2020 Volume 37 Pages 97-113
2020 Volume 37 Pages 97-113
Immunization can be traced back to classical China. Modern immunization reduces the risk of infection by attenuating or killing the pathogen or using non-infectious antigens to elicit the immune response. The challenge of immunization is to raise a robust protective response without infecting the individual or overstimulating the immune response, and this can be achieved by using nanoparticle delivery systems to specifically target the innate immune system with known antigens and where necessary include an adjuvant to enhance the efficacy. These systems can be targeted to mucosal sites that are located throughout the body with the nasal and pulmonary routes of administration allowing ease of access. Macrophages are the first line of defense of the innate immune system and are the host cell for primary intracellular infection by several respiratory pathogens notably mycobacteria and streptococci. The breadth of nanoparticle technology available to deliver vaccines has been explored and consideration of its value in nasal and pulmonary delivery is addressed specifically.
Protection from disease by immunization was documented in classical China (Arouet F.M., (Voltaire), 1733). Reports of the use of live organisms appeared in the eighteenth century most prominently by Jenner in preventing smallpox using a cowpox-based vaccine from which the term “vaccination” is derived (Smith K.A., 2011). Since the discoveries of Koch in the late nineteenth century vaccines have revolutionized disease prevention and have significantly decreased infant and child mortality (Gradmann C., 2006). The observation that individuals exposed to disease might develop immunity to future infection, and the initial steps at immunization were the foundation for the field of immunology and the desire to develop vaccines in the late nineteenth and early twentieth century. Advances in biotechnology combined with greater understanding of infectious disease and host immunity have resulted in sophisticated approaches to vaccine delivery and adjuvancy (Hoft D.F., 2008; Kaufmann S.H.E., 2000).
The most prominent route of administration of vaccines has been subcutaneous or intra-dermal injection. Vaccines also have been administered with adjuvant components intended to promote immunogenicity and elicit a protective immune response. The most common adjuvants were components of bacterial cell walls, such as Freund’s adjuvant that contains materials originating from mycobacteria, or particulates such as alum (aluminum salts) (Dvorak A.M. and Dvorak H.F., 1974; Shah R.R. et al., 2017; Stils H.F., Jr., 2005).
As drug and vaccine delivery systems and knowledge of immunity have improved new administration methods and routes have been considered (Saallah S. et al, 2018). Nanoparticle systems are known to offer unique biophysical opportunities to effectively deliver to antigen presenting cells at the site of administration. These particles may be composed of a core inert non-degrading (waxes and plastics) substance to which antigen is adsorbed, or matrix degrading (natural and synthetic polymers) material into which antigen is incorporated. It is now understood that there is a unique component of the immune system that is associated with mucosal sites (respiratory, gastro-intestinal and urogenital tracts). Consequently, as new nanoparticulate vaccine delivery systems have been developed new routes of administration also have been considered including the respiratory (nasal and pulmonary) and gastro-intestinal epithelia, sites of mucosal-associated lymphoid tissue. These alternative routes of administration offer the advantage of non-invasive ease of administration.
The history of nanoparticle manufacture overlaps with microsphere technology (Burgess D.J. and Hickey A.J., 2007). The key components of natural and synthetic polymers had been clearly identified and all that was required were processes to allow the manufacture of nanoparticles. Since functionality of nanoparticles is often associated with smaller sizes a practical definition of 1–100 nm has been adopted widely but clearly the scientific definition is derived from fundamental length dimension of particles in sizes smaller than 1,000 nm (Commissioner O.o.t., 2014; Nishijima N. et al., 2017).
Vaccines have been developed primarily to viral and bacterial pathogens. Natural immunity proceeds though the innate and adaptive/acquired immune systems. Processing of the whole vector or organism allows specific components to be isolated as identifying antigens that the immune system employs as recognition factors to drive clearance of infection and long-term immunity.
As the first line of defense the innate immune system of phagocytic cells have been the target for engineered solutions to vaccine delivery. Monocytes and macrophages are known to take up particles. From the later 1980s studies began to explore the interaction between particles and phagocytic cells to evaluate the ability to target these cells either through particle size alone or a combination of size and targeting moieties.
This review will consider manufacture and characterization of nanoparticle-based vaccine delivery systems, and the immunological consequences leading to protective immunity when delivered by different routes of administration.
The development of nanoparticle-based vaccine (nano-vaccine) delivery systems requires consideration of several factors: route of administration, formulations, passive or active targeted delivery, efficacious dosing, and required frequency of vaccine exposure. The material composition, the method of manufacture and processing parameters all contribute to key physicochemical properties including the architecture with respect to antigen presentation for nanoparticle-based vaccines. This section will briefly review the structures of nanoparticles and provide a concise overview of the design and composition of nanocarriers for delivery of vaccines, with an emphasis on those delivered through the mucosal route of administration. A description of the manufacturing and characterization of nanoparticle-based delivery systems will follow.
2.1 Mechanisms of controlled presentation from a carrierThe mechanism for controlled presentation from a carrier vehicle is intimately linked to various parameters of the system. Requirements for temporal and spatial control of the biologically active ingredient (BAI) in vivo ultimately dictate design features, including formulations (e.g., drug, antigen, excipient, adjuvant), carrier material (e.g., polymer), and the architectural arrangements of individual constituents. As reviewed elsewhere (Uhrich K.E. et al., 1999), temporally-regulated presentation of BAI is achieved by prolonging exposure of BAI to the surrounding environment. Two exemplary mechanisms can delay BAI presentation from a carrier: using the polymer vehicle to control diffusion of the BAI into the surrounding environment or by via slow erosion of the vehicle itself to delay delivery of BAI. For the diffusion-controlled mechanism, the release rates of BAI from a carrier vehicle is controlled by imposing physical barriers to transport (e.g., polymeric materials). In one example, reservoir-style vehicles have designated compartments for the BAI and carrier material and use a permeable polymer membrane to control BAI release. A wealth of examples exist for diffusion-controlled arrangements, including particle-based systems (Kamaly N. et al., 2016) and implantable systems (Bourges J.L. et al., 2006; Croxatto H.B., 2002; Htay T. and Liu M.W., 2005; Malcolm R.K. et al., 2012). The second temporally-regulated mechanism involves delaying the exposure of BAI into the environment through controlled erosion of the carrier polymer (e.g., polyester, polyanhydride). Degradation mechanisms typically involve hydrolysis to reduce the polymeric molecular weight and facilitate release of the BAI (Kamaly N. et al., 2016).
