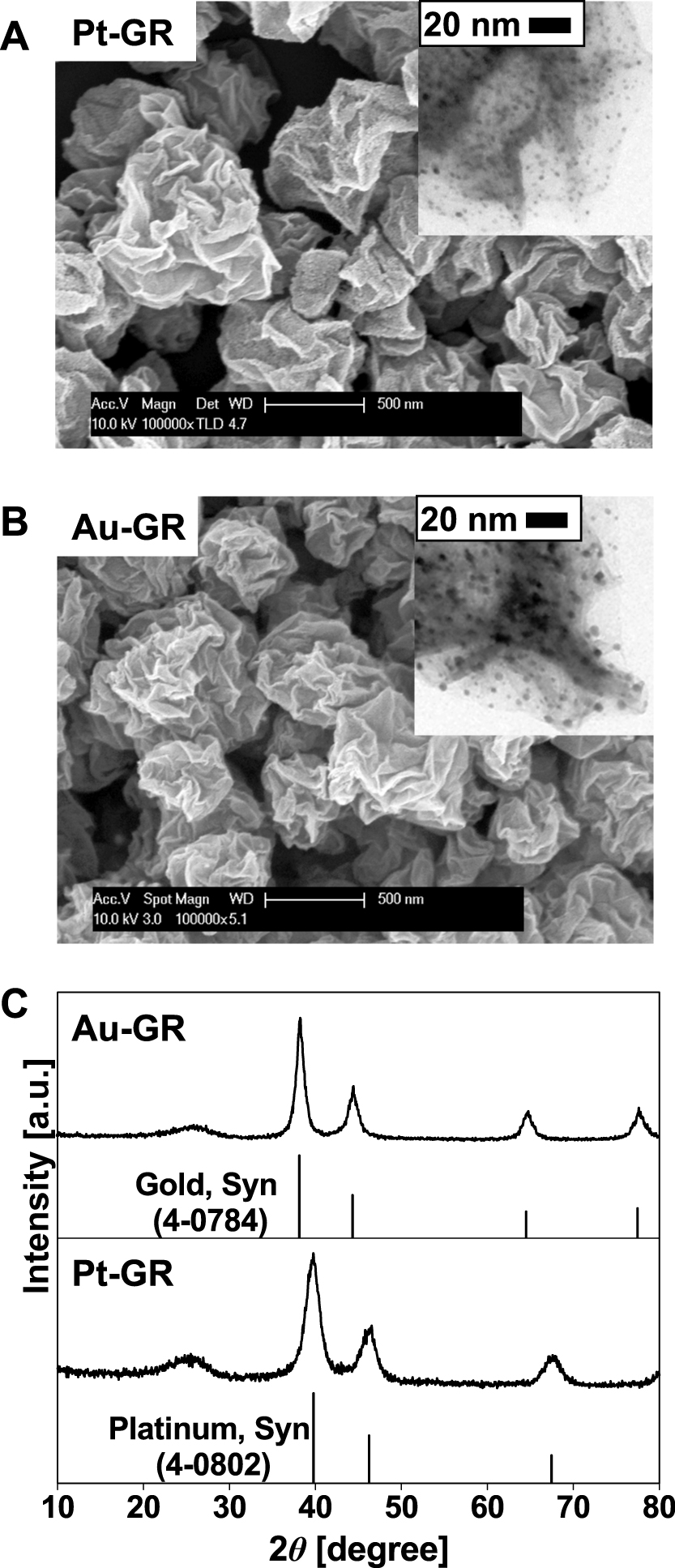
A: FE-SEM and TEM images of as-prepared crumpled Pt-GR composites. B: FE-SEM and TEM images of as-prepared crumpled Au-GR composites. C: The x-ray diffraction of patterns of as-prepared noble metal-GR composites (above: Au-GR and below: Pt-GR). Reprinted with permission from ref 45. Copyright 2013 ELSEVIER.
In a crumpled paper, the crumpled structure is stabilized by plastically deformed ridges made of kinked paper fibers, which prevent the structure from unfolding. The micro-structure analysis of the crumpled GR reveals that the plastic deformation in the crumpled graphene particles is due to strong π-π stacking at the ridges70–72). It is also found that the crumpled GR particles are compression-resistant because compressive stress makes them stiffer and harder.
