2016 Volume 33 Pages 33-47
2016 Volume 33 Pages 33-47
Magnetic nanoparticles provide a unique nanosystem for various smart therapy applications because of their biocompatibility, their nanostructures which can be prepared controllably to match with the interest of study and, specifically, their responses to an external magnetic field. Interests in utilizing magnetic nanoparticles for biomedical treatments originate from their external controllability of transportation and movement inside biological objects and magnetic heat generation which provide tremendous advantages for targeted drug delivery and controlled drug release as well as magnetic hyperthermia. Recent progress in synthesis and functionalization has led to the formation of various functional magnetic nanoparticles with controlled magnetic properties based on controlling their particle size, shape and composition. In this review, we focus on the synthesis, protection and functionalization of iron oxide-based magnetic nanoparticles in order to control magnetic properties of nanostructured systems. We also highlight the recent advances in the development of multifunctional therapeutic nanosystems combining magnetic nanoparticles and drugs as well as their superior efficacy in biomedical treatments with smart performance by including therapy and modulated drug delivery and release through magnetic heating.
Over the last decades, interest in magnetic nanoparticles has increased enormously. The magnetic nanomaterials are unique because of their magnetic behaviors with magnetic fields and field gradients. Fundamental researches exploiting various nanostructures with corresponding magnetic characteristics have utilized magnetic nanoparticles for various applications. The magnetic nanoparticles with appropriate modifications have been extensively investigated for biomedical and pharmaceutical applications such as drug delivery, hyperthermia, magnetic resonance imaging, tissue engineering and repair, biosensing, biochemical separations, and bioanalysis as widely reported and reviewed (Hervault and Thanh, 2014; Kumar and Mohammad, 2011; Laurent et al., 2008; Lu et al., 2007; 2011; Nguyen and Kim, 2014; Reddy et al., 2012). In the field of disease therapy, advances in magnetic nanoparticle technology resulted in the development of theranostic nanoparticles which consist of therapeutic and diagnostic agents within a single particle. These magnetic nanoparticles should possess very uniform morphology with high magnetization values which ensure the external controllability. They also must have good dispersion, stability and biocompatibility to prevent endocytosis by macrophages as well as to extend their residence time in circulating blood. The developments and applications of these nanoparticles strongly require synthesis of magnetic nanoparticles of customized size and shape with appropriate surface engineering. Precise control over the synthetic conditions and surface functionalization of magnetic nanoparticles is crucial because it governs their magnetic characteristics, colloidal stability, physicochemical properties, and biological responses. However, the magnetic nanoparticles obtained under different synthetic conditions may display large differences regarding their magnetic properties due to formation of structural disorder and phase boundaries, or the existence of a magnetically dead layer at the particle surface. Therefore, to understand, exploit and improve applications of magnetic nanoparticles as well as to develop new ones, careful studies for controlled synthesis and surface modification are utmost important.
Herein, we briefly discuss the backgrounds on physical properties of magnetic nanoparticles and then introduce the design strategies for magnetic nanoparticles as a smart platform for controlled drug delivery and hyperthermia treatment. We will highlight the recent progress on the synthesis of magnetic nanostructures with growth mechanisms in order to control their magnetic behavior. We also introduce and discuss some typical developments of mechanical actuation and manipulation of drug release. Finally, we will highlight recent state-of-the-art uses of magnetic nanoparticles in hyperthermia treatment.
The properties of magnetic materials are characterized by the alignment of atomic magnetic moments generated by the spinning of electrons. The atomic magnetic moments can be aligned in a parallel or antiparallel fashion with respect to the neighboring ones in the crystal lattice and this type of interaction gives rise to the macroscopic magnetic behaviors which are usually measured by magnetization, coercivity, and magnetocrystalline anisotropy constant (Leslie-Pelecky, 1996). A large-sized magnetic material usually consists of a number of domains in order to minimize its internal energy and exhibits ferromagnetic characteristics. When an external magnetic field of strength is applied to a multidomain magnetic particle, the magnetization increases with the increase of magnetic field until a saturation value is reached because the magnetic moments tend to align themselves in the same direction of applied field. When these particles are removed from the magnetic field, the magnetic domains do not return to their original orientations and cause a remnant magnetization. The remnant magnetization can only be removed by applying a coercive field in the opposite direction to the initially applied field. The coercivity is strictly related to the magnetocrystalline anisotropy constant which reflects the energy required to change the direction of magnetic moments.
When the particle size decreases to a certain level, the formation of a domain wall structure is not thermodynamically favored and thus the magnetic nanoparticles consist of all spins aligned in the same direction (Bean and Livingston, 1959). As the particle size decreases toward critical particle diameter for single domain structure, Dsd, the formation of domain walls becomes energetically unfavorable and the particles are called single domain. As the particle size continues to decrease and reaches a critical size where thermal energy exceeds the magnetic anisotropy energy barrier, the magnetization measured in a finite time interval or that in a removal of an external magnetic field is zero. This particle size represents the critical size for superparamagnetism behavior, Dsp, and such particles are superparamagnetic. The characteristic temperature which defines a transition from ferromagnetic to a thermally unstable or superparamagnetic behavior is called the blocking temperature, TB. The corresponding magnetization curve of the superparamagnetic material exhibits a saturation of the magnetization and no hysteresis around the origin. The negligible remnant magnetization of superparamagnetic nanoparticles is advantageous because the nanoparticles can avoid their aggregation during preparation and storage and after administration. The magnetism of nanoparticles is mainly determined by the type of material, the crystallinity of the structures, and also the particle size. The critical size for single domain structure and that for superparamagnetic behavior could be predicted as shown in Fig. 1 (Krishnan et al., 2006 and Krishnan, 2010) by using the physical theories for domain stability in crystalline particles (Kittel C., 1949) as well as the bulk properties available in the literature. Analysis and calculation for the critical sizes for magnetization behavior and that for single-domain behavior could be referred from Bean (1955) and Frei et al (1957), respectively.

Predicted size ranges for single domain structure and superparamagnetism behavior of common magnetic materials. For diameters (D) larger than maximum diameter for single domain structure (Dsd), D > Dsd, the magnetic materials split into multiple domains; for diameters (D) smaller than maximum diameter for super-paramagnetism behavior (Dsp), D < Dsp, they exhibit superparamagnetism; and for diameters in between, Dsd > D > Dsp, they are single domain and ferromagnetic (Krishnan et al., 2006; Krishnan, 2010).