2.2 Design and composition of nanoparticles for vaccine deliveryGiven the breadth of nanoparticle-based delivery of vaccines (Zhao L. et al., 2014), a comprehensive review of each system is outside the aim of this review. This section will instead highlight nanoparticle vehicles for delivery of vaccines via the mucosal route of administration. We will review two broad categories of nanocarriers: polymeric nanoparticles and lipid-based nanoparticles.
Polymer NanoparticlesNanoparticles comprising polymers are broadly categorized based on the source material: natural or synthetic. Naturally sourced polymers commonly include polysaccharides sourced from animals, plants, or microorganisms that are biocompatible and biodegradable (Tiwari P. et al., 2014). Natural polymers, however, can carry disadvantages including possibility for unpredictable rates of degradation, unintended immunogenicity, microbial contamination, differences between batches, and poor mechanical strength (Bhatia S., 2016). Despite the potential for undesirable attributes, naturally-sourced polymers are successfully employed in various applications for drug delivery, tissue engineering, and wound dressings.
Chitosan, which is sourced from crustaceans, exists as a cationic polysaccharide at low pH and can complex with negative polyions to produce polyelectrolytes useful as carriers for drug delivery (Hamman J.H., 2010; Nagpal K. et al., 2010; Pandey R. and Khuller G.K., 2004). Chitosan nanoparticles have been explored for various vaccines (Illum L. et al., 2001), including Mycobacterium tuberculosis (MTB) (Feng G. et al., 2013), diphtheria (van der Lubben I.M. et al., 2003), pertussis (Jabbal-Gill I. et al., 1998) influenza (Amidi M. et al., 2007; Sawaengsak C. et al., 2014), and pneumococcal diseases (Xu J. et al., 2011). Chitosan contains chemical groups, such as primary amines, which permit conjugation of different moieties onto the nanomaterial carrier for targeted applications (Chua B.Y. et al., 2012; Mohammed M.A. et al., 2017; Zhang X. et al., 2008). Another polysaccharide, the seaweed-derived alginate, comprises repeating units of α-L-guluronic acid and β-D-mannuronic acid and shows widespread utility as commercial wound dressings and drug delivery vehicles (Thomas S., 2000; Tønnesen H.H. and Karlsen J., 2002). Nanoparticles comprising alginate have been explored for vaccine delivery (Sarei F. et al., 2013), and also show utility as a coating for chitosan nanoparticles to improve stability of oral mucosally delivered vaccines (Borges O. et al., 2005; Liu Z. et al., 2013). Guar gum, a polysaccharide comprising segments of mannose and galactose, is resilient to human gut enzymes while remaining susceptible to degradation by microflora in the human intestine, which makes this polymer beneficial for targeted delivery to the colon (Prasad Y.V. et al., 1998). Formulations of guar gum have been used to deliver active pharmaceutical ingredients (APIs) to the colon, such as flurbiprofen to treat inflammation (Vemula S.K. and Bontha V.K., 2013) and methotrexate for colon cancer (Chaurasia M. et al., 2017) and for oral delivery of Ag85A antigen for tuberculosis vaccination (Kaur M. et al., 2015). A plant-based polysaccharide, pectin, is composed of a linear backbone of α-1,4-linked D-galacturonic acid (GalpA), in combination with diverse structural arrangements of branched regions (Liu L. et al., 2007). Pectin formulations can readily gel with mucin present within the nasal cavity, making this polymer useful for nasal drug administration (Morris G. et al., 2010; Watts P. and Smith A., 2009). Another natural polymer, gelatin, is produced through partial hydrolysis of collagen acquired from animal sources (Tiwari P. et al., 2014). Gelatin nanoparticles modified with polyethyleneimine (PEI), ovalbumin, and an immunostimulant were examined for a nasal administered vaccine (Lin S.F. et al., 2018).
Synthetic polymers represent the second source of materials used to fabricate polymeric nanoparticles. Contrary to natural polymers, synthetic polymers benefit from the ability to strictly tailor design parameters and synthetic steps to ultimately meet targeted product profiles in terms of mechanical properties, biodegradation timeframes, and purity. Synthetic polymers are either biodegradable or non-biodegradable with each category holding unique advantages for the intended drug delivery system. Biodegradable synthetic materials are briefly reviewed here in the context of mucosal vaccine delivery.
The well-known family of biodegradable polyesters that include poly (lactic acid) (PLA), poly (glycolic acid) (PGA), and the accompanying copolymer (DL-lactide-coglycolide) (PLGA) have been extensively studied and used in various biomedical applications and FDA-approved products, as reviewed elsewhere (Kapoor D.N. et al., 2015; Makadia H.K. and Siegel S.J., 2011). Delivery of BAIs from a PLGA copolymer involves bulk erosion via hydrolysis, which releases entrapped BAI. The hydrolytic erosion and the ensuing release rates of BAIs are tuned via polymer molecular weight, quantity of lactide and glycolide groups, crystallinity, and composition of endgroups. PLGA nanoparticles have been explored as carriers in a multitude of examples, including for oral and pulmonary vaccination against hepatitis B (Gupta P.N. et al., 2007; Mishra N. et al., 2011; Thomas C. et al., 2011), ocular and nasal delivery of influenza antigens (Alkie T.N. et al., 2018), and microparticles for pulmonary vaccination against tuberculosis (Shi S. and Hickey A.J., 2010). Another biodegradable polyester, Poly-(ɛ-caprolactone) (PCL), is a hydrophobic semicrystalline polymer that undergoes bulk degradation in aqueous environments. As compared to PLGA, the homopolymer of PCL typically exhibits longer timeframes for degradation and bioresorption (e.g., 2–4 years dependent on the molecular weight) (Woodruff M.A. and Hutmacher D.W., 2010). Nanoparticles comprising PCL have been explored for intranasal and intramuscular delivery of diptheria toxoid (Singh J. et al., 2006) and nasal administration of the influenza A H1N1 hemagglutinin protein (Gupta N.K. et al., 2011).
