2018 Volume 35 Pages 122-138
2018 Volume 35 Pages 122-138
The paper discusses essential engineering challenges related to the application of powder medicines for pulmonary delivery as inhaled aerosol. Starting from a physically based description of the complexity of aerosol dynamics inside the respiratory system, the paper discusses several technical factors responsible for efficient drug delivery to the lungs: (i) interparticle interactions—which can be tuned by selection and control of powder manufacturing methods, (ii) inhaler design—as a determinant of flow dynamics through the inhaling device and degree of powder dispersion, (iii) the dynamics of inhalation in a given inhaler—which is related to patient-device interaction. Basic information on the standard (compendial) methods of the quantitative evaluation of dry powder inhalers (DPIs) are presented with a special focus on the correct data interpretation and the consequences for in vivo-in vitro correlation (IVIVC) problems. Some issues regarding the development of inhalation products (including generics) are also briefly highlighted. Finally, possible strategies of powder particle functionalization to obtain the required bioavailability are outlined on the basis of knowledge on the physicochemical interactions of inhaled particles with the lung fluids.
The idea of using aerosol particles as drug carriers comes from the ancient times when the inhalation of smoke and fumes from burned plants appeared to induce favorable health effects (Sanders M., 2011; Stein S.W. and Thiel C.G., 2017). In modern times, it has been realized that the atomization of medicinal liquids to very small droplets helps to transfer them to the respiratory system beyond the upper airways (mouth and throat). Such a possibility of drug targeting to specific lung regions provided the opportunity to treat pulmonary diseases locally with low overall drug mass and minimized side effects. It also became clear that aerosolized drugs need not necessarily be a liquid, but can also be in the form of fine particles of solids.
The technical possibility of using dry powders as vehicles of drugs carried into the respiratory system is the topic of this paper. This apparently simple idea of drug delivery covers non-trivial challenges and many physical limitations which need to be overcome if a reproducible drug dose is to be delivered from a portable and inexpensive inhaling device.
This analysis starts with the overall characteristics of physical phenomena related to aerosol inhalation and particle deposition in the lungs. Next, the major challenges of aerosol formation in inhaling devices are discussed with the main focus on the proper design of dry powder inhalers (DPIs) and on the properties of powders as precursors of inhalable aerosols. The dynamic aspects of the above-mentioned problems are highlighted because very often they are ignored in the analysis of particle deposition in the lungs and powder inhaler performance. Some essential information on aerosol evaluation using the compendial (pharmacopeial) methods is also presented, indicating their limitations which can lead to the inaccurate interpretation of data. Finally, some facts regarding physical aspects of interactions between deposited particles and the lung surface are discussed to show the relationships between particle properties and drug bioavailability. All presented data make it possible to highlight potential new opportunities of inhalation therapy with the use of powder particles.
Drug delivery by the inhalation of aerosolized medicines is a therapeutic method which has several advantages but also some essential drawbacks (Table 1).
Main benefits and drawbacks of drug delivery by aerosol inhalation.
| Benefits | Drawbacks/limitations |
|---|---|
| 1. Drug delivered directly to the expected site of action in the respiratory system: the dose can be minimized, low side effects | 1. Uncertainty of dose delivered locally to the target area |
| 2. Low enzymatic drug deactivation in the lungs: the dose can be minimized | 2. Technical challenges related to drug formulation, dosing and aerosolization |
| 3. Non-invasive, pain-free and easy-to-use method of drug delivery | 3. Patient-dependent efficiency of drug delivery |
The main benefit is related to minimization of the dose which needs to be introduced to the organism to produce the expected concentration in the target site (i.e. on the surface of the respiratory system). For inhalation devices (inhalers), it is often enough to deliver only several micrograms of the active pharmaceutical ingredient (API) to result in the local lung dose comparable to almost one hundred milligrams delivered by injection or tablets (Alangari A.A., 2014). With a natural and painless application mode, inhalation therefore presents a safe and convenient method of pharmacotherapy. On the other hand, unlike other drug delivery systems (tablets, pills, injections), the inhalation of aerosolized medicine does not guarantee precise dosing. The reason is that none of the sequential processes of aerosol generation, aerosol evacuation from the inhaler and regional deposition in the respiratory system is 100 % efficient, which means that only a fraction of the drug initially loaded to the inhaler can be transferred to the target area, Fig. 1. Moreover, this number is noticeably variable since it depends both on the performance of the inhaling device and on the patient’s individual capabilities to use it correctly, Fig. 2. As stated earlier, there is a long list of technical problems which need to be solved to achieve a reliable and reproducible drug delivery system based on powder inhalation, and they will be addressed herein.
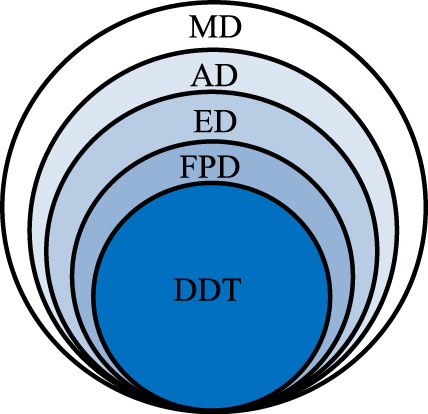
General relationship between the nominal (metered) dose–MD, aerosolized dose–AD, emitted dose–ED, fine particles dose–FPD and dose deposited at the target–DDT.
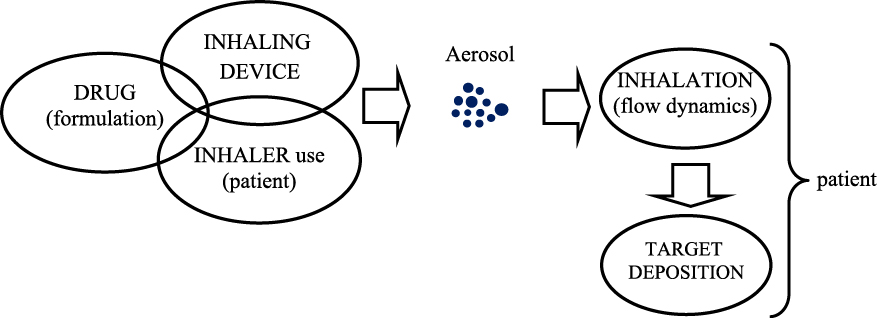
Schematic path from the drug to target deposition.
It is obvious that not all aerosol particles can be used as effective drug carriers. To play that role, particles must be inhalable, i.e. small enough to be drawn with air into the mouth or nose. Moreover, they must be capable of penetrating beyond the naso-oro-pharynx and the upper bronchial tree so they can reach the lower respiratory system and settle there.
Interestingly, the preferred deposition region may be different for various types of inhaled drug, since the occurrence and surface density of cellular receptors for drug binding/stimulation differs along the walls of the bronchial and pulmonary airways (Howarth P.H., 2001; Barnes P.J., 2004; Usmani O.S. et al., 2005; Pirożyński M. and Sosnowski T.R., 2016). For instance, muscarinic receptors (e.g. M3) are located mainly in the central airways, so the relevant drugs (bronchodilating cholinergic antagonists) should be preferentially delivered to these lung regions. The distribution of the receptors for beta2-mimetics requires the deposition of such drugs both in the central and distal respiratory tract, although the alveolar region can be omitted since no bronchodilating effect is expected there. Glucocorticoid receptors (GRs) are distributed all over the lower respiratory system, but steroids should not penetrate in large amounts into the lung periphery since we want to avoid their undesired absorption into the circulation from alveoli. It is therefore clear that different aerosol drugs can be formulated differently to obtain the most desired therapeutic effects.