The magnetic behavior of magnetic nanoparticles in a specific system is the results of both the intrinsic properties of the nanoparticles and the interactions between nanoparticles and surrounding materials. The biomedical applications of magnetic nanoparticles depend on a number of factors related to the size, shape and magnetism of the biocompatible nanoparticles as well as the physicochemical properties of the drug-loaded nanoparticles, field strength and geometry, depth of target tissue, rate of blood flow and so on. In order to maximize performance of magnetic nanoparticles in biomedical applications, it is necessary to consider the change of magnetic characteristics in the presence of various biocompatible coating or suspension media. In fact, surface modification and functionalization of magnetic nanoparticles is required to protect particle agglomeration and to provide functional groups for the conjugation of other materials such as drugs, targeting ligands, proteins and DNA sequences (Cuyper et al., 2007; Gupta and Curtis, 2004; Liu et al., 2008; Ma et al., 2006). Such modification can be achieved by a diverse group of materials such as polymeric stabilizers/surfactants like dextran (Bulte et al., 1992), carboxydextran (Briley-Saebo et al., 2006), poly(ethylene glycol) (PEG) (Butterworth et al., 2001; Lutz et al., 2006) or poly(vinyl alcohol) (PVA) (Liu et al., 2008); or by a layer of oxide surfaces (SiO2) (Chastellain, et al., 2004), or inorganic metals (gold) (Jeong et al., 2006).
When polymeric materials are employed as stabilizers, the adsorption of polymers onto the magnetic nanoparticles provides protective steric repulsion and acts as a barrier against the interaction between the particles and thus keeping the particles from aggregation (Duran et al., 2008). The polymeric coatings can also tailor the surface properties by introducing new chemical functionality and surface charge to the nanoparticles for further attachment of other materials. The key characteristics of polymeric materials should be considered including length and molecular weight, biodegradability and hydrophobic/hydrophilic nature, conformation, degree of surface coverage, and attachment mechanism to the particle surface. Several functional materials such as dimercaptosuccinic acid (Fauconnier et al., 1997), bisphosphonates (Portet et al., 2001) may be employed to facilitate the efficient attachment of the polymers onto the surface of magnetic nanoparticles.
Inorganic coatings such as gold and SiO2 are other useful coating materials. For instance, Au-Fe3O4 nanoparticles could be synthesized by controlling growth of Au on the surface of Fe3O4 (Goon et al., 2009; Nguyen et al., 2011; Xu et al., 2007) and the Au could provide new surface to be functionalized with various ligands such as protein, oligonucleotide, DNA sequences through Au–S covalent bonding as reviewed (Nguyen et al., 2011). Robinson et al. (2010) reported that surface coating of Fe3O4 by Au nanoparticles and subsequent functionalization with thiolated DNA slightly reduced the magnetization saturation due to addition of nonmagnetic materials but helped particles to remain stable in aqueous solution. It was also discussed that gold coating could modify the magnetic properties by isolating the magnetic cores and thus could provide possibility of producing very efficient nanostructures (Banerjee et al., 2011; Wang et al., 2005). For example, a gold coating of approximately 0.4 to 0.5 nm thickness around superparamagnetic iron oxide nanoparticles resulted in a four- to five-fold increase in the amount of heat release in comparison with uncoated nanoparticles on application of low frequency oscillating magnetic fields (Mohammad et al., 2010).
It should be noted that, in most cases, the presence of polymers or other nonmagnetic materials may induce the imperfections in crystal structure that, consequently, may negatively influence the magnetic properties of the nanoparticles. However, it has also been observed that it is possible to maintain the magnetic properties by coating the magnetic nanoparticles with phosphonates (Daou et al., 2008) which are supposed to reduce the spin canting in the particle surface. In fact, since the magnetic response of a system to the surface modification is complex and system specific, no correlation between surface coating and magnetic response of a system has been clearly established.
Many publications have described the efficient synthetic procedures to size- and shape-controlled, highly stable, and monodisperse magnetic nanoparticles. High-quality magnetic nanoparticles have been synthesized with a number of different methods such as chemical co-precipitation (Massart, 1981; Wu et al., 2007), thermal decomposition and/or reduction (Kimata et al., 2003), microemulsion synthesis (Chin and Yaacob, 2007), hydrothermal/solvothermal synthesis (Chen et al., 2008; Khollam et al., 2002). Among these methods, the magnetic nanoparticles prepared from thermal decomposition and hydrothermal/solvothermal approaches exhibit high uniformity in both size, shape with excellent magnetic properties and the preparation processes show high potential on a large scale fabrication. Herein, we highlight several typical synthesis processes to control magnetic properties of nanoparticles with the corresponding particle formation mechanisms, focusing on the Fe3O4-based material.
Monodisperse and superparamagnetic iron oxide nanoparticles have been widely synthesized by thermal decomposition of organometallic compounds in high-boiling organic solvents containing stabilizing surfactants. Since the system temperature is high, the magnetic nanoparticles are formed with high crystallinity and magnetization. Sun et al. (2002 and 2004) reported a synthesis of 4 nm monodisperse magnetite nanoparticles by refluxing a reaction mixture containing the iron (III) acetylacetonate, Fe(acac)3, 1,2-hexadecanediol, oleic acid, and oleylamine in diphenyl ether solvent (boiling point of 265 °C), while 6-nm monodisperse Fe3O4 nanoparticles were obtained in benzyl ether solvent (boiling point of 300 °C). The oleylamine acted as both reducing agent and stabilizer to obtain monodisperse nanoparticles. To make larger Fe3O4 nanoparticles, a seeded growth process was demonstrated for generating nanoparticles up to 16 nm by controlling the ratio of seed to additional precursor. The magnetic saturation values of 16 nm magnetite nanoparticles were experimentally determined to be excellent with a value of 83 emu g−1. In another study, monodisperse magnetic nanoparticles were synthesized by mixing iron oleate with octadecane and oleic acid, followed by heating to 300 °C (Jana et al., 2004). The diameter of the Fe3O4 nanoparticles could be controlled between 6 and 50 nm by changing the ratio of ferric oleate to oleic acid from 0.1 to 10. Similar to that in the synthesis of Fe3O4 nanoparticles, the high temperature reaction of Fe(acac)3 and Mn(acac)2 gives monodisperse MnFe2O4 nanoparticles with their size, composition, and shape controlled by the reactant concentrations (Zeng et al., 2004). It was reported that triethylene glycol directly reacted with Fe(acac)3 at an elevated temperature to prepare non-agglomerated Fe3O4 nanoparticles with uniform shape and narrow size distribution (Cai and Wan, 2007).