Polyanhydrides (PAs) are another class of synthetic polymers used in delivery systems (Tamada J. and Langer R., 1992). Since anhydride groups are highly susceptible to hydrolytic cleavage, polymer degradation occurs faster at surfaces exposed to aqueous environments resulting in BAI release from PA vehicles via surface erosion (Jain J.P. et al., 2008). Degradation rates of PAs vary based on the composition of the polymer, which is readily tailored by selection of monomers (e.g., aliphatic, aromatic) and use of copolymers (Jain J.P. et al., 2008). Numerous studies have used PA nanoparticles for intranasal delivery of vaccines (Irache J.M. et al., 2010; McGill J.L. et al., 2018; Zacharias Z.R. et al., 2018).
Lipid-Based NanoparticlesIn addition to polymeric nanoparticles, lipid-based nanoparticles are widely used for delivery of drugs and vaccines (Bulbake U. et al., 2017; Puri A. et al., 2009; Romero E.L. and Morilla M.J., 2011). Liposomes are one type of lipid-based nanoparticle, comprising spherical vesicles that self-assemble into a lipid bilayer around a core of BAI. The physicochemical properties of liposomes are readily customized though choice of amphipathic lipids, which typically comprise phospholipids (e.g., phosphatidylcholine) or cholesterol. Although intramuscular injection is the common route of administration for liposomal vaccines, intranasal delivery has also been explored (Bernasconi V. et al., 2016).
Solid lipid nanoparticles (SLNs) are another category of lipid-based carriers useful for delivery of BAIs. The composition of SLNs include biocompatible materials (e.g., triglycerides, waxes), which are assembled into nanoparticles using scalable manufacturing processes, such as high-pressure homogenization (see below). SLNs show utility as mucosally-administered delivery systems, as demonstrated with SLNs comprising carnauba wax for intranasal vaccines against tuberculosis (Stylianou E. et al., 2014) and as intranasal carriers of the HIV-gp140 antigen (Arias M.A. et al., 2011).
The association of BAIs within these nanoparticle systems is schematically shown in Fig. 1. The hypothetical location of the antigen and any molecular adjuvant within a nanoparticle system can vary, depending on the material composition and method of manufacture. Fig. 1(a) shows the embedding of the antigen in a polymer matrix resultant of a solvent evaporation technique of the in situ polymerization approach. Fig. 1(b) illustrates surface associated antigen that would occur as a result of passive physical adsorption or using covalent binding at the surface. Fig. 1(c–d) show the potential combinations of antigen and molecular adjuvant (often bacterial protein or cell wall component). Fig. 1(c) and (d) require consideration of the release rate of both antigen and adjuvant to ensure that the kinetics of presentation to the immune system assure the required synergy. Fig. 1(e) is unlikely to offer the full benefit of the presence of the antigen since it would likely clear from the surface before significant antigen presentation occurred. Fig. 1(f) would require mechanisms of long-term attachment to the particle surface that would allow time for antigen and adjuvant presentation. The kinetics of desorption or erosion, for covalently linked molecules should not be instantaneous for this structure to be useful. In general, the examples shown in Fig. 1(a) to 1(d) are more practical than 1(e) and 1(f).
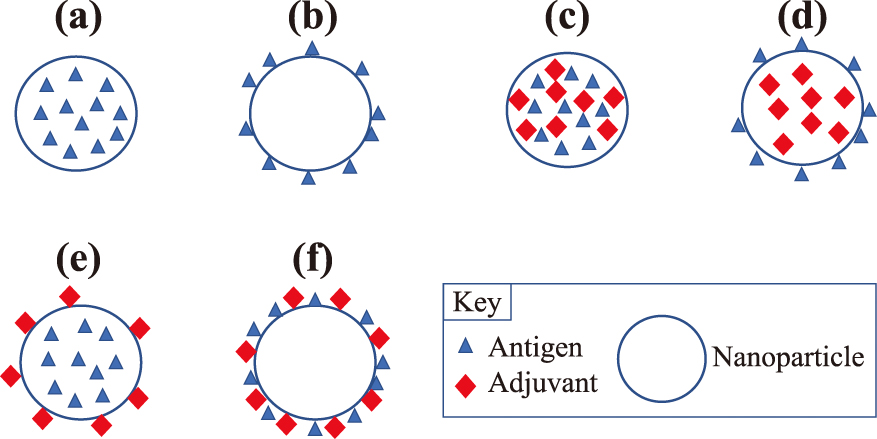
Schematic of general structure of nanoparticles with (a) matrix embedded antigen; (b) surface antigen; (c) matrix embedded adjuvant and antigen; (d) matrix adjuvant surface antigen; (e) matrix antigen surface adjuvant; and (f) surface antigen and adjuvant.
Development of nanoscale materials is considered a modern technology, but their first uses can be traced back to the 4th century (NNI, 2019). In more recent history, Michael Faraday described the optical properties of nanometer-scale metals in 1857 (Faraday M., 1857), and over fifty years later, in the first years of the 20th century, Beilby (Beilby G.T. and Neville F.H., 1904) and Turner (Turner T. and Poynting John H., 1908) separately demonstrated that visibly transparent highly conductive metallic films could be fabricated using Faraday’s methods. Nanoscale materials development gained momentum 42 years later, when La Mer and Dinegar published a process to grow monodisperse colloids, in 1950 (Lamer V.K. and Dinegar R.H., 1950), with nanoscience finally being internationally recognized later in the 20th century with the 1985 Nobel Prize for the development of the “buckyball” by Richard Smalley and his team at Rice University (Heath J.R. et al., 1985; Kroto H.W. et al., 1985; Zhang Q.L. et al., 1986). One year earlier, Bell Labs’ Louis Brus developed semiconductor nanocrystals (Brus L.E., 1984), known as “quantum dots”, which was later awarded the 2008 Kavli Prize in Nanotechnology. Completing the major developments in nanoscale materials in the 20th century, NEC’s Sumio Iijima developed the carbon nanotube in 1991 and shared the 2008 Kavli Prize with Brus.