2.2 Physics of aerosol particle flow and deposition in the respiratory tractThe whole respiratory tract acts as a filter for inhaled aerosols where particle flow, penetration and deposition in the respiratory system are governed by general physical laws (Heyder J. and Svartengren M.U., 2002; Darquenne C., 2012; Tsuda A. et al., 2013; Cheng Y.S, 2014). Obviously, particle ‘filtration’ in the respiratory system is sequential, i.e. only those particles which have avoided elimination in the previous generations of the bronchial tree can penetrate and deposit in the next ones. This factor must be considered if we try to target any specific region with inhaled aerosol medicine (Zanen P., 2003; Kleinstreuer C. et al., 2008). Fig. 3 shows all particle deposition mechanisms which are believed to predominate in respiratory airways.
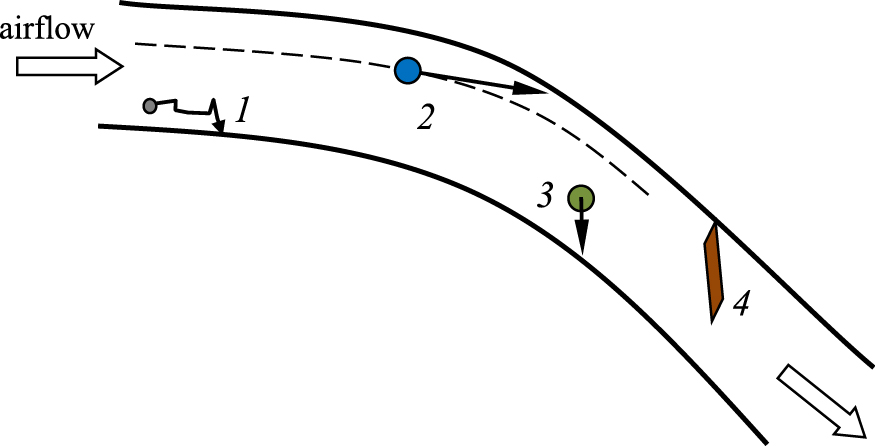
Basic deposition mechanism of airborne particles in the respiratory airways: 1 - diffusion, 2 - inertial impaction, 3 - sedimentation, 4 - interception.
Particles with a high momentum (mass × velocity) hit the walls of the air ducts due to inertial impaction, mostly when the airflow rapidly turns. It takes place, e.g. in the mouth (hard and soft palate), throat and at bifurcations of large bronchi (Moskal A. and Gradoń L., 2002; Sosnowski T.R. et al., 2007). Particles which are able to penetrate these regions but have a relatively high mass can still fall gravitationally at an appreciable rate. Therefore, the chance of their penetration beyond the small bronchi, where aerosol residence time is extended due to a decrease of the average flow velocity, is minute. Moreover, the diameter of the airways in this region is reduced so the gravitational settling becomes effective. Submicron particles, which have a low inertia and also a negligible sedimentation rate, penetrate to the lung periphery to a greater extent. They can reach the airway walls of that region by Brownian diffusion.
Presented facts allow the development of quantitative methods for predicting particle deposition in various lung regions (Longest P.W. and Holbrook L.T., 2012; Tu J. et al., 2013). Different approaches to that problem are presented in the next section.
2.3 Quantitative models of deposition of inhaled particlesThe deposition of inhaled drug particles can be quantitatively predicted by adaptation of the models which have been originally elaborated in the field of inhalation toxicology. Such semi-empirical models are often based on the experimental data fitted by relatively simple mathematical formulae which can estimate the deposition of particles with different sizes and for variable breathing regimes. Such models (e.g. ICRP (Annals of the ICRP, 1994)) calculate only the regional deposition efficiencies (in the upper airways, tracheo-bronchial region, small bronchi or alveolar zone) which, by summing up, give also the total deposition in the whole respiratory system (Fig. 4).
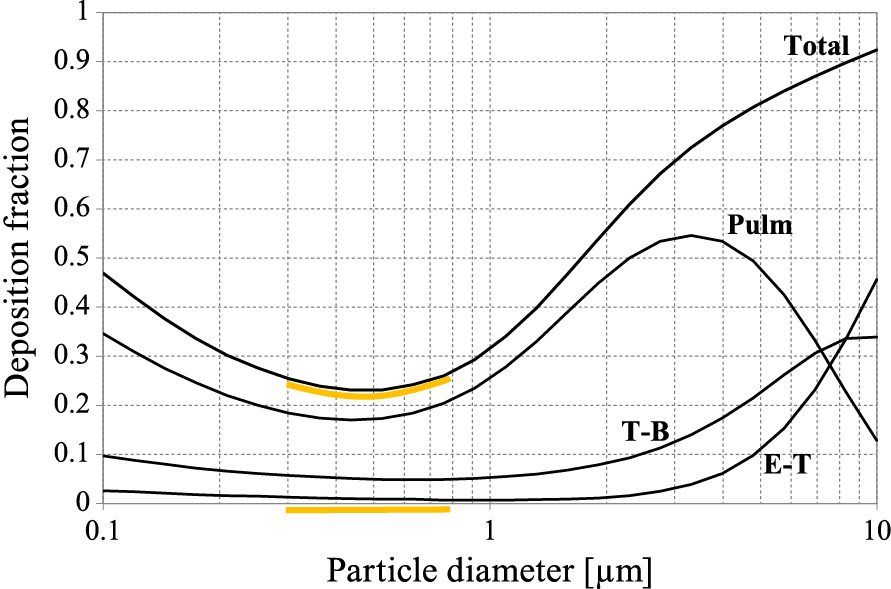
Regional and total particle deposition in the respiratory system as a function of particle size—calculations based on the ICRP model and Multi-Path Particle Dosimetry software (MPPD, 2015) for deep and slow oral inhalation (TV = 1500 ml, breathing cycle time = 12 s). Lung regions: Pulm = pulmonary (alveolar), T-B = tracheobronchial, E-T = extra-thoracic (airways of head and neck). The particle size range with the lowest deposition is highlighted.
Regional deposition can be derived also from other models which consider more precisely the predominating deposition mechanisms in various regions of the respiratory system. For instance, Finlay W.H. and Martin A.R. (2008) proposed mathematical formulae for the oro-pharyngeal region and the lower respiratory tract deposition, taking into account the residence time and the average local particle aerodynamics. In this model, the average Reynolds (Re) and Stokes (Stk) numbers are used to generalize air and particle flow in the respiratory system.
A more detailed approach to the quantitative description of particle deposition is based on particle tracking during their flight through the respiratory airways. Reliable quantitative data regarding bronchial tree geometry (airway lengths, diameters and branching angles) are needed to predict the average airflow rates and particle behavior in each generation. Symmetrical or asymmetrical lung geometry can be used (Yeh H.C. and Schum G.M., 1980) where the first leads to a single path while the second leads to multipath trajectories of aerosol particle transport (Asgharian B. et al., 2001). In the multipath transport, a random choice of geometry is assumed resulting in a so-called stochastic lung deposition model (Koblinger L. and Hofmann W., 1990).
In these models, the fractional deposition in each generation of the bronchial tree can be calculated which gives more detailed information on drug distribution in the lungs.
It may be mentioned that some of the above-discussed models are accessible in the user-friendly calculators which are freely available on the Internet (MPPD, 2015; ARLA, 2008).