The crumpled GR balls were remarkably stable against aggregation in solution as well as in a solid state due to the π-π stacked ridges and strain-hardening properties. When pelletized, crumpled GR formed a piece of isotropic, black solid with rough microstructures at both the surface and the cross section (Fig. 12(A, B)). In contrast, flat GR formed an anisotropic, shiny pellet with very smooth, nearly featureless surface but with a laminated micro-structure at the cross section (Fig. 12(C, D)). Moreover, the strongly compressed pellet of crumpled GR was readily redispersed in solvents upon shaking by hand (Fig. 12(E)). An SEM image of redispersed particles (Fig. 12(F)) shows that the crumpled GR morphology is not largely affected by the compression. However, pellets of the flat GR under the same pressure were not redispersed even after sonication due to extensive irreversible stacking (Fig. 12(G, H)). The electrical conductivity at the surface of crumpled GR pellets was similar to that of a flat GR. This reveals that crumpled GR can help to prevent aggregation without decreasing the overall electrical characteristics23).
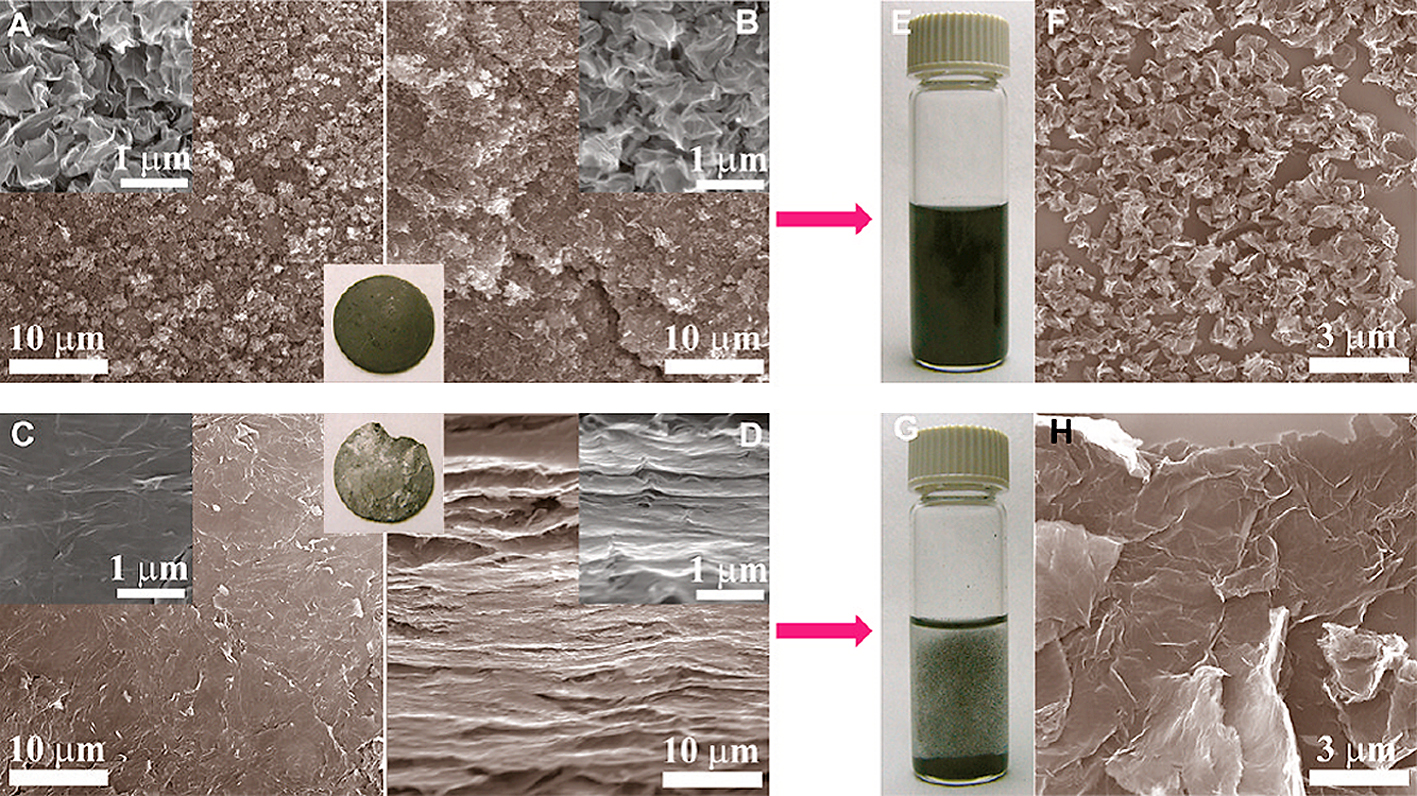
Compressed pellet of crumpled GR particles (inset of panels A and B) has rough and isotropic micro-structures as shown in the SEM images taken at both (A) the surface and (B) cross section due to their near-spherical, pointy shape. In contrast, flat GR sheets restack along the compressing direction, resulting in a highly anisotropic pellet (inset of C, D) with (C) very smooth surface and (D) lamellar cross section. (E, F) The pellet of the crumpled particles can be readily dispersed by gentle hand-shaking after being compressed at 55 MPa. (G, H) However, the pellet of regular GR sheets cannot be redispersed due to extensive aggregation. Reprinted with permission from ref 23. Copyright 2011 American Chemical Society.