Hyeon’s group prepared monodisperse iron oxide nanoparticles with sizes of 4, 8, and 11 nm by thermal decomposition of [Fe(CO)5] as iron precursor and oleic acid with different molar ratios, followed by controlled chemical oxidation with trimethylamine N-oxide as a mild oxidant (Hyeon et al., 2001). In this synthesis, monodisperse iron nanoparticles were initially generated, and these were further oxidized controllably by trimethylamine N-oxide to produce iron oxide nanoparticles. The particle size could be controlled by using different solvents with different boiling points. For instance, 5-, 9-, 12-, 16-, and 22-nm-sized iron oxide nanoparticles were synthesized by using 1-hexadecene, octyl ether, 1-octadecene, 1-eicosene, and trioctylamine which have the boiling point of 274 °C, 287 °C, 317 °C, 330 °C and 365 °C, respectively (Park et al., 2007). The current synthetic procedure is quite general, and nanoparticles of many transition-metal oxides, such as MnO, CoO, and ZnO, have been synthesized by using a similar procedure. Hyeon’s group also reported that sequential decomposition of iron pentacarbonyl and the iron oleate complex at different temperatures results in the formation of monodisperse iron nanoparticles in the size range of 6–15 nm (Park et al., 2005). The overall synthetic procedure was similar to seed-mediated growth. The thermal decomposition of [Fe(CO)5] at relatively low temperature induced short burst of nucleation from a supersaturated solution, and the decomposition of the iron oleate complex at a higher temperature facilitated subsequent growth of particles without any significant additional nucleation (Kwon et al., 2007). They also developed an ultra-large-scale synthesis of 40-g monodisperse Fe3O4 nanoparticles using Fe-oleate complex prepared from hydrated iron chloride and sodium oleate as reactants as shown in Fig. 2 (Park et al., 2004). The magnetic nanoparticles are dispersible in various organic solvents including hexane and toluene. The water-soluble magnetite nanoparticles could be prepared under similar reaction conditions with addition of α,ω-dicarboxyl-terminated poly(ethylene glycol) as a surface capping agent (Hu et al., 2006).

The overall scheme for the ultra-large-scale synthesis of monodisperse magnetic nanoparticles (Park et al., 2004).
Zhao et al. (2008) reported the formation of nanoporous Fe3O4 particles with excellent magnetic properties and various specific morphologies by simply changing the solvent system and amount of KOH. In a typical process, the FeSO4 was dissolved in ethylene glycol or glycerol to form a homogeneous solution, followed by a quick addition of KOH at room temperature. The mixture was then transferred into a Teflon lined stainless steel autoclave, sealed, and maintained at 200 °C for 24 h for the complete formation of product particles. The KOH concentration and solvent properties determined the growth of particles, leading to the evolution of different particle morphologies including cube, truncated octahedron, octahedron, sphere, truncated cube and equilateral octahedron. All particles exhibited magnetic saturation values of around 90 emu g−1 with negligible remnant magnetization and coercivity.
It is utmost important to synthesize monodisperse and superparamagnetic nanoparticles with high saturation magnetization values. A metal dopant of ferrite nanoparticles has been pursued to achieve high and tunable nanomagnetism (Beji et al., 2010; Hochepied and Pileni, 2000; Sun et al., 2004). Jang et al. (2009) synthesized Zn2+ doped ferrite nanoparticles ((ZnxMn1−x)Fe2O4 and (ZnxFe1−x)Fe2O4, with x = 0, 0.1, 0.2, 0.3, 0.4, and 0.8) by thermal decomposition of a metal chloride (MCl2, M = Zn2+, Mn2+, and Fe2+) with Fe(acac)3. The Zn2+ doping level was carefully controlled by varying the initial molar ratio of the metal chloride precursors. The structure investigation and magnetization saturation measurement revealed that, in both cases, the magnetization saturation value reaches its maximum of 175 emu g−1 (Zn + Mn + Fe) and 161 emu g−1 (Zn + Fe) at x = 0.4, which far exceeds the value of 127 emu g−1 (Fe) observed for undoped bulk iron oxide (Fe3O4).
Formation of Fe3O4 nanoparticles with porous/hollow structures is expected to integrate the valuable characteristics of porous/hollow structure and unique magnetic property of Fe3O4 material in a single platform which can provide opportunities to tune their properties for specific applications. Porous hollow magnetic nanoparticles are expected to have similar magnetic, chemical, and biological properties as the solid nanoparticles and their structures offer the additional opportunity to store and release drugs at a target. Hollow nanocapsules of either haematite or magnetite were produced through a wrap-bake-peel process, depending on the heat treatment conditions (Piao et al., 2008; Wu et al., 2011). The spindle-shaped β-FeOOH nanoparticles were prepared and then coated with a thin layer of silica by esterification of tetraethoxysilane in a base solution. The silica coated spindle was subject to a thermal treatment at 500 °C in air, leading to the formation of porous hollow nanocapsule structure with a rhombohedral haematite (α-Fe2O3). The haematite α-Fe2O3 was converted to magnetite Fe3O4 under a flow of hydrogen. Interestingly, the β-FeOOH nanorods and α-Fe2O3 nanocapsules were paramagnetic while the Fe3O4 nanocapsules were superparamagnetic at room temperature.
Recently, one template-free method to prepare Fe3O4 hollow nanoparticles has been developed based on the inside-out Ostwald ripening (Cheng et al., 2011; Hu et al., 2009; Lin et al., 2012; Liu et al., 2009; Zhu et al., 2008). The plausible mechanism was supported by several experimental observations, considering the chemical conversion as an important factor for the hollowing process (Nguyen and Kim, 2013). The chemical conversions of solid material caused a little shrinkage of the grain size and thus made more voids between the grains inside the aggregates and led to the formation of loose package of aggregates. The inner grains would dissolve into the solution and then diffuse to the outer stable shell by the Ostwald ripening process, resulting in continuous expansion of cavity space inside the aggregates (Fig. 3). The Fe3O4 porous/hollow nanoparticles with tunable particle size and porosity were synthesized controllably by simply adjusting the initial concentrations of Fe precursor and additive or varying other process variables such as processing time and temperature (Nguyen and Kim, 2013; Nguyen et al., 2014 and 2015).