The most precise predictions of local particle dynamics and deposition can be obtained from detailed calculations of the particle trajectory within the numerically reconstructed geometry of the selected fragments of the respiratory system. It can be conveniently done applying computational fluid dynamics (CFD) methodology (Tu J. et al., 2013). Commercial packages help to predict the actual three-dimensional airflow field in the defined airway geometry by numerically solving the Navier-Stokes equation within the domain decomposed into finite volume elements. Even with the use of a commercial computational package, the task itself is not trivial and requires correct construction and optimized resolution of the numerical mesh followed by the application of a suitable solver with appropriate boundary conditions. Tracking of each particle in the airflow field is done by the Lagrangian approach, i.e. solving the mass balance of a single particle. The most general description combines both deterministic and stochastic forces acting on particles, the latter being associated with Brownian diffusion, i.e. particle motion caused by random collisions with air molecules. The Langevin equation which describes particle acceleration under such conditions takes the form (Moskal A., 2011):
| (1) |
where up and u denote the velocity of particle and air, respectively, dp – particle diameter, ρp – particle density, μ – air viscosity, Fext – external forces affecting the particle trajectory (gravity, electrostatic or magnetic interactions, etc.) and FB – the Brownian force which plays a role mainly for submicrometer-size particles. For a spherical aerosol particle suspended in the air, the Brownian force is given by:
| (2) |
where αB is the characteristic magnitude of acceleration due to thermal excitation, and can be expressed as:
| (3) |
S0 is a spectral intensity of a white noise which can be conveniently used to model the stochastic Brownian effects:
| (4) |
where T is the absolute temperature, kB is the Boltzmann constant, and Cs the Cunningham slip correction factor. Z in eq. (2) is the dimensionless vector with random direction and Δt denotes the time step of numerical integration of the trajectory equation. It can be noted that the Brownian force acts on aerosol particles all the time but its direction and magnitude are randomly changed (through the random updating of value of Z) at the end of every time step Δt. The presented method of numerical computations can be used to analyse particle motion in a human breathing system also at realistic non-steady-state flow conditions (Moskal A. and Gradoń L., 2002). Numerical solutions allow finding the trajectory of any particle of the defined size and properties, and eventually finding the place of particle deposition on the airway wall. If the computations are repeated many times for thousands of particles with various sizes, the results of particle deposition probability (i.e. deposition efficiency) in the analysed region is obtained as a function of the particle size.
As in any type of particle separation process (e.g. conventional air filtration), a certain particle size range exists where none of the previously mentioned mechanisms is sufficiently effective. Such particles (typically 0.3–0.8 μm) can be exhaled without significant previous deposition in the respiratory system. In the example of the relationships of regional deposition efficiency vs. particle size shown in Fig. 4, the minimum of total deposition in the size range referred to above is clearly seen. Interestingly, some systems dedicated to aerosol delivery to the lungs may take advantage of a non-optimal deposition. For instance, e-cigarettes are designed in a way which allow a visible mist (typically composed of fine droplets of glycerine and propylene glycol) to be exhaled since this mimics the use of classic (i.e. tobacco) products. All droplets released from e-cigarettes are smaller than 2 μm (mass median diameter ~0.4 μm), for which the predicted deposition in the respiratory system is less than 50 %, independent of the breathing maneuver (Sosnowski T.R. and Kramek-Romanowska K., 2016).
2.4 Aerosol dynamics and other factors influencing particle deposition during inhalationIt is commonly agreed that the aerodynamic particle diameter da is the most suitable measure of the particle ability to penetrate and deposit within the respiratory system. The aerodynamic diameter is defined as:
| (5) |
where ρw denotes density of water, and χ the dynamic shape factor of the particle. Only particles with da < 5 μm are expected to be inhaled deeply to the bronchial tree where they eventually settle. Aerosol particle size distribution based on the aerodynamic diameter is typically assessed by standard compendial methodology using multi-stage (cascade) impaction separators: NGI (Next Generation Impactor), Andersen, Marple-Miller or impinging jet impactors (USP, 2008; European Pharmacopoeia, 2013). This type of determination of the aerosol particle size distribution is an indispensable step in the pharmaceutical development and validation of orally inhaled medical products (CHMP, 2009), and some details of such characterizations of aerosol drug particles will be discussed in section 3.3.
The aerodynamic diameter is a disputable criterion of particle behavior in the lungs if the particles are non-compact in shape, e.g. plate-like or needle-like, when interception can become the predominant deposition mechanism (Fig. 3). Moreover, the aerodynamic size properly describes the particle settling time under gravity or inertial forces, assuming that a particle is carried with air at constant velocity. In reality, it is obviously not true. Inhalation is a dynamic (time-dependent) process in which a certain volume of air is pumped through the airways at variable acceleration and deceleration. Therefore, the aerodynamic size has a different influence on particle behavior depending on the time-instant during inhalation. This complicates analysis of the processes of aerosol penetration and deposition in the respiratory system. In many theoretical and experimental analyses of aerosol inhalation and deposition, the constant flow rate is assumed (such as the mean flow of the inspiration, e.g. Matida E.Z. et al., 2004; Zhang Z. et al., 2005; Farkas A. and Balazhazy I., 2008; Ma B. and Lutchen K.R., 2009), which is an inaccurate simplification. Some studies show that particles in the realistic non-steady inhalation flow show dissimilar dynamics as compared to the constant flow conditions, and this leads to a different spatial distribution of particle deposition (Moskal A. and Gradoń L., 2002; Sosnowski T.R. et al., 2007). The influence of flow dynamics on particle deposition can be demonstrated by a simple example. Fig. 5 schematically shows the dynamics of the inhalation phase and the relationship between the temporal volumetric flow rate Q(t) and two characteristic values—the mean flow:
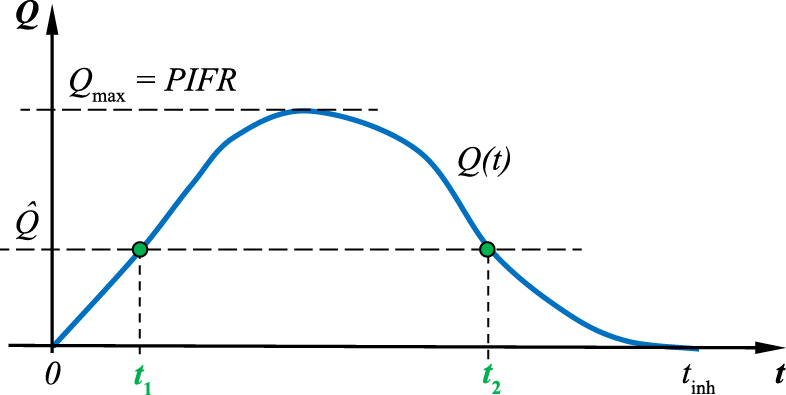
Relationships between real flow dynamics Q(t), average flow rate Q̂ and the peak inspiratory flow rate (PIFR).
| (6) |
and the maximum flow value, Qmax = PIFR (peak inspiratory flow rate). It is important to note that the flow value equal to Q̂ occurs in reality only at two instants of time (t1 and t2) during the whole inspiration.
The temporal dimensionless Stokes number, being an indicator of inertial deposition (impaction), is defined as:
| (7) |
where—in addition to already defined variables—L0 denotes the characteristic local dimension of the airways. It can be easily seen that the same local value of the Stokes number is obtained if:
| (8) |
Consequently, for a variable airflow rate during inhalation, a particle with dp = 10 μm which moves at a velocity equal to the air velocity u1 will be locally (L0 = const) deposited by impaction with the similar efficiency as a 5-μm particle at the velocity 4u1. The analogous analyses can be done also for other deposition mechanisms and they will show that at variable airflow, one cannot unambiguously link the regional deposition efficiency with the aerodynamic (or geometric) particle size.
The complexity of the description of dynamic behavior of inhaled powder particles in the lungs is additionally caused by other issues:
Flow dynamics influences not only the behavior of particles in the respiratory system but also the performance of inhaling devices (aerosol formation and emission). This problem will be discussed in section 3.
Several books, book chapters and review papers are focused on a variety of technical solutions required for drug delivery of aerosolized powders via inhalation (e.g. Dunbar C.A. et al., 1998; Zeng X.M. et al., 2000; Borgström L. et al., 2002; Vanbever R., 2003; Newman S. and Peart J., 2009; Hoppentocht M. et al., 2014; Berkenfeld K. et al., 2015; Carvalho S.R. et al., 2015; de Boer A.H. et al., 2017). Here we will highlight the critical issues. Assuming that aerosol particles within a certain size-range are required for effective lung treatment by inhalation, the following technical problems must be identified:
To be effective vehicles for inhaled drugs, powder particles must be de-agglomerated in the airflow to form micrometer-size particles as schematically shown in Fig. 6 The outcome of this process depends on inhaler design, applied flow rate and powder properties. This is why the pharmaceutical powder has to be properly prepared before it can be used as a drug carrier. The main cohesive interactions which act against powder redispersion are caused by van der Waals forces, electrostatic and capillary forces, by hydrogen bonds and mechanical interlocking due to surface roughness (Zeng X.M. et al., 2000; Ramachandran V. et al., 2015). These interactions depend not only on the particle properties and their preparation method, but also on the ambient conditions such as air humidity.