The hollow GR balls exhibit properties such as hydrophobic structure, light weight, large free volume, and many small pores on the surfaces. Those features make the hollow GR balls attractive for oil absorption and recovery43).
In order to compare the oil absorption capability of hollow GR balls against some high surface area carbon materials, such as commercial activated carbon (Norit Darco G60) and commercial carbon black powders (Cabot Vulcan XC-72), we performed a set of control experiments using vegetable oil colored with a red dye (Fig. 13). The specific surface areas of the hollow GR balls, carbon black and activated carbon were 84 m2/g, 230 m2/g, and 789 m2/g, respectively. The oil absorption experiments showed that the hollow GR had the highest oil absorption capacity among the three samples (Fig. 13(A)). The result demonstrates that a high specific surface area is advantageous for molecular adsorption, but not necessarily for oil absorption. This result also suggests a basic design principle for oil absorption materials, which can be used to prepare as high a free volume as possible. After oil absorbing, the oil filled GR balls were aggregated on the water surface. Fig. 13 (B–E) demonstrates that initially vegetable oil colored with a red dye was spread on the water surface. Then, hollow GR balls decorated with magnetic nanoparticles were dropped onto the oil film, which quickly shrank within a few minutes (Fig. 13(C)), and eventually transformed into a thick carbon-oil droplet (Fig. 13(D)), which could be recovered by a magnet. When undecorated hollow GR balls were used, the resulting blend could be directly picked up and removed by a glass rod (Fig. 13(F–I))43).
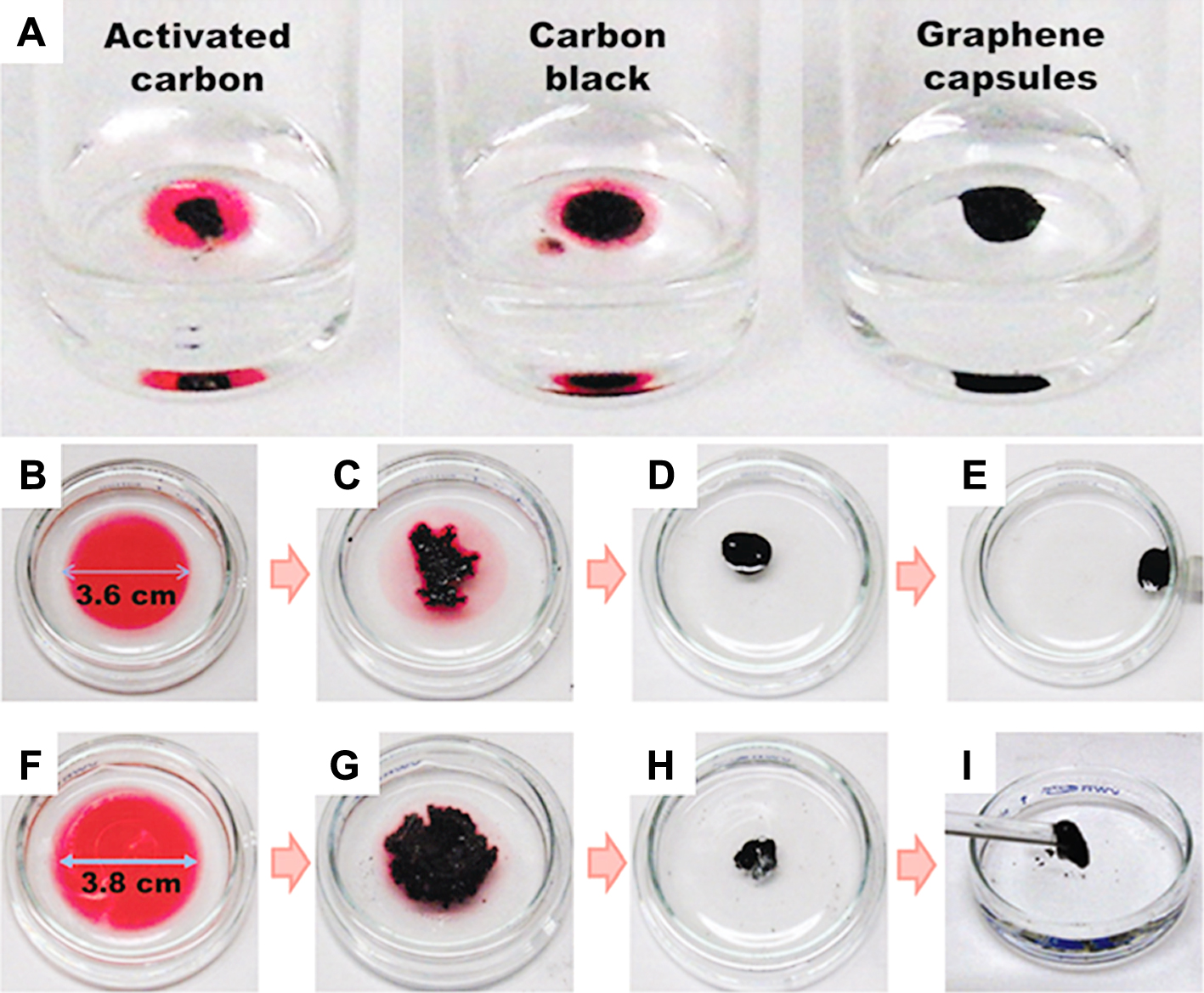
A: Photos showing oil absorption capability of the 3 carbon particles. In each vial, 1.0 mg of carbon particles was added to 17.5 ml of vegetable oil colored with a red dye. The oil-carbon blends were suspended on water for better view. B–E: Photos showing 100 ml of dyed vegetable oil absorbed by 10 mg of Fe3O4 decorated magnetic r-GO hollow capsules. F–I: 150 ml of dyed vegetable oil absorbed by 10 mg of r-GO hollow capsules. Reprinted with permission from ref 43. Copyright 2012 The Royal Society of Chemistry.