Since that time, synthetic nanoscale materials have seen continuous development using two major strategies: top-down/mechano-physical or bottom-up/chemo-physical methods. Top-down methods are generally subtractive techniques, where larger materials are ground down to smaller materials through milling or grinding. Conversely, bottom-up techniques arrange smaller constituents into larger and more complex assemblies. This latter approach enables the use of building blocks such as individual molecules that can self-assemble into larger organized arrays or monomers that can be polymerized into more targeted conformations and architectures such as nanoparticles for drug delivery (Antonietti M. and Landfester K., 2002; Kumari A. et al., 2010; Limayem I. et al., 2004; Reis C.P. et al., 2006). The nanoparticle formation process can be achieved through numerous techniques and processes that often include self-assembly and/or molecular-assembly polymerization techniques. Some of the more widely used methods involve microfluidics, dispersion of pre-formed polymers and polymerization of monomeric polymer pre-cursors.
Microfluidics is a technique that utilizes miniaturized systems to process extremely small volumes of fluids (as little as 10−14 ml), by means of flow channels with dimensions from ten to several hundred microns. A paper published in Nature in 2006 discussed how this emerging technique could be leveraged in molecular biology, pharmaceutical manufacturing, molecular analysis, biodefence, and microelectronics (Whitesides G.M., 2006). Over 12 years later, microfluidics has been utilized as a processing technique in many different areas, including the production of nanoparticles (Baroud C.N. et al., 2010; Beebe D.J. et al., 2002; Di Carlo D., 2009; Lee J.N. et al., 2003; Lin Y.W. et al., 2015; Stroock A.D. et al., 2002). The microfluidic technique enables the rapid mixing of reagents and precise control of temperature and reagent dynamics, resulting in the production of a much narrower size distribution than bulk synthesis methods (Lim J.M. et al., 2014). The fine control and high degree of homogeneity provided by the microfluidic synthesis are responsible for the resulting smaller particle size and lower polydispersity (Valencia P.M. et al., 2012). Typically, microfluidic synthesis is used to produce small volumes of nanoparticles, but some high throughput systems have been developed that are capable of scale-up (Lim J.M. et al., 2014).
Several nanoparticle manufacturing methods have been developed that involve the controlled dispersion of preformed polymers. Solvent evaporation was one of the first methods developed to form nanoparticles from a preformed polymer (Vanderhoff J.W. et al., 1979). Further development has been fostered by pharmaceutical chemists in the production of drugs (Gurny R. et al., 1981). This method uses solutions of polymers in volatile organic solvents into which the drug is loaded and dispersed. To this organic phase, water and surfactant are added and the complex mixture is agitated to from an emulsion (Allemann E. et al., 1993; Anton N. et al., 2008; Beilby G.T. and Neville F.H., 1904). Upon evaporation of the organic solvent from the emulsion, via agitation or reduced pressure, a nanoparticle suspension is formed. Emulsion methods can employ a typical single emulsion route involving an oil-in-water approach (o/w), as described above, or a double emulsion (water-in-oil) in-water (“W/O/W”) approach. In either case, the aqueous phase contains the stabilizer/surfactant and the organic solvent contains the pre-formed polymer phase. Both methods require high shear agitation methods, such as ultrasonication or homogenization, to ensure that the emulsified reaction contains dispersed domains on the nanoscale, as these become the nanoparticles upon removal of the organic phase by evaporation under reduced pressure or via extended stirring. The resulting nanoparticles can then be removed and collected from the aqueous phase using ultracentrifugation or lyophilization. Scale-up of this approach can be challenging due to the need for high shear agitation and the requirement that the drug be oil-soluble. A second method that utilizes pre-formed polymers has been termed the nanoprecipitation or the solvent displacement method (Fessi H. et al., 1989). This method involves the interfacial deposition of a polymer via the displacement of a semi-polar water-miscible solvent such as ethanol or acetone which can be easily removed by evaporation with continued stirring (Mishra B. et al., 2010; Reis C.P. et al., 2006). The surfactant again resides in the aqueous phase and a narrow size distribution can be achieved by slow addition of the organic phase to the surfactant-containing aqueous phase, or vice-versa, while the size of the nanoparticles is a function of the type of surfactant used (i.e., ionic, amphiphilic or non-ionic) (Fessi H. et al., 1989; Limayem I. et al., 2004). The natural pre-formed polymers chitosan and alginate require a different synthesis method involving ionotropic gelation. To prepare chitosan nanoparticles, chitosan is acidified to a pH of 4–6 to render it water-soluble to which an alkaline solution of sodium triphosphate (pH 7–9) is added. To produce a high yield of stable solid nanometric structures, the chitosan to sodium triphosphate weight ratio should target a range of 3:1 to 6:1. The synthetic conditions required to produce a high yield of nanoparticles with a targeted nanoscale size vary significantly as a function of the purity, acid-salt form and overall molecular weight of chitosan utilized, which requires that the formulation parameters be optimized for each chosen chitosan type (Janes K.A. et al., 2001). Alginate particle formation is triggered by divalent metal containing salts, such as calcium chloride. Like chitosan, the reaction to form alginate nanoparticles of targeted size involves precisely controlling the concentration/viscosity of the alginate solution, counter-ion concentration, and the speed of its addition to the alginate, making it difficult to replicate the resulting particles with the degree of precision that other techniques afford (Kaloti M. and Bohidar H.B., 2010). One way to mitigate the synthetic challenges that chitosan and alginate pose is to use them as surface modifiers in pre-formed nanoparticles made from a different polymer. Using this approach, researchers were able to decorate the surface of PLGA-based nanoparticles with cationic chitosan and anionic alginate to facilitate the controlled release of resveratrol (Sanna V. et al., 2012) using a layer-by-layer nanoprecipitation method (Zhou J. et al., 2010). Images of particles produced via this approach are shown in Fig. 2. PLGA-based nanoparticles, like related PLA, Poly-L-Lactic Acid (PLLA), and PCL and polyanhydride nanoparticles, release their cargo via a hydrolysis mechanism.