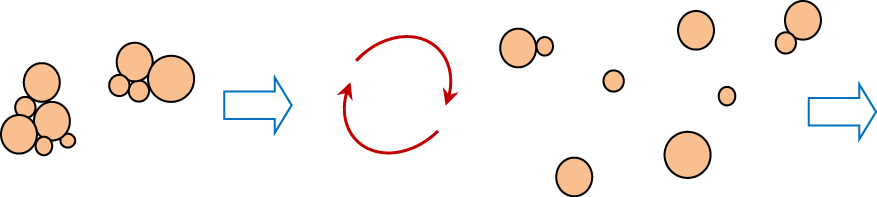
De-agglomeration of powder drug in the airflow.
Strategies which are used to prepare powder particles with the properties that facilitate their de-agglomeration include: (i) controlling the surface smoothness of the particles (Chew N.Y.K. and Chan H.-K., 2001; Geller D.E. et al., 2011), (ii) increasing interparticle distance, e.g. by adding nanoparticles (Begat P. et al., 2005), (iii) changing the properties of the particle surface, e.g. by coating with, e.g. leucine, magnesium stearate or surfactants as force-control agents (Begat P. et al., 2009; Sosnowski T.R. and Gradoń L., 2010). It can be also be beneficial to use micrometer-size nanostructured (partly porous) particles instead of compact ones with the same size (Tsapis N. et al., 2002; Jabłczyńska K. et al., 2015). All these strategies can be used both for drugs prepared as interactive mixtures (API-excipient) and for excipient-free formulations. Inhalable particles can also be developed with API embedded in a polymer matrix with PLGA (poly(lactic-co-glycolide) acid) being the most common matrix builder (Liang Z. et al., 2014). This drug delivery system has the additional advantage of sustained drug release in the target area. We should also mention a novel concept of inhalation powders based on the application of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) forming a dry lipid matrix which can be loaded with the API and transferred to the lungs from DPIs without additional excipients (Weber S. et al., 2014; Li Y.Z. et al., 2010).
Spray drying, spray-freeze drying and enhanced crystallization/precipitation (ultrasonic-assisted or utilizing the supercritical fluid technology) are applied to obtain engineered powder particles. We will skip the detailed characteristics of these methods as their thorough description can be found elsewhere (Gradoń L. and Sosnowski T.R., 2014; Kaialy W. and Nokhodchi A., 2015). Due to the technological feasibility and easy scale-up, spray drying is probably the most convenient method of producing inhalable powders. It also allows control of the particle structure, size and aerosolization properties (Vehring R., 2008; Weers J.G. and Miller D.P., 2015). Correct selection of the composition and concentration of the precursor (i.e. liquid solution or suspension), as well as of the drying parameters are decisive in obtaining powders with the desired particle size and shape (Nandiyanto A.B.D. and Okuyama K., 2011; Kramek-Romanowska K. et al., 2011). Fig. 7 shows some examples of different powder structures which can be obtained by tuning the parameters of the spray-drying process into a conventional laboratory-scale device with pneumatic atomization of liquid precursors.
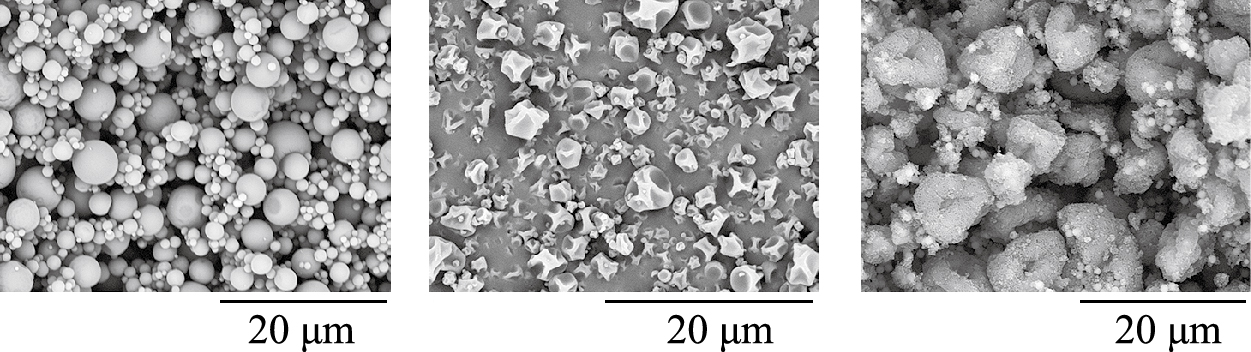
Examples of different shape and structure of powder particles obtained by the controlled spray-drying of various precursors (unpublished SEM images from author’s laboratory).
In all powder inhaler devices, aerodynamic stresses are used to fluidize the powder and de-agglomerate particle clusters to maximize the number of individual fine particles. The majority of DPIs currently available in the market belong to so-called ‘passive’ devices (Islam N. and Gladki E., 2008), where the aerosol is formed solely due to powder interaction with the flow generated by the patient’s inhalation. In all such devices it is necessary to maximize the transfer of airflow energy to the powder, possibly without useless dissipation. This can be effectively done by the creation—in a logical way—of local turbulence or other effects such as flow focusing or fluctuations (Gac J. et al., 2008; Sosnowski T.R. et al., 2014). However, flow arrangements of this type always increase the aerodynamic resistance (the pressure drop) in the inhaler, so the final design of a DPI must be optimized. It is important to note that erroneous concepts of aerosolization chambers can result in completely inefficient powder redispersion. This obvious rule is shown schematically in Fig. 8 by presenting two hypothetical inhaler chambers of identical design and aerodynamic resistance, but with totally different performance regarding the aerosol emission.
Comparison of two hypothetical aerosolization systems with the same aerodynamic resistance but disparate dispersion efficiencies due to a different location of the powder layer (grey rectangle).
Aerodynamic resistance of the inhaler is a very important issue which needs to be well understood since it often leads to ambiguities. The intrinsic inhaler resistance is defined by the equation (Clark A.R. and Hollingworth A.M., 1993):
| (9) |
where ΔP denotes the pressure drop (typically in hPa) and Q the volumetric airflow rate (typically in liters per minute, L/min).
Eq. (9) can be derived from the well-known relationship describing the local pressure drop:
| (10) |
where γ denotes the local resistance coefficient, which can be assumed independent of the airflow rate in the range relevant to inhalation (30–120 L/min). This equation can be rewritten as:
| (11) |
where A denotes the cross-sectional area for the airflow. After substituting:
| (12) |
Eq. (9) is obtained directly.
Passive DPIs have different degrees of aerodynamic resistance, typically in the range of 0.05–0.2 hPa0.5min/L (or: 30–120 Pa0.5s/L). At the given inspiratory effort, low-resistant (L-R) DPIs allow high flow rates to be obtained at which good aerosolization is achieved. In the case of high-resistant (H-R) DPIs, similar efforts will produce lower flow rates, however, the flow restriction obtained in such inhalers should cause strong aerodynamic effects and a similar powder deaggregation as in the low-resistant devices at higher flows. This is why DPIs need to be tested at the fixed pressure drop (the standard value is 4 kPa (USP, 2008; European Pharmacopoeia, 2013) not at a fixed flow rate.