Therefore, the hollow GR balls prepared by the ASP process have a high free volume determined by the size of the template, and show promising oil absorption properties. In addition, hollow GR balls with many opening pores with a hydrophobic structure have an excellent oil absorption ability43).
4.2 Glucose biosensorsAs GR is expected to enhance bio-electronic devices in the near future, many studies have been conducted to find potential applications of GR73). Chen et al. (2008) showed that GR sheets may be biocompatible and therefore suitable for biomedical applications based on the cell culture experiments74). Yang et al. (2011) showed that no obvious toxicity of GR was observed in blood biochemistry, hematology, or histology analysis75). Park et al. (2010) proved that GR sheets are noncytotoxic to mammalian cell lines76). Thus, GR is expected to play an important role in improving the catalytic performance of biosensor applications due to its biocompatibility and unique properties.
Recently, glucose biosensors fabricated with GR and GR-based composites have been a great concern among enzyme-based biosensors since they can monitor blood glucose for the treatment and control of diabetes77,78).
In case of glucose biosensors fabricated with GR, Nafion-flat GR/GOD film-modified electrodes were developed for the detection of glucose and showed good stability. The glucose sensor exhibited a linear response in the range of 2.0 × 10−6 to 1.0 × 10−4 M with a detection limit of 1.0 × 10−6 M. The good response was due to the fast electron transfer in the GR composite film79). The self-assembled GR platelet-GOD nanostructures were reported as an enhanced glucose biosensor. The linear detection range of their sensor was estimated to be from 2 to 22 mM, and the detection limit was estimated to be 20 μM, which revealed that the GR platelet-GOD had a notable catalytic performance for detection of glucose80).