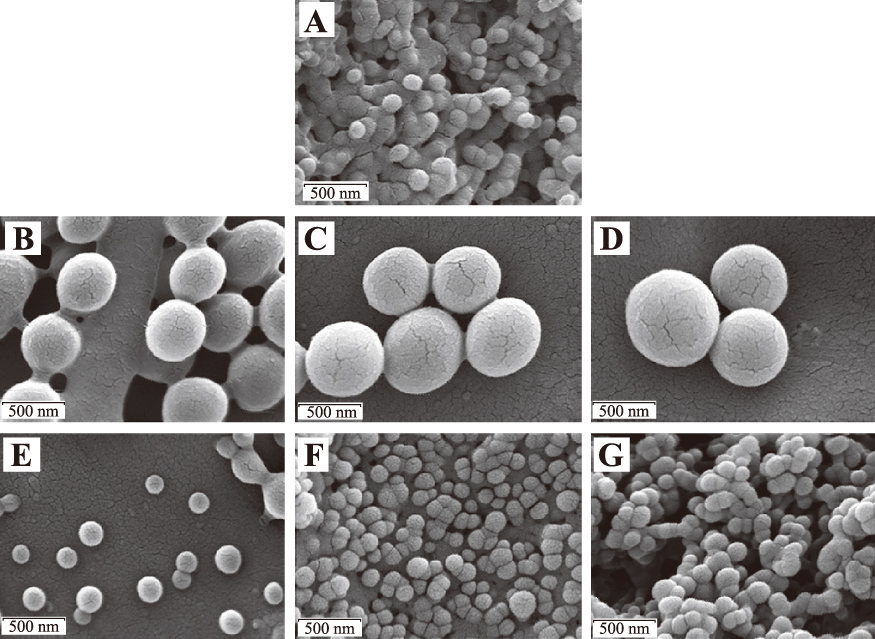
Scanning electron microscopy images of PLGA (poly(d,l-lactide-co-glycolide)) (A), PLGA-CS1 (chitosan) (B), PLGA-CS2 (C), PLGA-CS3 (D), PLGA-Alg1 (alginate) (E), PLGA-Alg2 (F), and PLGA-Alg3 (G) Resveratrol-loaded nanoparticles. All scale bars represent 500 nm (Sanna V. et al., 2012). Reprinted with permission from the original publisher Dove Medical Press LTD.
When exposed to water, the polymer chains are hydrolyzed, or cut, at the ester-bond (Ikada Y. and Tsuji H., 2000) (PLGA, PLA PLLA, PCL), or anhydride-bond (Whitaker-Brothers K. and Uhrich K., 2006). This chain scission event results in degradation of the mechanical properties of the polymer, with continued hydrolysis events degrading molecular weight to the point that the products can be removed from the body and excreted (Uhrich K.E. et al., 1999).
Most of the methods used to prepare polymeric nanoparticles use organic solvents. In order to minimize risks associated with their use, supercritical fluids can be substituted (Aboubakar M. et al., 1999). Supercritical fluids possess a critical temperature, above which the fluid remains a fluid, regardless of pressure. Supercritical carbon dioxide (SCO2) is used very frequently due to its relatively mild supercritical conditions of 31.1 °C and 72.9 bar (1057.3 psi). The low polarity of SCO2 facilitates high solubility of hydrophobic drugs which can then be sprayed through a capillary in a method called the Rapid Expansion of Supercritical Solution (RESS) process (Larson K.A. and King M.L., 1986). The rapid expansion of the solution into an ambient environment results in the generation of nanoparticles. This method was first developed for inorganic particles before being used with pre-formed polymeric materials (Al-Kassas R. et al., 2017; Anton N. et al., 2008; Padrela L. et al., 2018; Williams H.D. et al., 2013). Conversely, SCO2 can also be used as an anti-solvent, or precipitant, during the nanoparticle fabrication process. This method is applicable to drugs that do not dissolve well in SCO2 in a method called the Supercritical Anti-Solvent Process (SAS), where the SCO2 is passed through the drug solution. Addition of SCO2 expands the organic drug solution, causing the drug to precipitate, thereby leaving the precipitated nanoparticles free from any residual solvent (Reverchon E. et al., 2007).