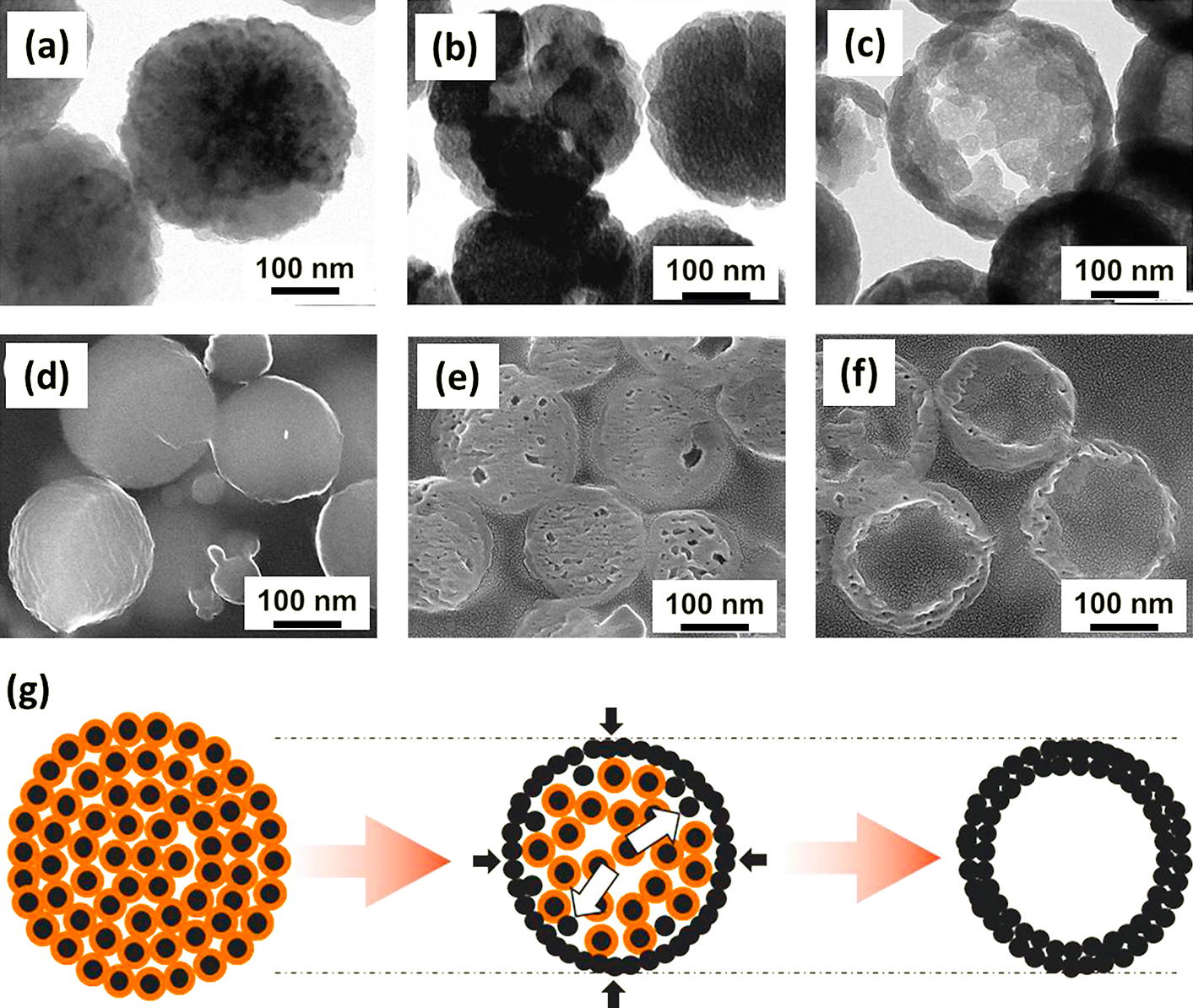
(a, b, c) TEM images and (d, e, f) corresponding SEM images of cross-section of Fe3O4 nanoparticles prepared by solvothermal process for 6 h, 8 h and 12 h of processing times, respectively, showing the evolution of porous/hollow structures, and (g) schematic of the hollow structure development in solvothermal process (Nguyen and Kim, 2013).
It has been reported that a magnetic coating on a magnetic nanoparticle leads to new magnetic nanocomposites containing exchange of anisotropy between core and shell materials which causes a specific magnetization (López-Ortega et al., 2015; Nogus et al., 2005). For example, Yoon et al. (2011) presented the formation of nanoparticles consisted of an elemental iron (Fe) core and a ferrite shell (Fe@MFe2O4, M = Fe, Mn, Co) by a seed-mediated growth approach. In the first step, the Fe cores were prepared by thermally decomposing iron complex [Fe(CO)5] in the presence of oleylamine under air-free conditions. After that, by co-injecting Fe-oleate complexes with Mn-oleate or Co-oleate complexes into Fe nanoparticle suspension then annealing the mixture at elevated temperatures, the Fe@Fe3O4, Fe@MnFe2O4 or Fe@CoFe2O4 core-shell structures were obtained. The core-sell nanoparticles displayed a hysteresis loop with high saturation magnetization of about 150 emu g−1 but with negligible coercivity and remanence. Increasing the crystallinity of ferrite shells could further improve the magnetization of such core-shell nanoparticles. For example, highly crystalline Fe/Fe3O4 nanoparticles exhibit 50 % higher magnetization than amorphous Fe/Fe3O4 nanoparticles (Lacroix et al., 2011).
Controlled drug delivery by nanostructured functional materials is attracting great attention because of the high opportunities in cancer therapy. The magnetic nanoparticles have been widely used for targeted delivery and controlled drug release due to their response to a magnetic field. Design of magnetic nanoparticles for biomedical applications required careful considerations for the physicochemical stability, targeting ability, drug loading and release. The functionalized magnetic nanoparticles could carry other active targeting moieties, drugs and imaging agents by both physical interactions and covalent linkages (Gupta and Gupta, 2005; Mahmoudia et al., 2011; Oh and Park, 2011). A controlled drug delivery system should be able to deliver drugs to a targeted location in the body, and to maintain drug levels within the required concentration range for therapy. A high-gradient of external magnetic fields could be used to guide and concentrate the magnetic nanocarriers at targeted site in order to reduce the systemic distribution of cytotoxic compounds, and to enhance drug uptake, resulting in more effective treatment at lower doses (Chertok et al., 2011; Todd et al., 2014).
Magnetic nanoparticles embedded in a polymer matrix which could be mechanically deformed upon application of a static magnetic field or generate heat under an alternating current field was used to deliver drug and control drug release. For example, upon applying a static magnetic field, the magnetic particles in the polymer matrix aggregated instantly and produced a bulk magnetic moment, leading to a “close” configuration and resulted in a slower drug release than that when the static magnetic field was off (Hu et al., 2007; Liu et al., 2006). This magnetic-induced mechanical deformation of the polymer could be utilized to sustain drug levels within the desired therapeutic range for a long time. On the contrary with the static magnetic field, drug release could be enhanced under an alternating magnetic field. The thermal energy from magnetic nanoparticles could be used as an external and remotely controlled trigger for controlled drug release.
The magnetic nanoparticles conjugated with thermoresponsive polymers offered a class of “smart” nanocarriers which have ability to respond to a change in surrounding temperature. The poly(ethyleneoxide)-poly(propylene- oxide)-poly(ethylene-oxide) (PEO-PPO-PEO) block copolymers were used to squeeze out drugs with magnetic nanoparticles and then was investigated for drug release at different temperatures (Liu et al., 2009). The profiles of drug release rate were very favorable: very slow at 4 °C and 25 °C, modest at 37 °C, much faster at 45 °C and bursting upon magnetic heating. It has been revealed that, under magnetic heating, burst-like release was observed due to the irreversible and disruptive changes of the magnetic nanoparticle core (Liu et al., 2009).