In case of glucose biosensors fabricated with a GR-based composite, Chitosan-flat GR/Au nanocomposite films were also reported as a potential glucose biosensor due to their good amperometric response to glucose with wide linear ranges81). The glucose biosensor fabricated with Pt-graphite nanoplatelets was suggested as an enhanced glucose biosensor as well and showed a high sensitivity and selectivity82). The results revealed that the graphite nanoplatelets incorporated into the biosensor interface increased the effective electrode surface area. Using a fabricated Au-flat GR composite, a prominent electrochemical response to glucose with wide linear ranges and a high sensitivity due to the synergistic effect of GR and Au nanoparticles was reported83,84).
In the above studies, flat GR and graphite platelets were employed to fabricate a GR or metal-GR composite-based biosensor electrode and improved properties for glucose biosensors were obtained. Therefore, if the glucose biosensor with the crumpled GR is used, more enhanced properties are expected.
Recently, it has been reported that a glucose biosensor fabricated with a crumpled GR-TiO2 composite demonstrated enhanced properties such as sensitivity and stability31). When cyclic voltammogram of glucose biosensors prepared with crumpled GR, TiO2, and the crumpled GR-TiO2 composite was measured, the biosensor prepared with the crumpled GR-TiO2 composite had the highest current flow as well as clear oxidation, with reduced peaks at −0.5 V and −0.6 V (Fig. 14(A)). The amperometric response by the glucose biosensor based on the crumpled GR-TiO2 composite was linear against the concentration of glucose ranging from 0 to 8 mM at −0.6 V (Fig. 14(B)). The highest sensitivity of the glucose biosensor based on the crumpled GR-TiO2 composite was about 6.2 μA/mM·cm2. This reveals the synergistic effect of TiO2 nanoparticles with crumpled GR as the electrode materials for biosensors31).
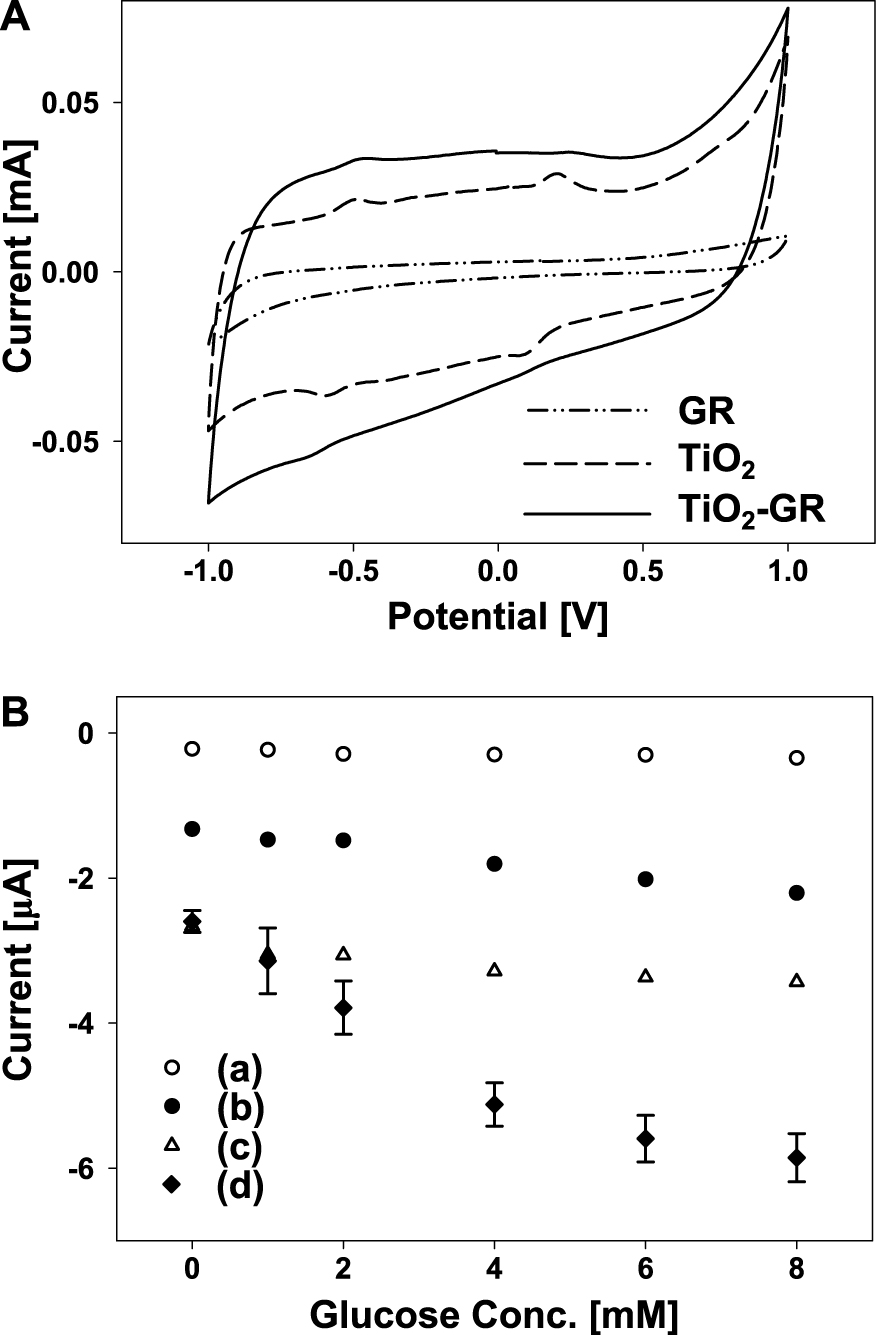