In contrast to the methods described above that utilize pre-formed polymers, polymerization of monomeric materials provides a much greater degree of control over the final properties of the produced nanoparticle. Emulsion polymerization, a free-radical chain growth method, is the most common route used in global production of polymers and is also a widely used nanoparticle production route. In conventional emulsion polymerization, water is used as the dispersion medium, a surfactant is used to disperse the water-insoluble monomers in the water and a water-soluble free radical initiator is used to trigger the polymerization chain reaction (Thickett S.C. and Gilbert R.G., 2007). The conventional emulsion approach typically results in particles that are in the range of 50–250 nm (Asua J.M., 2004; Garay-Jimenez J.C. et al., 2009; Reis C.P. et al., 2006). The use of surfactants with this approach requires the removal of the surfactant materials from the final product that can be costly at scale. To address this, surfactant-free emulsion techniques have been developed where the initiator is water soluble and the monomers are typically vinylic or acrylic. While this approach is attractive as a polymerization method, nanoparticle size and polydispersity are more difficult to control, due to the absence of surfactant (Zhang G.Z. et al., 2001). In contrast, the mini-emulsion polymerization technique also utilizes water as the dispersion medium, as well as water-soluble surfactants and water-insoluble monomers. This approach differs in its use of a high shear device, such as an ultra-sonicator, and low a molecular weight co-stabilizer, due to the high interfacial surface tension of the system. For the polymerization of methacrylate (Mouran D. et al., 1996) and n-butylacrylate (Leiza J.R. et al., 1997), sodium laurylsulfate/dodecylmercaptan and sodium dodecylsulfate/hexadecane was used, respectively, as the surfactant/co-stabilizer systems. Micro-emulsion polymerization, while similar to the mini-emulsion approach, produces particles of smaller size and with fewer polymer chains per particle. In some cases, particles as small as 5–50 nm can be produced using this technique (Antonietti M. and Landfester K., 2002). Despite the potential of this nanoparticle synthesis method, micro-emulsion has seen limited commercial use due to the dilute concentration of polymer and the use of surfactants that typically exceed the monomer concentration in this polymerization approach. Interfacial polymerization is a step growth polymerization approach that can be used to generate nanoparticles (Aboubakar M. et al., 1999; Vauthier C. et al., 2003). Interfacial polymerization involves the step-growth polymerization of two monomers that are soluble in two phases, one dispersed and one continuous, where the reaction occurs at the interface of the two liquid solvents (Karode S.K. et al., 1998). Nanocapsules containing an oil-based core can be formed (Fallouh N.A. et al., 1986), as can nanocapsules with an aqueous core (Gasco M.R. and Trotta M., 1986; Watnasirichaikul S. et al., 2000). Though the production of polymer-based nanoparticles has been well demonstrated, precise control of nanoparticle size and polydispersity remain as challenges with this polymerization technique.
From the brief review described above, it can be understood that neither the top-down nor the bottom-up synthetic approach is preferable when scale-up to manufacturing volumes is considered. Rather, the applicable synthetic technique is driven by considering the solvation conditions required to properly drive the cargo into the encapsulation system. Therefore, solvent considerations, in tandem with nanoparticle dimensional requirements, ultimately drives the choice of the synthetic scale-up approach.
2.4 Methods of nanoparticle characterizationThe quality of the particulate system employed to deliver antigen and elicit an immune response must be assured and depends upon the particle size distribution, the inoculum (dose), and morphology (shape and surface characteristics). Specialized techniques are required to assess each of these features. For nanoparticle systems the primary method of particle size determination is dynamic light scattering (DLS). This is typically the first technique used to characterize the nanoparticles and provides information about their size and size-distribution, with the latter typically referred to as the particle polydispersity. Results from the DLS method can be qualitatively confirmed by transmission (TEM) and scanning electron microscopy (SEM), which also allows assessment of the morphology and shape of the nanoparticles. SEM generates an image of the particles by scanning it with a focused beam of electrons which, when scattered, gives information on surface morphology and surface composition. TEM accelerates electrons to nearly the speed of light and transmits them through the sample in a fashion similar to an optical light microscope. This technique provides information about the morphology of the sample, as well as information about the internal composition of the particle to identify crystallinity and magnetic domains. Atomic force microscopy (AFM) provides information about the particle surface via the use of a sub-micron probe tip. Using the probe tip in contact mode, a topographical map of the particles can be generated. Using non-contact mode requires the tip to hover over the sample area of interest and is very useful for analyzing fragile biological and polymeric nanostructures (Mu L. and Feng S.S., 2003).
A variety of surface analytical methods can be performed and the release of the antigen and adjuvant can be conducted in dissolution media under appropriate conditions (pH, simulated gastric or pulmonary fluid) for the route of administration with a relevant analytical method. Methods include mass spectroscopy and electrophoretic approaches, such as sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
In order to be capable of engaging the key elements of innate and adaptive immunity, antigens must be processed and presented to immune cells. Antigen presentation is mediated by MHC class I molecules, and the class II molecules found on the surface of antigen-presenting cells and certain other cells. The Major Histocompatibility Complex-Class I (MHC-I) processing pathway is largely geared toward presentation of endogenous antigens encountered in the cytoplasm of most cell types. Peptides derived by cytosolic proteolysis of such antigens are translocated into the endoplasmic reticulum where they encounter and may assemble with newly synthesized MHC-I molecules. MHC class II molecules are expressed by antigen presenting cells, such as dendritic cells and macrophages. MHC class II molecules bind to peptides that are derived from proteins degraded in the endocytic pathway (Mantegazza, A.R. et al., 2013).
The most common routes of administration for vaccines are parenteral, notably intra-muscular, intra-dermal or sub-cutaneous. However, there is increasing interest in mucosal routes of administration (Youngren-Ortiz, S.R. et al., 2017). Targeting of M-cells in the nasal mucosa, macrophages and dendritic cells in the lungs, and Peyer’s patches in the gastro-intestinal tract have been studied extensively in the last 25 years (Calderon-Nieva D. et al., 2017; Jia Y. et al., 2015; Padilla-Carlin D.J. et al., 2008). It is generally believed that nanoparticles are the most effective way to target the antigen presenting cells and the cells of the phagocytic mononuclear cell system (Fig. 3). There are safety considerations related to the use of nanoparticle therapeutics that are beyond the scope of the present review (Somasundaran P. et al., 2010; Sayes C.M., 2014).

Schematic depiction of antigen presenting cell interaction with pathogen/vaccine particulate subsequent cell response. Grey shading indicates antigen processing pathways by the lysosome or following lysosomal escape promoting MHC-II and MHC-I antigen presentation.