A frequent mistake is the comparison of different DPIs based on the criterion of the optimum volumetric flow rate (Qopt needed to get aerosol with maximized FPD). It is often believed that a low Qopt is always equivalent to a better performance of the DPI. But a low Qopt actually means a high resistance of the inhaler, which may limit correct operation by all groups of patients due to the demands of high inspiratory efforts. It is easier to obtain high flow rates in L-R DPIs, so even if they work sub-optimally at Q < Qopt, they can be used with some success by patients who may be not able to use H-R DPIs. The problem of L-R inhalers is that they are typically flow-dependent, i.e. the mass and particle size distribution of the aerosol they release are significantly improved when flow increases (Weuthen T. et al., 2002; Azouz W. et al., 2015). Powder de-agglomeration is better at high airflow rates but at the same time the aerosol velocity at the inlet to the mouth becomes higher, which increases undesired drug deposition in the oro-pharynx. For H-R DPIs, the airflow through the device is limited, and dependence of their performance on the flow rate is lower. Concluding this analysis, it may be stated that there should be an optimum of flow resistance in DPIs. The flow must be restricted to produce the high aerodynamic stresses required for good aerosolization of the powder, however, if the overall resistance of the device is too high, it can exclude a large group of patients from proper use of such device. Elimination of the problem of DPIs flow-dependence is achieved in ‘active’ DPIs which utilize, for instance, the compressed gas to facilitate powder de-agglomeration to fine particles. More than a decade ago, Exubera® was developed as an active DPI for insulin delivery, however—due to various reasons—it was withdrawn from the market in 2007 (Heinemann L., 2008). Another active DPI—Spiromax®—has emerged recently for the delivery of drugs for asthma and COPD patients (Canonica G.W. et al., 2015).
Similarly to particle deposition in the lungs, drug inhalation from passive DPIs is a dynamic process. The aerosol is formed and released from the device during the given (rather short) period of the inhalation. Let us recall the flow curve already shown in Fig. 5 to analyse this process. Two different situations are depicted in Fig. 9. Assuming that the two DPIs denoted as 1 and 2 have a similar aerodynamic resistance but a different principle of powder release, we can see that the aerosol is emitted from inhaler 1 during time period t1 ÷ t1′, but from inhaler 2 during time period t2 ÷ t2′. Fig. 9a shows the corresponding flow rate, i.e. inhalation curve, while Fig. 9b shows the corresponding volume of air aspirated to the lungs.
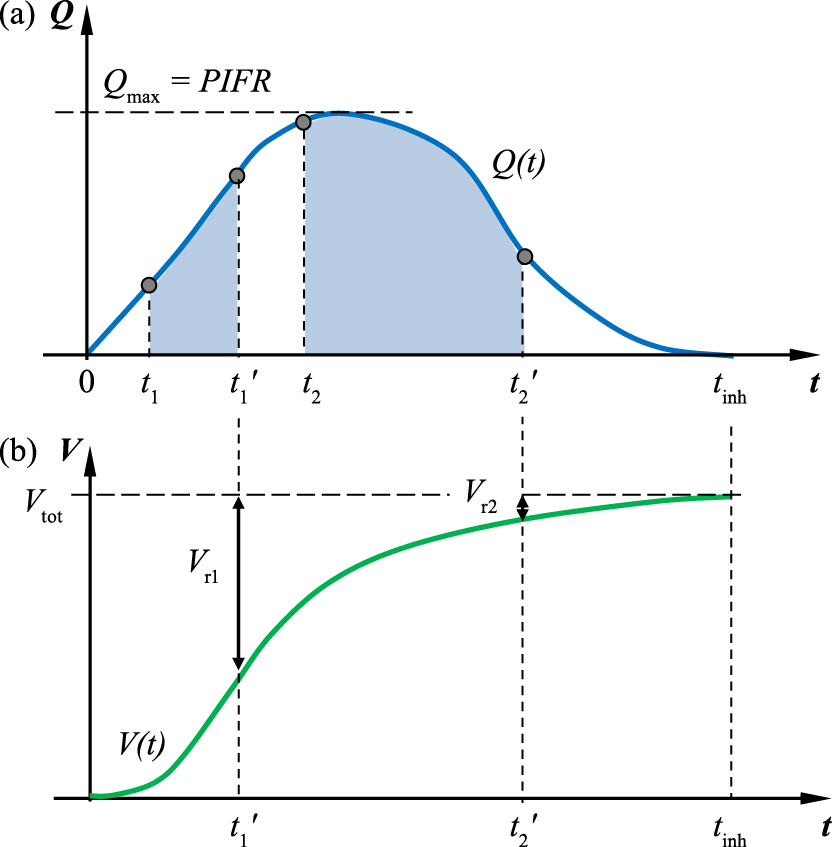
(a) Inhalation curve Q(t) and periods of aerosol release (gray fields) for two DPIs; (b) the corresponding inhaled volume curve V(t) = ∫Q(t)dt and indicated remaining volumes of air inhaled after complete release of aerosol from both DPIs (Vr1 or Vr2).
Let us note that aerosol is emitted from inhaler 1 during a short period which is finished at t1′, i.e. before the maximum flow rate Qmax is achieved. In contrast, inhaler 2 is activated later (at t2) by the flow rate close to Qmax and the release time is longer, so the aerosol is introduced to the air stream until t2′, which is not far from the end of inhalation, tinh. It can be expected that the quality (size distribution) of aerosol produced at higher flow rates, i.e. in case 2, will be more favorable than in case 1.
The graph of inhaled volume (Fig. 9b) shows that when the aerosol is totally emitted from inhaler 1, the remaining volume of air to be inhaled is still large (Vr1), which means that the released particles can be carried even to the lung periphery. In contrast, after the entire dose of aerosol is released from inhaler 2 (at t2′), a small volume of air Vr2 remained for inhalation, so probably these particles can penetrate only the very first generations of the bronchial tree before the exhalation will begin.
The presented example shows another, often overlooked, factor which should be considered for the rational design of inhalation systems based on powder redispersion.
3.3 Understanding the compendial methods of aerosol characterizationAerosol particles of medicines intended for inhalation must be evaluated by specialized methods to confirm that they have the proper size characteristics and reproducibility (USP, 2008; European Pharmacopoeia, 2013). Use of impactors instead of other measuring techniques is necessary because the majority of formulations contain particles with different chemical composition, e.g. in DPIs based on API-lactose blends or in combined products (two or more drugs delivered in one inhalation). In such cases, optical methods which are typically used for aerosol assessment (e.g. time-of-flight or light scattering/light diffraction) cannot be used because they do not distinguish particles that carry different chemicals. Since only the mass of API is of interest from the therapeutic perspective, all other particles should be ignored in such analyses. Cascade impactors can sort particles into several (typically 5–8) size classes based on their inertial deposition which is caused by flow acceleration at the consecutive separation stages. In the testing of DPIs, the aerosolized drug is drawn to the impactor via the inlet and the additional vessel (preseparator) capable of capturing large grains of the excipient which usually also contains some API is attached. Impactor inlet and preseparator are believed to mimic the upper airways (mouth and throat), however, the particle separation efficiency of these impactor elements is not strictly defined. Material collected at each stage of the impactor is then quantified by sensitive chemical assays – typically high-performance liquid chromatography (HPLC) – against the pharmacopeial standard of the API under test. Chromatographic analysis ensures that the API concentration is evaluated selectively and assigned to the given size class of particles captured at each separation stage. Thanks to this technique, the size distribution based on drug mass is obtained, which allows finding the most important indicators of the quality of medical aerosol: fine particle dose (FPD), fine particle fraction (FPF) and mass median aerodynamic diameter (MMAD). Due to their principle of operation, all impactors classify particles based on their aerodynamic size da defined by equation (5). Assuming a quasi-spherical particle shape and density in the range of 800–1200 kg/m3, the aerodynamic particle diameter is almost the same (± 10 %) as the geometric diameter, dp. However, these two diameters can be very different in the case of, e.g. porous particles with an effective density in the range of hundreds of kg/m3. In such cases, large particles (larger than 10 μm) may still be effective as drug carriers for inhalation since their aerodynamic behavior and inertial deposition is equivalent to smaller particles with a typical density.