A: Cyclic voltammograms of glucose biosensors prepared with GR, TiO2, and TiO2-GR composite, respectively. B: Amperometric responses of the glucose biosensor based on TiO2-GR composite prepared at different weight ratios of GO/TiO2 (b) 0.5, (d) 0.1, (a) pure TiO2, and (c) pure GO. Reprinted with permission from ref 31. Copyright 2012 ELSEVIER.
Furthermore, a crumpled GR-noble metal composite was developed for glucose biosensor application45). Fig. 15(A) shows the cyclic voltammetry result of the glucose biosensor prepared with the crumpled GR-noble metal composites. The current flow of the biosensor prepared by the crumpled GR-Pt was higher than other composites, crumpled GR-Au or crumpled GR. The redox peak of the electrochemical reaction by the crumpled GR-Pt was the strongest at −0.07 and −0.25 V among the three electrodes. The sensitivity was 62 and 15 μA/mM·cm2 for the crumpled GR-Pt and the crumpled GR-Au composites, respectively (Fig. 15(B)). This result showed that the noble metal-crumpled GR composite covered with uniformly distributed noble metal nanoparticles had higher electro-chemical activity, as noble metals can enhance the electron transfer between the enzyme and the electrode45). The superior electrochemical activity could have originated from the crumpled GR that can tightly pack without significantly reducing the area of accessible surface and can also deliver much higher specific capacitance ability and better rate performance44).
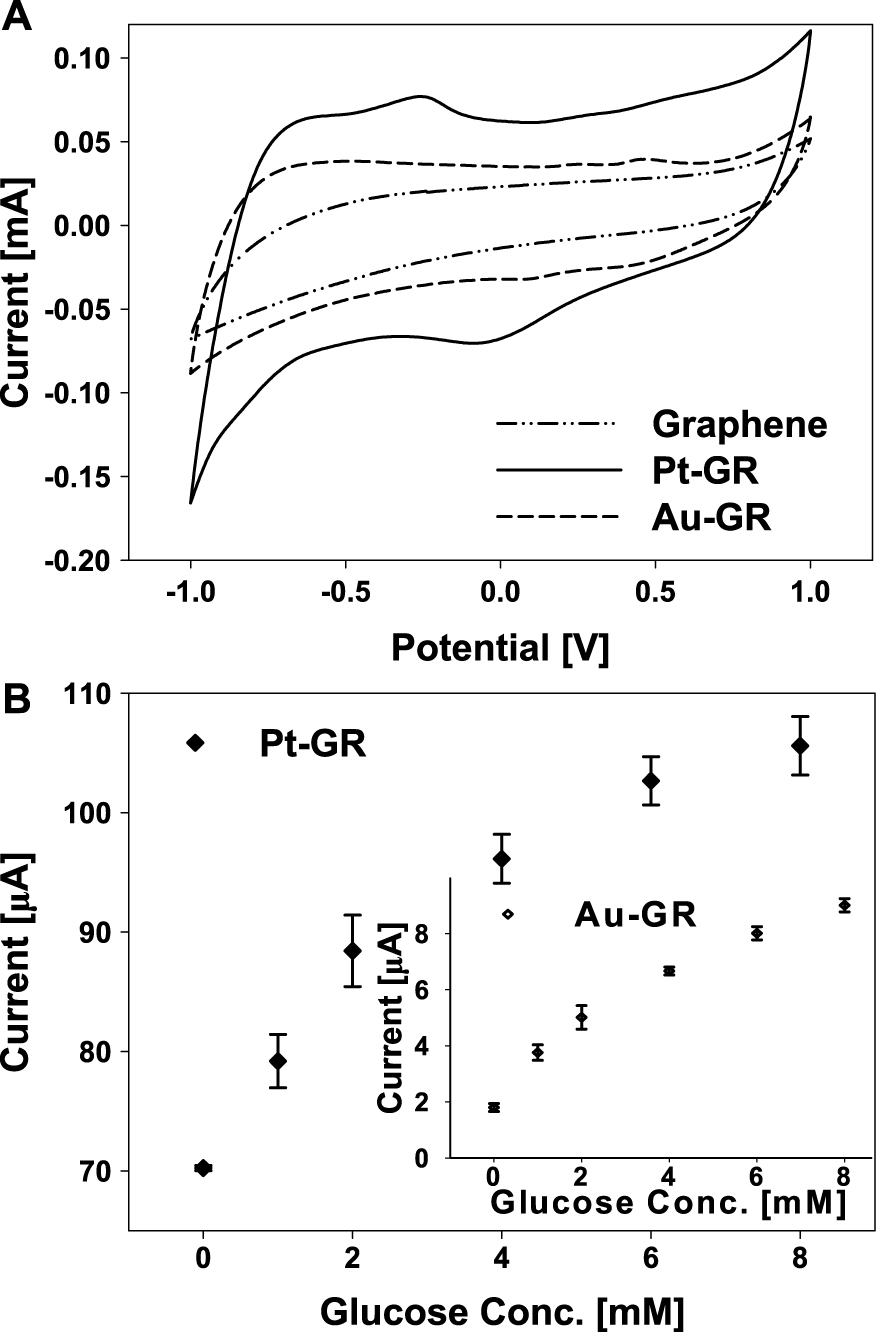
A: Cyclic voltammograms of the glucose biosensor prepared by noble metal-GR (Pt-GR and Au-GR). B: Amperometric responses of the glucose biosensor based on Pt-GR composite, Inset graph indicate that of Au-GR composite. Reprinted with permission from ref 45. Copyright 2013 ELSEVIER.
This review first introduced crumpled GR and its composite fabricated by aerosol spray pyrolysis (ASP). We then discussed the material properties of crumpled-GR such as strain-hardening, compression and aggregation-resistant behavior. Finally, we introduced potential applications of a crumpled GR ball and its composite such as oil absorbent and glucose biosensors.
