2021 Volume 38 Pages 269-280
2021 Volume 38 Pages 269-280
Extension of photocatalytic activity within the visible light range has an immense importance on the ability of manufacturing self-cleaning textiles that are active indoors. This study focuses on assisting problems, which have delayed the commercialization of the photo-catalytically active textiles by following innovative technological developments in nanotechnology. Polymeric additives are utilized to prepare composite photocatalytic particles with the ability of extending light absorption in the visible light range. Techniques are introduced to avoid the deterioration of the composite particles during their application on textiles in addition to uniform coating strategies to enable an optimized concentration for improved photocatalytic efficiency. It is demonstrated that the titania (TiO2) particles in anatase form extended absorption in the visible light range in the presence of branched titania particles. Correspondingly, an optimized dip coating process is evaluated for textile manufacturing, providing a systematic methodology to enable the production of self-cleaning textiles to be able to manufacture them with commercialization potential.
Increasing environmental pollution, inevitable mass consumption of raw materials and, the most recently faced challenges in hygiene standards due to the Covid-19 pandemic keep forcing societies to make smart use of resources, including textiles. Notably, the high ecological cost of the conventional cleaning methods utilized for textiles encourages the market to introduce new and more technologically smart products. Photocatalytically active materials are known for their efficiency in self-cleaning, and their implementation can promote fast sanitization in the cases of efficient cleaning as well as sanitization against the spread of diseases (Saunders-Hastings et al., 2017). One fundamental challenge remains to be promoting the photocatalytic activity in the visible light range to enable applications indoors through understanding nano-technology principles and the photocatalytic activity of the selected additives.
Catalysts are substances that can accelerate reactions through lowering the free activation enthalpy of the reaction (Wubbels, 1983). Accordingly, photocatalysis can be defined as a photoreaction in the presence of a catalyst where a chemical modification of one molecular entity results from the initial absorption of radiation by another molecular entity called a photosensitizer. The catalyst might speed up the photoreaction by interaction with the substrate in its ground state or with a primer photoproduct (Castellote and Bengtsson, 2011). The nature of photocatalysis is based on solid-state chemistry and knowledge on the crystal structure. Hence, nonstoichiometric and any other alternative imperfections such as impurities that can act as dopants that change the electronic properties affect the photocatalysis functionality of materials (Castellote and Bengtsson, 2011). The most commonly utilized photocatalytic materials are zinc oxide (ZnO), cadmium sulfide (CdS), iron(III)oxide (Fe2O3), tungsten trioxide (WO3), tin oxide (SnO2), zinc sulfide (ZnS) and titanium dioxide (TiO2) (Cerhan et al., 2018; Dumitrescu et al., 2015). It is also common to use blends of these primary compounds in manufacturing. Among all the alternatives, titanium dioxide (TiO2) is the most commonly used photocatalyst for the textiles due to its superior performance and light color that is the most suitable for the manufacturing of the optic white textile products (Patra and Gouda, 2013). TiO2 has three polymorph forms varying in crystal structures as rutile, brookite, and anatase. When considering the enhancement of the photocatalytic activity, surface structure, composition, atomic and molecular scale surface dynamics, as well as the electronic properties play an important role. It is necessary to evaluate the adsorption and bonding of atoms and molecules on the surfaces to understand the mechanisms of self-cleaning ability. Among the polymorphs of titanium dioxide, anatase is the most thermodynamically stable form of titania amid the three nanocrystal types. The anatase powders with higher crystallinity are favored for photocatalysis since higher crystallinity provides a smaller quantity of defects acting as recombination sites in the middle of photogenerated electron and hole combination (Filippo et al., 2015). It is more active than the rutile phase as a photocatalyst, although its band gap value, 3.27 eV, is higher than the rutile’s bandgap, which is 3.05 eV (Filippo et al., 2015; Hümmelgen, 2012; López and Gómez, 2012). Anatase powders tend to agglomerate despite the high photocatalytic performance (Kiwi and Rtimi, 2016; Qiu et al., 2012). The commercial Degussa-P25 powder contains anatase and rutile forms blended in a ratio of 3:1 and has a bandgap energy of 3.26 eV (López and Gómez, 2012). P-25 titania is commonly used as a benchmark for photocatalysts (Ohtani, 2008; Rui et al., 2014).
The challenge of standard photocatalytic semiconductor activity is known to be the fact that it takes place in the ultraviolet light range. In order to enhance the photocatalytic activity into the visible spectrum the formation of bonds between photocatalytic particles and pure elemental dopants or synthesized particles are needed. Beyond the standard dopants (Cerhan et al., 2018; Dumitrescu et al., 2015) advanced particles are introduced in this study named as branched particles with controlled synthesis and particle size distribution. The core-shell particle decorated with polymeric branches can actively control the binding event by pairing ion, metal complex recognition, and hydrogen-bond recognition (Randazzo et al., 2012).