The innate immune system provides the first line of defense against foreign microbes, particularly on mucosal surfaces, skin and at other interface areas. The mononuclear phagocytic system, or reticuloendothelial system, is characterized by monocytes and macrophages. The cell types are often identified specifically by historical reference, e.g. Kupfer or Langerhans cells in liver and skin, respectively, or anatomical location, e.g. alveolar or peritoneal macrophages. Phagocytic cells are responsible for the initial uptake and clearance of pathogens and exogenous debris. They are considered housekeeping cells as their role is to maintain the integrity of the physiological milieu. A variety of processes are mediated by phagocytic cells. These phagocytic cells are complemented by ubiquitous antigen presenting, dendritic cells. Thus, monocytes, macrophages, neutrophils and other granulocytes, Kupfer and Langerhans cells, and dendritic cells are key players in the innate immune response. A variety of processes are mediated by phagocytic cells. In the context of eliciting an immune response, these cells are active against infectious materials through two major pathways: Phagolysosomal trafficking and the production of cytokines and chemokines. Phagolysosomal trafficking initiates intracellular killing of internalized pathogens through targeted production of reactive oxygen and nitrogen species, while cytokine and chemokine secretion such as TNF-α and IFN-γ can kill pathogens by recruiting and inducing specific cytotoxic immune cells (Newton A.H. et al., 2016; Ritchie A.I. et al., 2016).
Furthermore, the structure and composition of the adsorbed vaccine targets on the nanoparticle surfaces could be important for optimal MHC I and MHC II antigen presentation. In this regard, nanoparticles may elicit responses after surface interactions or after becoming internalized by the various phagocytic cell types. Nanoparticle characteristics such as size, shape, composition, degradation profile and overall consistency may influence uptake and subsequent immune responses. For example, it has been shown that TiO2 nanotubes with a diameter of 30 nm produced less inflammation compared to 80 nm (Lu W.L. et al., 2015). Alternatively, similar surfaces with a diameter of ~78 nm attenuated the inflammatory activity of macrophages (Ariganello M.B. et al., 2018). Therefore, outcomes cannot simply be predicted based only on the size or surface features, but rather nanostructured topography must undergo an evaluation to obtain a reliable indication of inflammatory potential (Fig. 3).
In addition, complement activation and the inadvertent binding of lipopolysaccharide to nanoparticles are important to consider as this may skew the outcome and interpretation of studies and have an impact on safety. Thus, a better understanding of the induction mechanisms for the specific processes could pave the way for improved vaccination strategies via nanoparticles.
3.3 Acquired immunityAcquired or adaptive immunity is driven by T and B-lymphocytes, the former generating natural killer and other T-cells, and the latter producing antigen specific antibodies. This component of the immune system results in a memory of previous exposures that generates protective immunity to future infections. Additionally, citrate-buffered silver coated nanoparticles were shown to be phagocytosed by dendritic cells and macrophages, either derived from the peripheral lymph nodes or as cell lines (Njoroge J.M. et al., 2018). Work with the J774 mouse macrophage cell line demonstrated that the endocytosis of 57 nm PEG-treated silver-coated nanoparticles is dependent upon actin and clathrin-mediated endocytosis (Kuhn D.A. et al., 2014). Also, 24-hour exposure to these nanoparticles will induce elevated oxidative stress as demonstrated by ROS formation in Jurkat T cells (Manke A. et al., 2013). U937 human monocytic cells exposed to polyvinylpyrrolidone-treated silver nanoparticles demonstrated apoptotic cell death and ROS formation, however, the cellular effects were dependent upon the size of the particle (Abdal Dayem A. et al., 2017). Other studies using primary human monocytes have similarly demonstrated a particle size-dependent effect. In these studies, the smaller coated nanoparticles induced greater levels of the pro-inflammatory cytokines (Elsabahy M. and Wooley K.L., 2013). Active uptake of nanoparticles by alveolar macrophages is enhanced by their presentation in micron-sized aggregates whereas nanoparticles are preferentially taken up by DCs in the respiratory tract.
As described earlier T cell activation by coated nanoparticles is impacted by the size, other physical aspects, and the ability of the nanoparticles to stimulate innate immunity. However, many questions remain for therapeutic vaccine development requiring acquired immunity. For example, it is critical to determine how to promote a T effector response, but not a T regulatory response, in an immunosuppressive microenvironment. Fine-tuning the balance between the strong T cell responses and auto-immune side effects will also be a key challenge.
3.4 VaccinesThe strongest immune response will be raised to whole organisms that present all antigens such that a robust heterogeneous immunity can be achieved. Live virulent organisms typically generate natural immunity in immunocompetent individuals. The risk of infection is such that living organisms are usually attenuated in terms of virulence to give the host immune system an advantage. An alternative that preserves the entire antigen library but mitigates risk entirely is the use of whole inactivated organisms.
The use of nanotechnology in vaccinology has been leading to the birth of “nanovaccinology” (Zhao L. et al., 2014). In both prophylactic and therapeutic approaches, nanoparticles are used as either a delivery system to enhance antigen processing and/or as an immunostimulant adjuvant to activate or enhance immunity. Therapeutic nanoparticle vaccines currently are mostly being applied to cancer treatment (Krishnamachari Y. et al., 2011), and less for treating infections. Alternatively, prophylactic nanoparticle vaccines have been applied for the prevention of different diseases, particularly infectious diseases.
3.5 Nasal and pulmonary vaccine deliveryNanovaccines can be delivered to the mucosal surfaces in the respiratory tract as liquid suspension or micro-aggregated particles (Irvine D.J. et al., 2015). There is a long history of nasal delivery of vaccines (Arouet F.M., (Voltaire), 1733). Deposition in the nose is efficient with particles greater than 5μm being easily collected. A suspension of nanoparticles might be delivered from a nasal spray with a droplet median diameter of approximately 20μm, FlumistTM (Medimmune) is an example of this strategy (Yusuf H. and Kett V., 2017). The concentration and stability of the nanoparticle suspension will dictate the nature of presentation to local immune cells (as nanoparticles or nanoparticle aggregates). However, an ideal suspension product would be formulated to allow a stable homogeneous dispersion of nanoparticles. Pulmonary vaccine delivery required particle and droplet sizes below 5μm (Hickey A.J., 2004). Nebulized droplets containing nanoparticles or dry micro-aggregated particles can be used to deliver vaccines to the pulmonary mucosa (Osman N. et al., 2018).