Several systems have been developed to demonstrate the remotely controlled pulsatile drug release for a number of different drugs as well as for different “on–off” durations of an alternating magnetic fields. Hoare et al. (2009) developed nanocomposite membranes based on poly(N-isopropylacrylamide)-based nanogels and magnetite nanoparticles entrapped in ethyl cellulose to achieve “on-demand” drug delivery upon the application of an oscillating magnetic field as shown in Fig. 4. A small-scale device made by gluing two 1 cm diameter membrane disks to the ends of a 1cm length of silicone tubing filled with a sodium fluorescein solution was mounted inside a flow cell placed in a solenoid coil. Under an oscillating applied field, heat generated by magnetite nanoparticles caused the shrinkage of nanogels and permitted drug diffusion out of the device. When the magnetic field was turned off, the nanogels reswelled and refilled the membrane pores, resulting in the return to a near-zero value of drug flux. This study demonstrated new development of adaptable therapeutic tools which could provide smart control of drug release over real time drug dosing. In another study, poly(D,L-lactic-co-glycolic acid) (PLGA) microcapsules (1–3 mm in diameter) with a 250 nm thick membrane containing a high density of iron oxide nanoparticles loaded with doxorubicin were prepared and then used in cancer treatment (Chiang et al., 2012). It was demonstrated that, for magnetic nanoparticles loading of at least 25 wt%, an application of alternative magnetic field of 100 kHz and 2.5 kA m−1 could induce heating above the glass temperature of PLGA and thus could allow controlled pulsatile release of the drug.
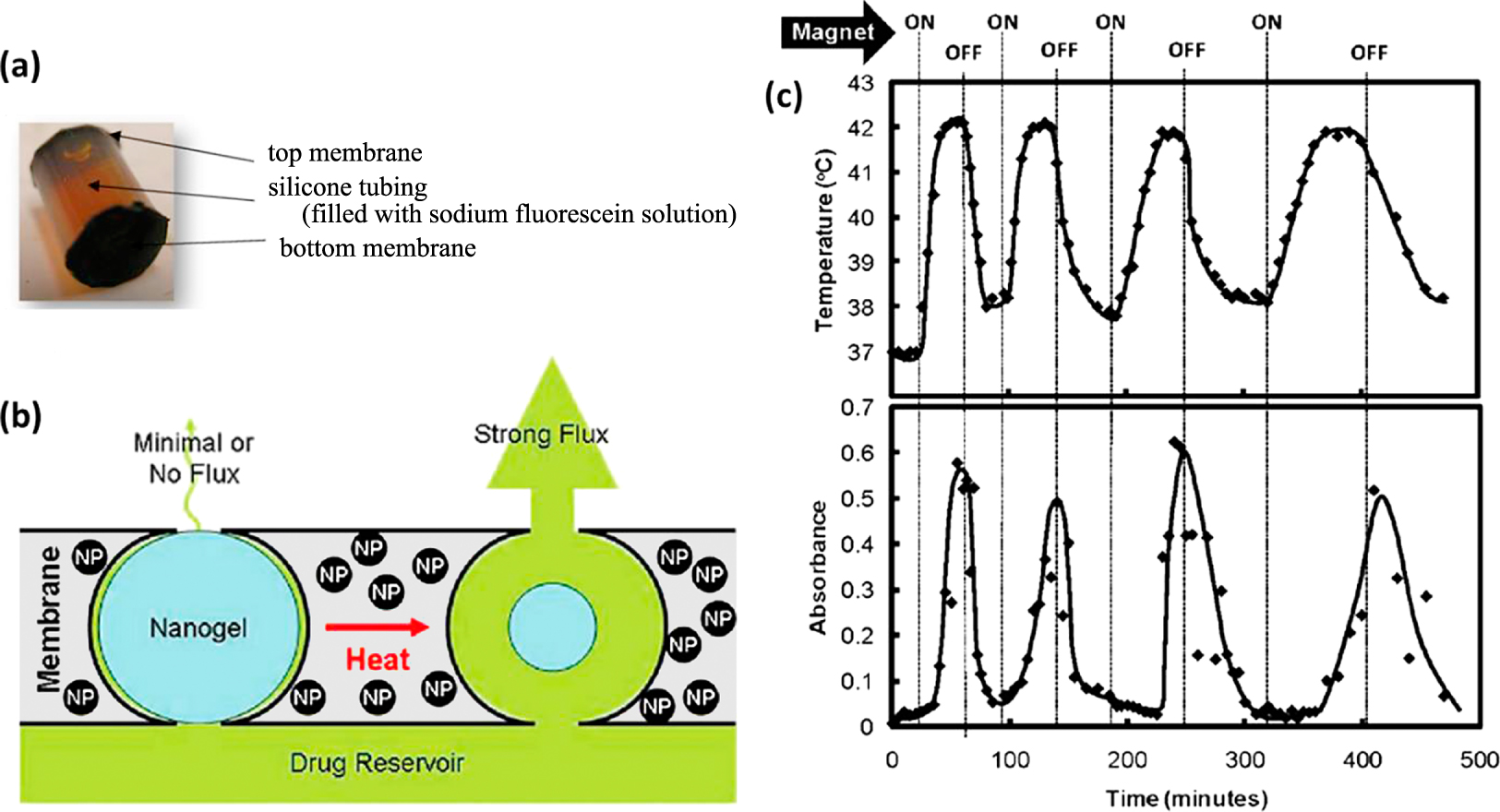
(a) Picture of membrane capped capsules used for magnetic flux testing. (b) The concept of the “on-demand” drug delivery upon the application of an oscillating magnetic field. (c) Temperature profile in the sample chamber and differential flux of sodium fluorescein out of membrane-capped devices as a function of time over four successive on/off cycles of the external magnetic field (Hoare et al., 2009).
The thermal energy from magnetic heating was utilized to open the gates of drug carriers which contain drugs for therapy as shown in Fig. 5. Thomas et al. (2010) synthesized 15 nm (Zn0.4Fe0.6)Fe2O4 nanoparticles and then incorporated these nanoparticles inside porous drug carrier nanoparticles. The drug molecules were loaded and kept by nanovalves consisted of N-(6-N-Aminohexyl) aminomethyl-triethoxysilane and cucurbit[6]uril capped onto the pores. When an external alternating magnetic field was applied, heat generation and subsequent pressure buildup inside the porous nanoparticles caused the rapid removal of molecular valves and rapid release of drug molecules. These magnetic nanoparticles were considered as effective actuators for controlled drug release from carriers.