There are two major drawbacks to impactor particle size analysis:
Until the Next Generation Impactor (NGI) was developed, inhalers could be tested only at several standard airflow rates (e.g. 28.3, 60 and 90 L/min in the Andresen cascade impactor) due to a lack of certified calibration for other flows. The NGI allows characterization of the aerosol particle size at any flow rate in the range of 30–100 L/min, but also at lower values (down to 15 L/min). The aerodynamic cut-off size, dci, at each stage i depends on the applied flow rate and for 30–100 L/min, they can be calculated from the equation:
| (13) |
where xi is the unique exponent for the given separation stage (MSP Corporation, 2008). A slightly modified correlation is valid for flow rates in the range 15–30 L/min. Cut-off sizes for selected airflow rates are listed in Table 2.
Nominal cut-off sizes [μm] for NGI impactor at selected airflow rates [L/min].
| Stage no. | 15 L/min | 30 L/min | 60 L/min | 90 L/min |
|---|---|---|---|---|
| 1 | 14.1 | 11.7 | 8.06 | 6.12 |
| 2 | 8.61 | 6.40 | 4.46 | 3.42 |
| 3 | 5.39 | 3.99 | 2.82 | 2.18 |
| 4 | 3.30 | 2.30 | 1.66 | 1.31 |
| 5 | 2.08 | 1.36 | 0.94 | 0.72 |
| 6 | 1.36 | 0.83 | 0.55 | 0.40 |
| 7 | 0.98 | 0.54 | 0.34 | 0.24 |
As seen from Table 2, the NGI cannot determine the size of particles larger than those captured at stage no. 1, and this maximum diameter depends on the flow rate applied during DPI testing. According to the standard, the mass median aerodynamic diameter (MMAD), which is often considered the important parameter in comparing different inhalation products, is calculated only taking into account the drug particles within the range of cut-off sizes specified for the numbered stages, thus ignoring the API mass collected inside the inlet port and preseparator. This leads to a risk of misinterpretation of the experimental data. Since the range of particle sizes used for calculation of the MMAD depends on the applied flow (Table 2), it will be incorrect to compare the MMAD obtained for DPIs characterized by different resistance, RD. According to the standard, they must be compared at the same pressure drop (4 kPa), hence at different flow rates. As particles eliminated before stage no. 1 are not considered in the mass balance, then it is possible to achieve a low MMAD (apparently a good value) even if a large amount of aerosol drug is contained in big particles deposited in the impactor inlet port and preseparator, as shown in Fig. 10. Therefore, the MMAD should be considered rather as a quality parameter which allows verification if different batches of a given inhalation product (i.e. with a given DPI) deliver—at the fixed airflow rate—the aerosol with a similar particle size distribution. According to the presented discussion, it is incorrect to use MMAD as a criterion of comparison of different DPIs and drugs.
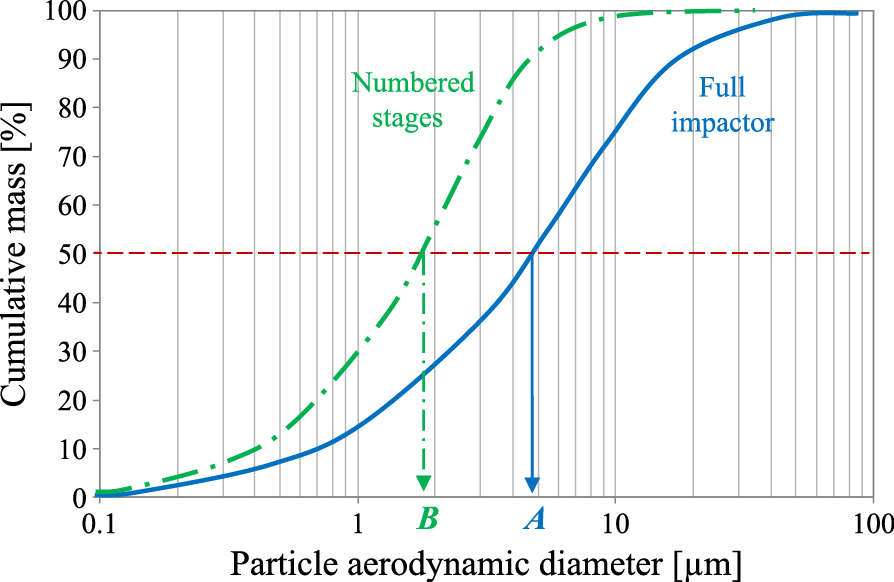
Comparison of the real MMAD (A) of the aerosol and the MMAD calculated only on the basis of particles in the range limited to 0–11.70 μm, i.e. the sizes classified inside the NGI at 60 L/min (B).
The troublesome aerosol particle sizing by cascade impactors can sometimes be replaced by fast and accurate optical on-line measuring techniques such as laser diffraction, however, only under certain conditions. These methods can be used, e.g. in carrier-free DPIs which aerosolize only a single API. It is also reasonable to apply optical methods for pMDIs (pressurized metered dose inhalers), SMIs (soft mist inhalers) and nebulizers, assuming that all particles/droplets have a statistically identical composition. In such cases, they contain the API mass which is proportional to the cube of the particle diameter. For aqueous droplets ρp ≈ ρw, according to eq. (5), we will directly obtain the aerodynamic diameter. For other materials, the geometric diameters dp can be easily recalculated to da if the material density is known. Although optical methods are not compendial, for some inhalers (e.g. nebulizers), they have been demonstrated to be equivalent to pharmacopeial impactors (Kwong W.T.J et al., 2000; Ziegler J. and Wachtel H., 2005; Mao L. et al., 2010).
3.4 In vitro-in vivo correlation (IVIVC) problem in aerosol inhalationThe aerodynamic size of drug particles is needed not only to verify the reproducibility of the inhalation product (quality assurance) but also to confirm that the drug can be expected to effectively penetrate to the target region in the respiratory system. FPD (da < 5 μm) informs about the dose which has the best chance to reach the lower airways (USP, 2008; European Pharmacopeia, 2013). However, the applicability of this criterion is still discussed within the issue of IVIVC, i.e. in vitro-in vivo correlation (Clark A.R. and Borgström L., 2002; Newman S.P. and Chan H.-K., 2008). This problem tries to find a possibly precise link between the particle size of inhaled drugs and the expected health benefit and must, in fact, acknowledge the complexity of particle deposition discussed in section 2. Some researchers claim that the mass of extra-fine particles (1–3 μm) is more relevant to the real deposition and health effects (de Boer A.H. et al., 2015). However, as shown in Fig. 4, it is difficult to unequivocally indicate the optimum particle size, in particular considering inter-subject variability in inhalation flow dynamics and airway geometry where both can be additionally altered in lung disease. Mitchell J. et al. (2007) additionally pointed out that the clinical response (the real in-vivo effect) will depend not only on the actual deposition of drug particles but also on the drug potency and the severity of lung disorder. These authors also discussed other limitations of application of the results of aerodynamic particle sizing to predict the regional aerosol deposition in the respiratory system. Cascade impactors are the technical devices which operate at ambient temperature and at a constant flow rate, which is a completely different situation compared to real aerosol inhalation. Laboratory testing conditions may influence the particle size distribution, e.g. due to evaporation of nebulized droplets (Zhou Y. et al., 2005) or additional deagglomeration of powders (Sosnowski T.R., 2008), which make such aerosols incomparable with the ones entering the lungs during drug inhalation. Although some additional technical improvements of cascade impactors have been proposed such as a more realistic mouth-throat model instead of the standard inlet (Copley M. et al., 2011), the problem of a direct conversion of the measured particle size to the real in-vivo drug delivery and efficacy still remains unsolved (Clark A.R. and Borgström L., 2002; Mitchell J. et al., 2007).