One area of current interest is the use of the lungs as a route of administration for vaccines to prevent tuberculosis. MTB is the primary causative organism of this disease. The mycobacteria present antigens that elicit natural immunity and can therefore be used for immunization. A variety of approaches have been attempted including attenuated whole organism (attenuated Mycobacterium bovis, Bacille Calmette Guerin) (Garcia-Contreras L. et al., 2008), sub-unit proteins (Lu D. et al., 2007), fusion proteins (Shi S. et al., 2010,) and viral vectored genes (Tameris M.D. et al., 2013). The whole organism has one dimension in the nanoscale and viral vectors are nanosized. It may be possible to adopt a nanoparticle strategy to deliver mycobacterial antigens to the lung mucosa and protect against disease. In this context the M. tuberculosis heparin-binding haemagglutinin antigen has been adsorbed to wax particles and shown to successfully immunize mice (Stylianou E. et al., 2014). Delivery of particles of this nature to the lungs may be a means of inducing an enhanced innate immune response.
Vaccines for preventing or treating serious infectious diseases have been employed for millennia. The emergence of knowledge in microbiology and immunology at the end of the nineteenth century drove eradication of several otherwise deadly diseases. Research in the twentieth century improved vaccines and expanded their application to many otherwise untreatable viral diseases. However, some diseases have resisted significant vaccine development:—Prominent among these are mycobacterial diseases, such as tuberculosis and viral diseases such as influenza. The advent of nanoparticle technology and experience with the delivery of antigen and adjuvant in or attached to an organic matrix material potentially advances vaccine development. Moreover, interest in targeting the cell mediated immune system located at accessible nasal and bronchial mucosal sites of the respiratory tract offers a strategy for disease prevention that has rarely been considered and might be advanced using nanotechnology.
Leah M. Johnson
Dr. Leah Johnson is a Senior Research Chemist in the Engineered Systems (ES) Division at RTI International. Dr. Johnson leads a research team to develop advanced polymeric materials for controlled delivery applications. She has experience with designing polymeric systems for applications in biosensing, tissue engineering, and drug delivery, with recent programs involving subcutaneous implants for prevention of infectious diseases, controlled-release dental materials, and polymeric implants for islet transplantation. In her role at RTI, she collaborates with various groups from private industry, academic institutions, and government agencies to advance material systems to early stage technical products. Dr. Johnson received her B.S. (Biochemistry) from the University of Michigan and her Ph.D. (Biochemistry) from the University of Colorado. She held a Postdoctoral Fellowship at Duke University (Biomedical Engineering), where she developed a bio-separation processes for acoustic-mediated rare cell isolation and triggered-release systems for tissue engineering. She previously held positions at Pfizer working in the nuclear magnetic resonance (NMR) laboratories and at Gilead Sciences where she researched biological pathways of new pipeline antiretroviral (ARV) drugs and led small molecule screening campaigns for ARV discovery efforts.
Jeffrey B. Mecham
Dr. Mecham is a Research Chemist in RTI International’s Center for Engineered systems, in the Technology Advancement and Commercialization Division. In this capacity, he is responsible for the design and synthesis of monomers, polymers and particles (at the micro- and nanoscale) with targeted performance capabilities for a wide array of applications. His team are contributors to the Center’s successful development of particle-based controlled release technologies for triggered release of therapeutics and chemistries for environmental remediation. The development of such materials over the last several years at RTI has resulted in IP that has been licensed by interested parties for further development and subsequent commercialization of particles with controlled release properties.
Frederick Quinn
Dr. Quinn earned a BS from Marquette University, a MS and PhD in Microbiology and Biochemistry from Indiana University, Bloomington and completed a postdoctoral fellowship in Microbial Pathogenesis at Stanford University. He oversaw several laboratory groups at the Centers for Disease Control and Prevention in Atlanta investigating bacterial disease outbreaks including Brazilian Purpuric Fever, Cat Scratch Disease, meningococcal meningitis, Buruli ulcer, and ultimately tuberculosis (TB). In 1999, Dr. Quinn completed a Fullbright Fellowship studying TB pathogenesis at the University of Bristol in Great Britain. In 2002, he became Professor and Head of the Department of Medical Microbiology and Parasitology (now Infectious Diseases) in the College of Veterinary Medicine at the University of Georgia. His research focuses on understanding the pathogenesis of Mycobacterium tuberculosis, with the ultimate goal of developing improved vaccines and diagnostic tests for TB. Current collaborative activities include novel TB vaccine development and animal efficacy testing, animal model development for disease transmission, and understanding TB social networking and zoonotic transmission patterns in TB endemic areas including Africa.
Anthony J. Hickey
Dr. Hickey is Distinguished RTI Fellow, at the Research Triangle Institute and Director of the UNC Catalyst for Rare Diseases of the Eshelman School of Pharmacy He obtained Ph.D. and D.Sc. degrees in pharmaceutical sciences from Aston University, Birmingham, UK. He is a Fellow of the Royal Society of Biology, the American Association of Pharmaceutical Scientists, the American Association for the Advancement of Science and the Royal Society of Biology. He received the Research Achievement Award of the Particulate Presentations and Design Division of the Powder Technology Society of Japan, the Distinguished Scientist Award of the American Association of Indian Pharmaceutical Scientists; the David W Grant Award in Physical Pharmacy of the American Association of Pharmaceutical Scientists; Thomas T Mercer Joint Prize for Excellence in Inhaled Medicines and Pharmaceutical Aerosols of the American Association for Aerosol Research and the International Society for Aerosols in Medicine and the Ralph Shangraw Memorial Award for Excipient and Excipient Technology of the International Pharmaceutical Excipient Consortium Foundation. He is founder (and formerly President and CEO) of Cirrus Pharmaceuticals, Inc., acquired by Kemwell Pharma; founder (formerly CSO, 2002–2007) of Oriel Therapeutics, Inc, acquired by Sandoz and founder and CEO of Astartein, Inc.; member of the Pharmaceutical Dosage Forms Expert Committee of the United States Pharmacopeia and formerly Chair of the Aerosols Expert Committee of the USP. Dr. Hickey conducts a multidisciplinary research program in the field of pulmonary drug and vaccine delivery for treatment and prevention of a variety of diseases.