In the section on the fabrication of crumpled GR, we described the preparation of colloidal GO by the oxidation of graphite powder using a modified Hummers’ method. Then, we presented the experimental apparatus and schematic procedure of ASP for fabrication of crumpled GR, hollow GR, and GR-based composites.
In the section on the materials properties of crumpled GR, we demonstrated that crumpled GR is stabilized by external influences, and thus does not unfold or collapse during various types treatments. We further discussed the difference in specific surface area between flat GR and crumpled GR processed under the same conditions. Finally, we discussed strain-hardening behaviors of crumpled particles, which make them remarkably resistant to aggregation in both solution and dried states.
In the section on the applications of crumpled GR, first, we discussed application of hollow GR balls for oil absorption and recovery since the hollow GR balls have properties, such as hydrophobic structure, light weight, large free volume, and many small pores on the surfaces, and introduced the superior capacity for oil absorption of hollow GR capsules. Second, we introduced glucose biosensors fabricated with crumpled GR-TiO2 and crumpled GR-noble metal composites since GR-supported composites are known for their superior biocompatibility, electrical conductivity, and chemical and electrical properties. The biosensor prepared with the crumpled GR-Pt composite showed the highest current flow as well as clear oxidation, which results in high sensitivity. The results for the biosensor prepared with the crumpled GR-noble metal composite showed that the noble metal-crumpled GR composite covered with uniformly distributed noble metal nanoparticles had higher electrochemical activity, as noble metals can enhance the electron transfer between the enzyme and the electrode.