3.5 Innovative and generic drugs developmentAt the end of the discussion of challenges and opportunities in using inhalable powders for drug delivery, we should take a short look at the problems of development of inhalable medical products. Completely new inhalation concepts (i.e. the new molecules with new inhalers) require strictly defined, demanding and expensive procedures to become a candidate for the medial product. In addition to precisely documented development steps, clinical studies to confirm the efficacy and safety are always required. Since many patents for therapeutic molecules are expiring, there is a trend to develop the generic products as cheaper substitutes of drugs originally developed some years ago. The procedure required to introduce a generic inhalation product to the market is simplified, and—according to some regulations (e.g. in Europe)—clinical studies are not required if therapeutic equivalence is convincingly demonstrated (CHMP, 2009). Accordingly, the whole procedure is cheaper and faster, although there is concern whether such an approach is fully justified. It should also be noted that the legislation regarding generic inhalation products is different worldwide (Pirożyński M., and Sosnowski T.R., 2016). In any case, an important issue is the quality of the powder drug and the inhaling device, and both have to match the original product closely regarding the aerodynamic performance (e.g. DPI resistance) and the API aerosol size distribution obtained at different flow rates through the inhaler (CHMP, 2009).
Consequently, all key problems highlighted in the previous sections should be taken into account when the development of innovative or generic powder inhalation products is considered.
Powder properties should also be optimized regarding the bioavailability of the drug they carry into the lungs. Inhaled particles land on the wet surface of bronchi, bronchioles or alveoli, so they directly interact with the fluid layers of the lungs. In conducting airways, particles come into contact with mucus and the drug molecules eventually reach the epithelium after penetrating across the layer of this complex viscoelastic liquid. Deposited particles can be simultaneously transported along the airways due to the action of ‘mucociliary escalator’, i.e. the longitudinal flow propelled by beating cellular cilia (Smith D.J. et al., 2007). Since the underlying epithelium is the main target for inhaled bronchodilating and anti-inflammatory drugs, it is important to prepare the aerosol drug carriers in a way which facilitates API penetration through the mucus blanket. In diseased lungs, the mucus is abundant and more viscous (Cone R.A., 2009), so less permeable for inhaled drugs. In such a situation, inhalable powders with a specific composition and properties can be useful. According to a recent idea (Odziomek M. et al., 2012), the excipient particles might contain a mucolytic agent such as N-acetylcysteine together with the stabilizing additive (e.g. dextran). Deposited particles of the drug bonded to such a carrier (in a similar way as in typical API-lactose blends) are capable of rheologically thinning the mucus due to interactions with the mucolytic agent which is released locally. This allows the drug to penetrate faster through the mucus layer (Odziomek M. et al., 2015). In the alternative concept, multicomponent powders combining both the mucolytic agent and the drug in a single particle were proposed (Gradoń L. et al., 2009). The described functional particles can be effectively prepared by spray-drying through the adjustment of process conditions to obtain powders with good aerosolization properties. Mucus rheology and mass transfer phenomena in raw or modified mucus can be analysed by selected physicochemical methods (Schuster B.S. et al., 2013; Odziomek M. et al., 2017).
Particles deposited in the respiratory (alveolar) region interact not with the mucus but with the pulmonary surfactant (PS). The PS produced by type II alveolar cells contains lipids (mainly lecithins) and some unique proteins (Parra E. and Perez-Gil J., 2015). True to its name, the PS demonstrates strong surface-active properties. They have important consequences for lung physiology, due to:
It is important to note that PS is highly surface-active under the dynamic conditions of breathing, i.e. during oscillatory variations of the air/liquid interfacial area. Both temporal and spatial fluctuations of the surface tension along the interface take place due to surfactant mass exchange between the bulk liquid and the free interface, but also due to flow and diffusion at the interfacial boundary. Marangoni effects are generated due to the surface tension gradients providing the driving force for the hydrodynamic clearance of deposited particulates from the alveolar zone (Gradoń L. and Podgórski A., 1989; Gradoń L. et al., 1996) - Fig. 11.
Dynamic surface tension in alveolar region. Marangoni flows and particle transport are due to dynamic surface tension gradients Δσ (t).
Such behavior of the air-liquid interface can be reproduced in the laboratory using specialized measuring devices, e.g. Langmuir-Wilhelmy balance, oscillating bubble or oscillating drop tensiometers (e.g. Sosnowski T.R. et al., 2017; Kondej D. and Sosnowski T.R., 2013; Kondej D. and Sosnowski T.R., 2016; Wüstneck R. et al., 2002). These experimental systems allow determination of the dynamic surface tension variations during the breathing-like compression and expansion of the interface with the PS. Experimental measurements can also directly demonstrate the occurrence of the Marangoni flows induced by the variations of interfacial area. For some types of inhaled medicinal aerosols (e.g. drugs with the desired systemic absorption), it may be beneficial to extend their residence time in the alveolar region, so this kind of fast transport would be undesired. Tuning the surface properties of particles (e.g. total surface area, porosity, wettability) may influence their interactions with the surfactant which can extend the residence time and improve the local bioavailability of inhaled drugs (Sosnowski T.R. et al., 2003).
The results shown in Fig. 12 confirm that local disturbance of the air/liquid interface with a model PS generates the surface tension gradient Δσ and, as a consequence, the superficial flows. In Fig. 12, the surface tension gradient is produced by the unidirectional compression of the interface, and the superficial flow is illustrated by a lateral displacement of tracer particles.
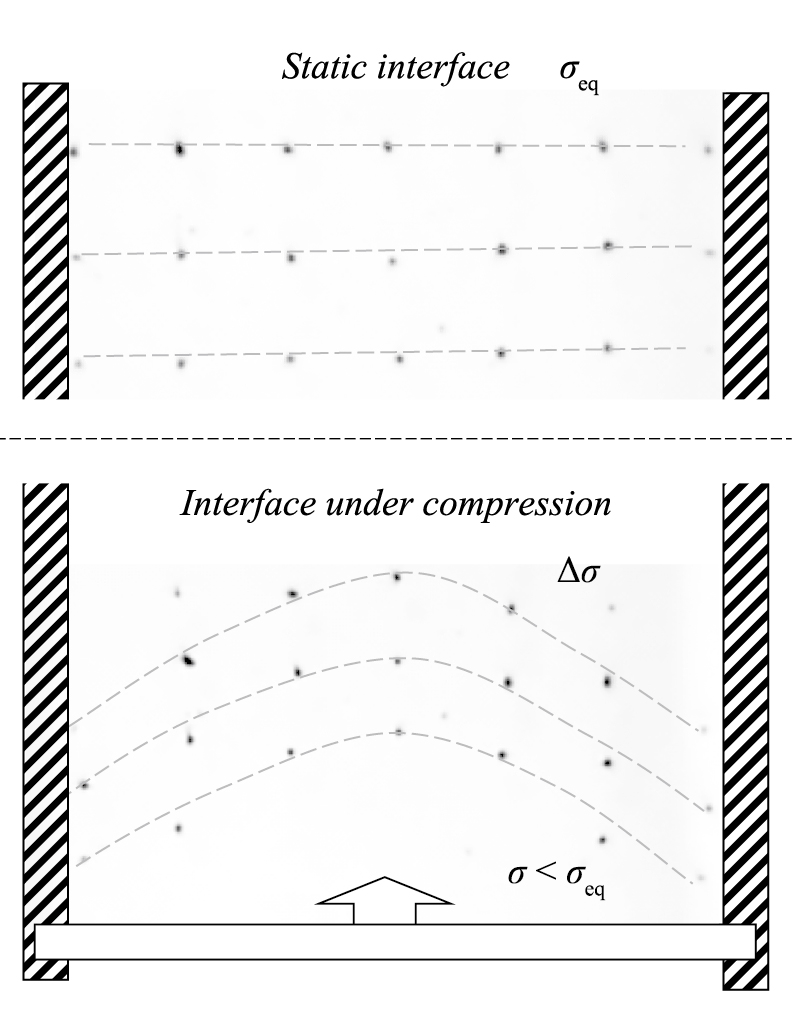
Experimental results (top view of the Langmuir-Wilhelmy balance) showing the flow of tracer particles at the air-water surface with the phospholipid monolayer (as the model of PS) during unidirectional surface compression.