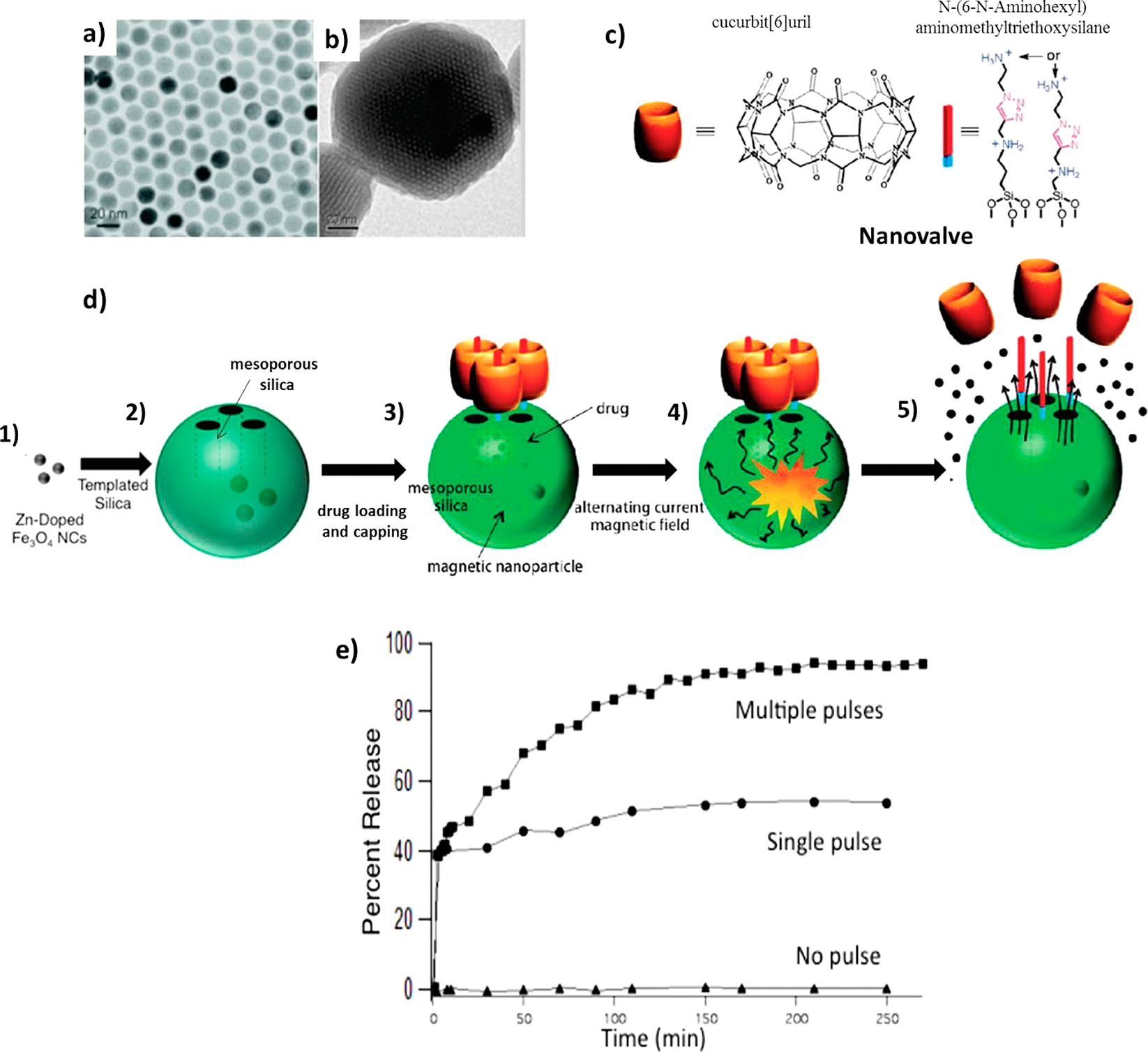
a) TEM image of (Zn0.4Fe0.6)Fe2O4 nanoparticles. b) TEM image of (Zn0.4Fe0.6)Fe2O4 encapsulated silica nanoparticles. c) Chemical structure of nanovalve. d) Schematic of nanoparticles formation (1), encapsulation (2), drug loading and nanovalve assembly (3), pore opening by the local internal heating (4), and drug release (5). e) Drug release profile monitored during the AC field application with multiple AC field pulses, single initial pulse, and no pulse. The sample was kept at 0 °C to determine whether internal heating alone causes the valve opening or not (Thomas et al., 2010).
The use of magnetic nanoparticles for hyperthermia has shown great promise in the field of disease treatment. By raising the temperature of tissues to between 42 °C and 46 °C, the viability of the disease tissues is reduced and their sensitivity to chemotherapy is increased (Chan et al.,1993; Jordan et al.,1993; Gilchrist et al.,1957; Overgaard and Overgaard, 1972). In addition to selectively killing tumor cells, a potential of developing antitumor immunity after hyperthermia treatment has been also suggested (Ito et al., 2003; Sivasai et al., 2010; Suzuki et al., 2003). In fact, it should be considered that the impact of the alternative magnetic field on human body is connected with heating effects due to eddy currents induced in the electrically conducting tissue. It has been suggested that the upper limit for the magnetic field (the product of amplitude, H, and frequency, f) should not exceed 5 × 109 A/m s−1 (Hergt and Dutz, 2007).
As shown in Fig. 6 (Nguyen and Kim, 2014), when exposed magnetic nanoparticles to an alternating magnetic field, the external magnetic field is dissipated with the relaxation of the magnetic moment to its equilibrium orientation, or so-called Néel relaxation for superparamagnetic nanoparticles. A so-called hysteresis loss mechanism dominates heat generation of ferromagnetic materials whose sizes exceed the domain wall width. Under application of an alternative field, the magnetic moments oscillate and cause domain wall displacement which generates heat. The heat generation depends not only on the applied field but also on the magnetic pre-history of material. It has been found that, when the applied field is small, the hysteresis loops differ from full hysteresis loops in that they are minor loops having relatively smaller areas and the amount of heat generated is considered to be proportional to the frequency multiplied by the area of the loop (Kita et al., 2010; Vallejo-Fernandez et al., 2013). Considerable heat generation can be expected if we apply a magnetic field with appropriate frequency which is sufficient to saturate the magnetization of magnetic material. For all types of magnetic nanoparticles, heat generation is also caused by the rotational Brownian motion within a dispersed media because of the torque exerted on the magnetic moment by the external alternating magnetic field. In this condition, the thermal energy is characterized by the viscosity of medium and the global hydrodynamic diameter of particle. However, due to the high viscosity of the intracellular medium, the Brownian relaxation mechanisms could be significantly hindered and consequently the performance of the nanoparticles mainly depended on their primitive mechanisms (Fortin et al., 2008; Rivière et al., 2007; Wilhelm et al., 2007).