This research was supported by the General Research Project of the Korea Institute of Geoscience and Mineral Resources (KIGAM) funded by the Ministry of Science ICT and Future Planning. J.H. thanks supports from the Initiative for Sustainability and Energy at Northwestern (ISEN) through an Early Career Investigator Award (initial seed fund) and the Office of Naval Research (ONRN000141310556).
Sun Kyung Kim
Sun Kyung Kim received her B.S. (2006) and M.S (2010) degrees from Chungnam National University and Sogang University in Korea, respectively. She is currently working toward her Ph.D. degree in Rare Metals Research Center at Korea Institute of Geoscience and Mineral Resources and Interdisciplinary Program of Integrated Biotechnology at Sogang University. Her current research interests include aerosol synthesis of nano-materials and their applications for electrochemical sensors.
Hankwon Chang
Hankwon Chang received his B.S. (1998) and M.S. (2000) degrees from Yeungnam University, Korea and Ph.D. (2003) degree in Chemical Engineering from Hiroshima University. In 2004, he returned home country, Korea and joined in Nano-Materials Research Team of Korea Institute of Geoscience and Mineral Resources (KIGAM) as a Senior Researcher. He is now a Principal Researcher in Rare Metals Utilization Research Team of KIGAM and an Associate Professor of Nanomaterials Science and Engineering Major of University of Science and Technology (UST). As a societal service, he has been working as an Editorial Board Member of Advanced Powder Technology from 2009 and a Chair for Nano-Materials Division of the Korean Association for Particle and Aerosol Research (KAPAR) from 2012. His current research interests include aerosol synthesis of functional nano-materials and their applications for energy conversion and storage materials.
Jeong-Woo Choi
Jeong-Woo Choi received his B.S. (1982) and M.S. (1984) at Sogang University, Korea, and Ph.D. (1990) at Rutgers University, USA. He worked at the IBM Almaden Research Center and Mitsubishi Electronics Advanced Technology R&D Center as a visiting researcher from 1993 to 1996. He also received D. Eng. (2003) from Tokyo Institute of Technology, Japan, as well as MBA (2005) from the University of Durham, UK. He has been a Professor of the Department of Chemical & Biomolecular Engineering, Sogang University, South Korea, after 1990. His main research area is nanobioelectronics including biomemory device, nanoprotein chips, and cell chips based on nanotechnology. He has published over 313 peer-reviewed papers including in Angewandte Chemie, Advanced Materials, Biomaterials, Biosensor and Bioelectronics, etc. His current research interest is the application of nanoparticles to nanobioelectronic device.
Jiaxing Huang
Jiaxing Huang is an Associate Professor of Materials Science and Engineering at Northwestern University. He received a B.S. in Chemical Physics from University of Science and Technology of China (USTC) in 2000 and a Ph.D. in chemistry from the University of California, Los Angeles (UCLA) in 2004, and became a Miller Research Fellow at the University of California, Berkeley before joining Northwestern in 2007. His research interest is in the general area of materials chemistry, processing and manufacturing. Some materials of interests include 2D materials, organic crystals and metal nano-structures. He has been a recipient of the IUPAC Young Chemist Prize, the Alfred P. Sloan Research Fellowship, the NSF CAREER Award, the inaugural ISEN Early Career Investigator Award at Northwestern, and the Gustav Olling Outstanding Young Manufacturing Engineers Award from the Society of Manufacturing Engineers.
Hee Dong Jang
Hee Dong Jang received his B.S. (1982), M.S. (1984), and Ph.D. (1993) at Sogang University in Korea, and Dr. Eng. (2005) at Hiroshima University in Japan. He has worked as a postdoctoral researcher at the University of California at Los Angeles from 1996 to 1997 and a visiting scholar at the Northwestern University from 2009 to 2010. He has been appointed as a President of Korea Association for Aerosol and Particle Research from 2012, and an Executive Editor of Advanced Powder Technology from 2009. He has been working at Korea Institute of Geoscience and Mineral Resources (KIGAM) from 1985, and he is currently a distinguished principal researcher and a Director of Rare Metals Research Center at KIGAM, and Professor in the Nano-Materials Science and Engineering Major of University of Science and Technology. His area of expertise is the aerosol processing and application of nanostructured materials. His current interests involve the synthesis of Graphene based nanocomposite materials and their application for energy, environmental and biomaterials.