Similar flows can contribute to alveolar clearance in vivo, i.e. to the effective removal of deposited particles (or particles already captured by alveolar macrophages) from alveoli as shown in Fig. 11.
Surface tension hysteresis measured in vitro for PS shown in Fig. 13 is another characteristic dynamic feature of the pulmonary surfactant, and it can be used to trace alterations in the dynamic surface tension of the PS caused by deposited particles (Kondej D. and Sosnowski T.R., 2013). In this way, physicochemical methods help to assess potential disturbance of the alveolar mass transfer caused by inhaled particles, including powder drugs (Kramek-Romanowska K. et al., 2015). The results may indicate which properties of the inhaled powders are required for the best drug bioavailability. These specific powder properties can be obtained during their production by particle engineering methods highlighted in section 3.1.
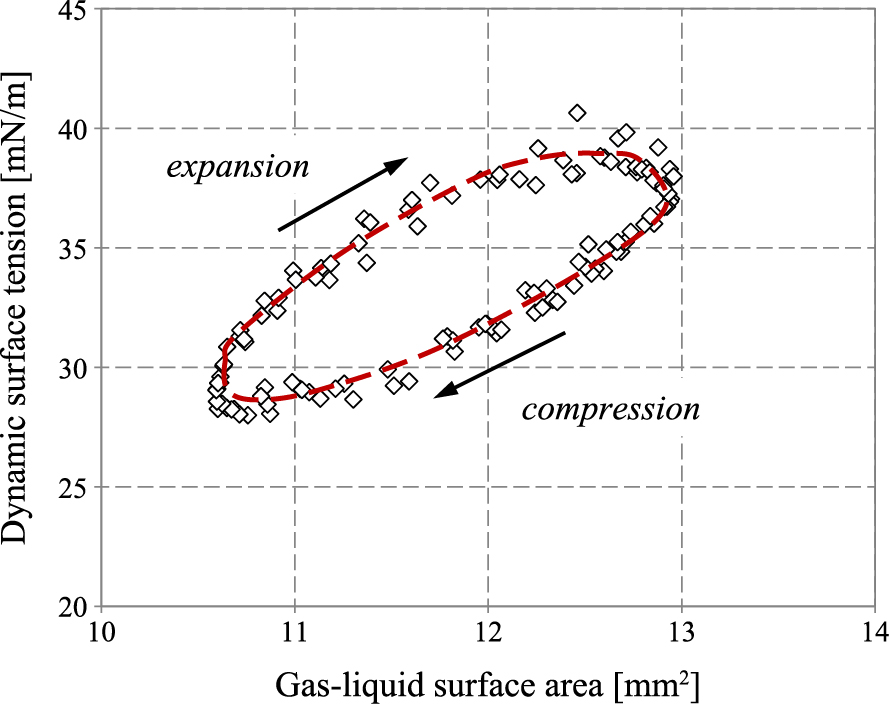
Example of surface tension hysteresis recorded in the PS during oscillation of the air/liquid surface at a physiological rate and temperature (results obtained with PAT-1M device, Sinterface, Germany).
The main intention of this paper was to indicate important technical issues of the use of aerosolized powder particles as vehicles of drugs delivered to the lungs. Starting from the overall physical overview of the aerodynamic particle transport in the respiratory system, the importance of dynamic effects related to the non-steady airflow during breathing was pointed out. Other difficulties and uncertainties in predicting particle flow and deposition in the respiratory tract are related to the specific phenomena (e.g. particle coagulation, hygroscopic growth) which can influence the dynamic behavior of particles in the lungs. The properties of powders and the selected issues related to their preparation for inhalation purposes were briefly presented to indicate the key factors in optimizing the inhalation product. The proper design of powder inhaling devices, i.e. DPIs, is another vital condition of successful drug delivery. Again, special attention was given here to the dynamic processes of aerosol formation and entrance into the organism. This allowed demonstration of the fact that for particles which are not drawn in sufficiently early during the inhalation phase of the breathing cycle, their deep penetration to distal lung regions will not be possible. Discussion of the unique methods of the characterization of aerosols produced in the inhalers (including DPIs) was used to indicate common misinterpretations of certain experimental data. Finally, the possibility of designing powder particles with a better pharmaceutical outcome was highlighted on the basis of a brief description of physicochemical interactions between deposited particles and lung fluids (mucus and pulmonary surfactant).
It should be realized that the physics of all processes of powder aerosolization, penetration through the respiratory system, deposition and interaction with the lung surface are so complex that we are still lacking their complete quantitative description. Classic considerations which are often based on strong simplifications overlook some key features of these processes and can lead to erroneous conclusions. It may have certain consequences in choosing the appropriate steps in product development by the pharmaceuticals industry, but also in non-optimal therapeutic decisions made by physicians. It is therefore always important to take into account the latest achievements in understanding aerosol generation from powders and their deposition in the lungs.
This scientific work was partly supported by the budget for sciences in the years 2015–2018 as Research Project No. 2014/13/B/ST8/00808. The author thanks Katarzyna Jabłczyńska, M.Sc., for preparation of the spray-dried powders and their SEM images used in this paper.
Aerosolized Dose
APIActive Pharmaceutical Ingredient
CFDComputational Fluid Dynamics
COPDChronic Obstructive Pulmonary Disease
DDTDose Delivered to Target
DPIDry Powder Inhaler
EDEmitted Dose
E-TExtra-Thoracic (deposition)
FPDFine Particle Dose
H-RHigh-Resistant (inhaler)
ICRPInternational Committee for Radiological Protection
L-RLow-Resistant (inhaler)
MDMetered Dose
MMADMass Median Aerodynamic Diameter
MPPDMulti-Path Particle Deposition (model)
NGINext Generation Impactor
PIFRPeak Inspiratory Flow Rate
pMDIPressurized Metered Dose Inhaler
PSPulmonary Surfactant
SMISoft Mist Inhaler
T-BTracheo-Bronchial
TVTidal Volume
Across-sectional area (m2)
CsCunningham slip correction factor (–)
daparticle aerodynamic diameter (μm)
dcicut-off diameter of stage i (μm)
dpparticle diameter (μm)
FBBrownian force (N)
Fextexternal forces (N)
kBBoltzmann constant (J K−1)
L0characteristic length (m)
mmass (kg)
ΔPpressure drop (Pa)
Qairflow rate (m3s−1)
Qmaxmaximum airflow rate (m3s−1)
Qoptoptimum airflow rate (m3s−1)
mean airflow rate (m3s−1)
RDintrinsic aerodynamic resistance of a DPI (hPa0.5min L−1)
ReReynolds number (–)
S0spectral intensity of fluctuations (m2s−3)
StkStokes number (–)
ttime (s)
tinhtime of inhalation (s)
Δttime step of numerical integration (s)
Ttemperature (K)
uair velocity (m s−1)
upparticle velocity (m s−1)
Vinhaled volume (L)
xiexponent of the correlation in NGI (–)
Zdimensionless vector (–)
αBmagnitude of the acceleration of thermal excitations (m s−2)
γlocal resistance coefficient (–)
μair viscosity (Pa s)
ρpair density (kg m−3)
ρwwater density (kg m−3)
σsurface tension (N m−1)
σeqequilibrium surface tension (N m−1)
χdynamic shape factor (–)
Tomasz R. Sosnowski
Tomasz R. Sosnowski received his PhD (1997) and DSc (habilitation - 2006) in chemical engineering from Warsaw University of Technology (WUT), Poland. In 1999–2000 he was a post-doc fellow at Lovelace Respiratory Research Institute in Albuquerque, USA. Currently he is a full professor at the Faculty of Chemical and Process Engineering WUT. His main scientific interests are: (i) interfacial phenomena in human lungs, including the role of the pulmonary surfactant, (ii) technical aspects of aerosol systems used in drug delivery by inhalation. He is a co-author of more than 70 scientific papers, several books and chapters, 2 granted patents and 6 patent applications.