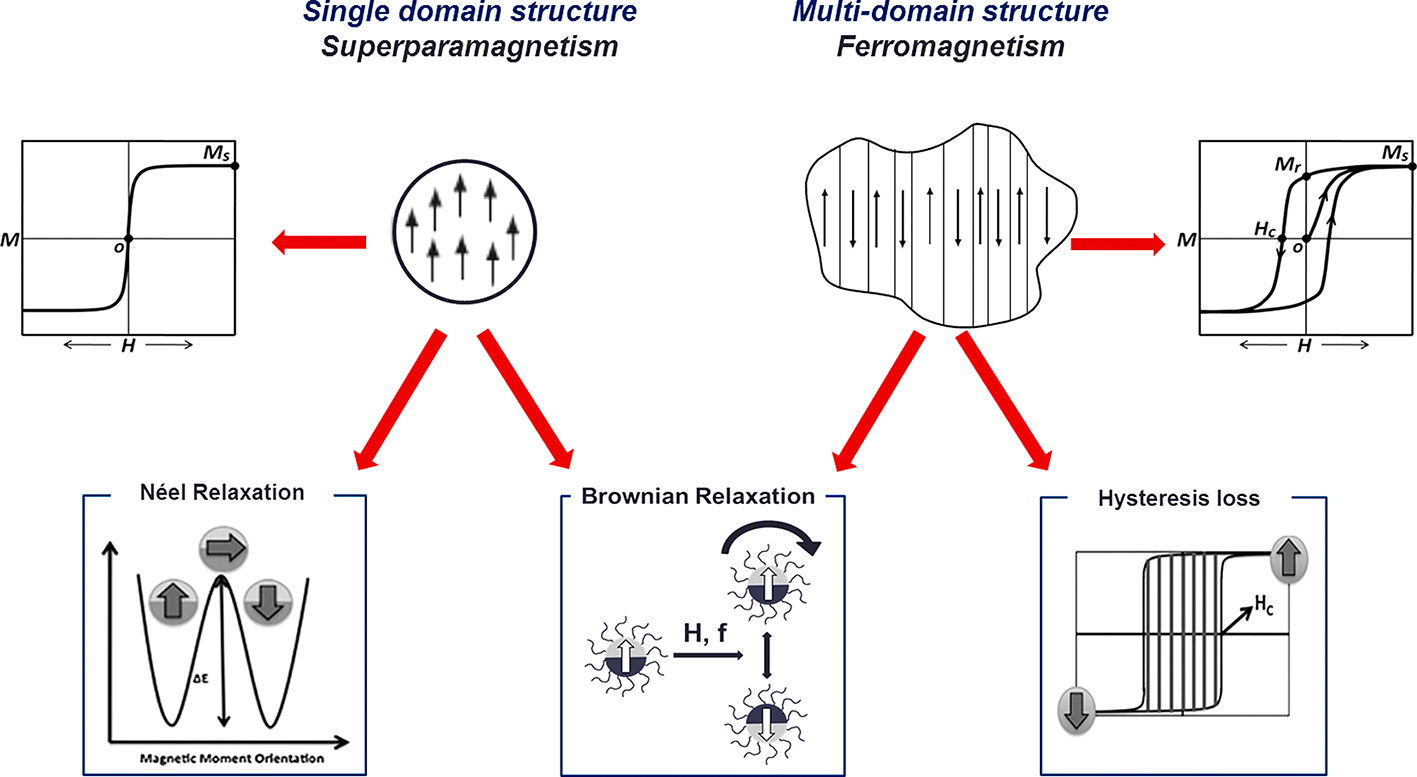
General mechanisms for heat generation of magnetic nanoparticles (Nguyen and Kim, 2014).
Magnetic particles in hyperthermia treatment have been focused on the magnetite Fe3O4 and also on the nanoparticles related with cobalt, nickel, or other substitutions in a size range from several nanometers to a few tenths of a micron. The magnetic iron oxide nanoparticles are still the most studied magnetic material for hyperthermia applications, due to their low toxicity, good biocompatibility, ease of synthesis and surface functionalization. The main parameter to determine the heating of tissue is the specific loss power (SLP) or specific absorption rate (SAR) which can be calculated based on the following equation.
| (1) |
where C represents the sample specific heat capacity, m is the amount of magnetic material per mass or volume of sample used in the experiment and (ΔT/Δt) is the initial slope of the time-dependent heating curve.
The hyperthermia efficiency which could be reflected by heating rate or SLP values depended on various factors, including the intrinsic factors of material such as particle structure, magnetic properties and the external factors such as amplitude and frequency of applied magnetic field (Deatsch and Evans, 2014; Laurent et al., 2011; Mornet et al., 2004). Rosensweig (2002) formulated and computed heating rates for various magnetic samples and reported that the highest heating rates depended on the particle size. In this work, the very sharp maximum of the heating rate was observed for monodisperse Fe3O4 nanoparticles of about 14 nm diameter. An increase of the size distribution caused a very fast decrease of the heating rate. In another study, by using available data for domain magnetization and magnetic anisotropy constant, Habib et al. (2008) predicted the dependence of SLP values on particle size for various materials in single domain regime as shown in Fig. 7 wherein the maximum heating rate of monodisperse Fe3O4 nanoparticles was achieved for particles of about 19 nm diameter.
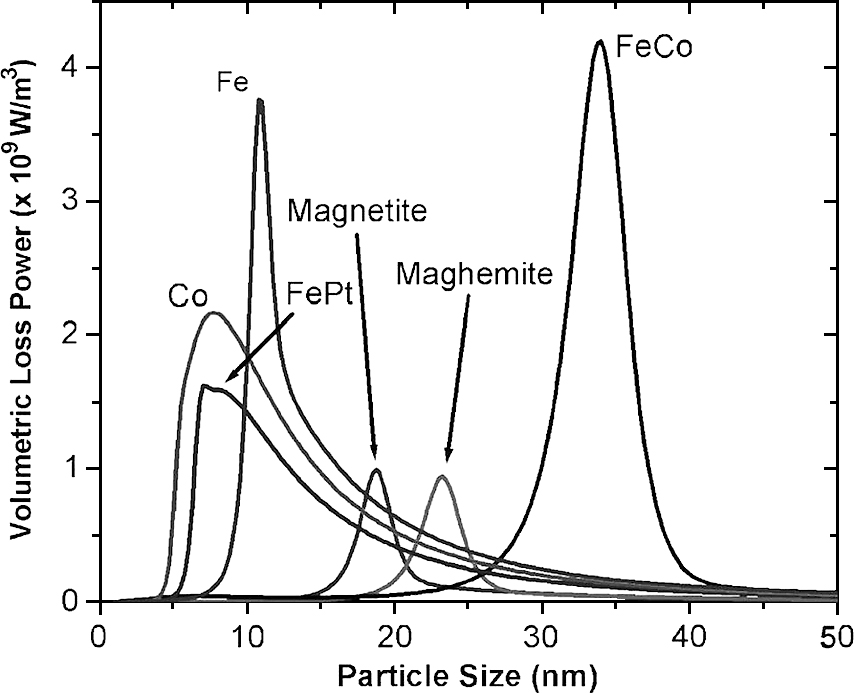
Size-dependent volumetric SLP values for different magnetic nanoparticles in single domain regime (Habib et al., 2008).
Practically, effect of each significant parameter such as particle size and shape, functionalization, magnetic properties on the hyperthermia efficiency has been independently investigated from study to study with different particle synthesis methods and different magnetic-induced heating systems. Ma et al. (2004) reported that the SLP values depended on the particle size and the coercivity of Fe3O4 nanoparticles. A maximum SLP was recorded for sample containing 46 nm diameter of Fe3O4 nanoparticles which also has the highest coercivity under an applied field of 80 kHz frequency and 32.5 kA m−1 amplitude. Gonzales-Weimuller et al. (2009) demonstrated that the highest SLP was measured for 11.2 nm particles under an applied field of 400 kHz frequency and 24.5 kA m−1 amplitude while Gonzalez-Fernandez et al. (2009) showed a maximum of SLP for particles of around 30 nm diameter by applying an alternative magnetic field of 260 kHz frequency and 100 Oe (about 8 kA m−1) amplitude to induce heat generation.
The choices of the ligand and thickness of coating are important because it can modify the magnetic properties of the magnetic nanoparticles. Larumbe et al. (2012) showed that silica coating on magnetite nanoparticles resulted in the decreases of the magnetic properties and the SLP values, due to an enhancement of spin disorder caused by the silica coating. In another recent study, the heating efficiency was observed to decrease with increasing polymer chain length. However, with a proper coating, a ferromagnetic nanoparticle could give a fast heating rate for disease treatment. For instance, chitosan oligosaccharide-stabilized iron oxide nanocubes (Chito- FIONs) were developed for cancer hyperthermia (Bae et al., 2012). The magnetic heating revealed that Chito- FIONs encapsulated with multiple 30-nm-sized iron oxide nanocubes showed the higher hyperthermal efficiency than single nanocube and more than 30 times higher than that of the Feridex particles as reference. In this study, it should be noticed that an evolution of aggregation of the nanoparticles caused a modification of the SLP values due to the effect of dipolar interparticle coupling with Néel relaxation.
There are potential opportunities to enhance hyperthermia through manipulation of magnetic properties utilizing core-shell architecture. The combination of different magnetic nanoparticles such as MnFe2O4/CoFe2O4 or CoFe2O4/MnFe2O4 or CoFe2O4/NiFe2O4 has been developed to enhance hyperthermia efficiency compared to single phase nanoparticles (Lee et al., 2011). Remarkably, further enhancement of the SLP values has been obtained in cubic Zn0.4Fe2.6O4/CoFe2O4 core/shell nanoparticles for achieving SLP values 14 times higher than the spherical single Fe3O4 nanoparticles (Noh et al., 2012).
The temperature control during heating is essential but still remains challenges because heat conduction and energy adsorption in vivo are widely unknown and local overheating may damage the healthy tissue and, therefore, the magnetic materials with a maximum self-heating temperature are very attractive for hyperthermia applications. Achieving self-controlled and self-regulated heating by developing materials with Curie temperature (TC) slightly above the therapeutic temperature has been considered as an effective method to prevent such local overheating problem. The Curie temperature is the maximal temperature reachable by magnetic particles to maintain their magnetic properties. Above this temperature, the magnetic particles lose their magnetic properties, and thus they do not convert electromagnetic energy into heat. Syntheses of several materials including M1−xZnxFe2O4 (with M = Ni, Cu, Co, Mn) and alloy such as Ni1−xCrx with adjustable TC were reported. It has been also reported that the TC of Gd-substituted Zn-ferrite (ZnGdxFe2−xO4) increased with an increasing Gd content and was found to be around 45 °C at x = 0.02 (Yao et al., 2009) while the TC of Ni1−xCrx increased with decreasing Cr content and was found equal to 44 °C at x = 5.63 (Akin et al., 2009). Other studies reported that the TC of 46 °C could be achieved for Mn1+xTixFe2O4 with x = 0.55 (Barati et al., 2013) and for Mg1+xFe2−2xTixO4 with x = 0.37 (Ferk et al., 2014). Certainly, an ideal material with optimal physical features for self-controlled and self-regulated magnetic heating has not been well developed yet but, indeed, this concept offers a smart way to control hyperthermia treatment.
The combination of hyperthermia and traditional chemotherapeutic functions in the same system could utilize the great potentials of individual hyperthermia and controlled drug delivery as well as could raise synergistic effects in the treatment of cancer. Kulshrestha et al. (2012) developed paclitaxel loaded magnetoliposomes and achieved a better cell killing efficacy than individual magnetoliposomes or paclitaxel loaded liposomes. The drug release behaviors were studied under an AC magnetic field at 37 °C and 43 °C. At 37 °C, only 1.2 % of paclitaxel were released compared to 55.6 % at 43 °C. The hyperthermia was performed on HeLa cells at 42.5 ± 1 °C for a duration of 30 min with magnetoliposomes containing 6 mg mL−1 of magnetic nanoparticles only or in combination with 100 nM paclitaxel. The cell viability was decreased by 37 % after the heat treatment with magnetic particle alone and by about 50 % and 89 % for paclitaxel loaded magnetoliposomes without and with application of an alternative magnetic field, respectively. This study demonstrated that the magnetoliposomes exhibit promising potential for combined thermo-chemotherapy due to its good biocompatibility, slow drug release at 37 °C, burst drug release at 43 °C and synergistic cancer cell killing effect.
Controlled drug delivery and hyperthermia treatment by magnetic nanoparticles exhibited high opportunities in biomedical treatments. High-gradient, external magnetic fields could be utilized to guide and concentrate the magnetic nanocarriers at target locations where the therapy has to act specifically. A combination of hyperthermia- based therapy and controlled drug release has strong potential to develop an intelligent therapy for cancer treatment. The hyperthermia and traditional chemotherapeutic functions combined in the same system could utilize the great potential of individual hyperthermia and controlled drug delivery as well as could raise the synergistic effects in the treatment of cancer. It is still a great challenge to develop a system that minimizes nanoparticle dose for actual treatment. In order to conquer this challenge, it is crucial to develop systematic strategies of controlled synthesis and surface modification of magnetic nanoparticles to obtain the nanoparticles of customized size and shape with high homogeneity as well as sufficient magnetization. However, the magnetic nanoparticles obtained under different synthetic conditions may display large differences due to formation of structural disorder and antiphase boundaries, or the existence of a magnetically dead layer at the particle surface and, thus, the synthesis and functionalization of high-quality magnetic nanoparticles in a controlled manner are still the critical challenges to be faced in the coming years. It will also be desirable to develop the large-scale synthesis of highly functional magnetic nanoparticles with the requirement of reproducible and industrial processes without any laborious purification step to ensure cost effective synthetic procedures.
This research was supported by the Converging Research Center Program through the Ministry of Science, ICT and Future Planning (Grant number 2014048827).
Dung The Nguyen
Dung The Nguyen received his BS degree in chemical engineering from VNU University of Science, Vietnam National University, Vietnam in 2006 and then obtained the PhD degree in chemical engineering from Kangwon National University in 2014. He is now working as a full time researcher in Department of Chemical Engineering, Kangwon National University. His current research interests include controlled synthesis of multifunctional nanoparticles and engineering of chemical reactions and processes.
Kyo-Seon Kim
Kyo-Seon Kim is currently a Professor of Chemical Engineering Department at Kangwon National University, Chuncheon, Korea, where he has been working from 1989. He received the BS, MS and PhD degrees all in Chemical Engineering from Seoul National University, KAIST and University of Cincinnati, USA in 1979, 1981 and 1989, respectively. He worked at Korea Institute of Energy and Resources as a researcher for 1981–1985. On his sabbatical leaves, he was a Visiting Professor at Mechanical Engineering Research Laboratory, Hitachi, Ltd, Japan for 1993–1994 and at DuPont Central Research Laboratory, USA for 2003–2004. His research interests are mainly focused on preparation and modification of nanoparticles by the gas, liquid and plasma processes for the applications to medical devices, pollution control and energy harvest.