Application of the visible light range photocatalytic activity enhancing nano-systems on textiles requires modifications to the textile manufacturing processes. Manufacturing of self-cleaning textiles at an industrial scale requires the integration of the selected photocatalytic system to the standard coating processes. Traditionally, this will be performed by following two steps; (i) development of photocatalytic additives that can be added into the selected finishing solutions without settling and, (ii) enabling their effective and homogeneous coating on the textiles at the optimized dosages. It is agreed that self-cleaning textiles with photocatalytic activity should maintain high-quality textile standards with the application of functionalized films promoting the increase in the potential market applications and meeting the variations in demands. Self-cleaning textiles decorated with photocatalytic materials possess the self-cleaning property, and hence they can actively clean themselves without a need for conventional laundry. This attribute provides significant benefits concerning the prevention of environmental pollution and cost savings in addition to the spontaneous active cleaning of the textiles even throughout their utilization before and after washing. Consequently, many scientific studies conducted on the textiles are linked to the desire to produce eco-friendly photocatalysis reactions. Although there are potential solutions proposed to widen the diversity of applicable photocatalytic materials, there is still room to perfect the applications due to the variation in the demand and the limitations in the activity within the visible light range (Kiwi and Rtimi, 2016).
In this study, photocatalytic materials and self-cleaning textiles are evaluated in the context of composite nano-scale additives promoting secondary bonds to adjust initial energy levels for enhanced photocatalysis action. Titanium oxide particles in anatase crystalline structure were made into composite photocatalytic agents through polymeric additives names as branched titania were coated on textiles in DI-water as well as standard finishing solution to support the implementation of the final combination suitable for self-cleaning textile standards. A commercial titania (Degussa P25) coating was compared to the anatase with and without the polymeric branching at various selected concentrations to optimize photocatalytic activity, particularly under visible light. The branched titania particles were synthesized and characterized for particle size and stability in addition to optimization of the most effective coating concertation for photocatalytic activity by analyzing absorbance and stain removal ability in solutions as well as post coating on textiles.
The procedure for the synthesis of branch titania particles is adapted from an earlier study by S.A. Simakov and Y. Tsur. The technique is based on the hydrolysis-precipitation procedure, by using TiOSO4·2H2O as a precursor and water as a hydrolyzed preparation medium. Diethylene glycol monomethyl ether (DEGME) was used as a surface modifier to form the desired branches utilized for stability as well as an enhanced attachment onto the textile surface. The necessary chemicals were obtained from Sigma-Aldrich and used as received to conduct the particle synthesis reactions.
The advanced textiles utilizing nanotechnology in the coating processes, including the self-cleaning textiles, are generally preferred to be manufactured from either cotton or elastin type fibers and their blends (Akbar and Bahar Basim, 2019). Thus, the textile was selected to be a blend of 35 % elastin and 65 % cotton for the coating procedure. In addition, a hydrophobic finishing solution was preferred by the manufacturer’s recommendation based on its common implementation in textile production. The selected finishing solution is composed of Setasil KF 1320 (amino-functional micro silicon emulsion) Setas Chemicals, Serisoft 210 (non-ionic softener) Serboy Chemicals, Walline PE (polyethylene) Geochem Chemicals and acetic acid (Sigma-Aldrich for pH balance) at a total concentration of 2.7 % (w/v) in DI-water and measured pH of 3.52 (Akbar et al., 2017). These polymeric and polyethylene structures provide holding on the textile surface for additives by crosslinking (Andrienko, 2015).
2.2 Methods 2.2.1 Synthesis and particle size characterization of branched titania particlesBranched titania preparation procedure was carried out in a continuously stirred closed glass flask at room temperature by adding 20 g of TiOSO4·2H2O powder (≥ 29 % Ti Sigma-Aldrich) to 180 ml of diethylene glycol monomethyl ether solution (≥ 99 % Sigma-Aldrich) and 40 ml of DI-water. After mixing for 90 hours, the solution pH was increased by the dropwise addition of a dilute NH4OH solution diluted from ammonium hydroxide solution of 26 % (Sigma-Aldrich) to pH 10. The addition of the NH4OH solution changed the mixture into a white color suspension, and the resulting precipitates were separated by centrifugation. Following the separation by centrifugation, particles were washed three times with a diluted NH4OH solution followed by DI-water to avoid creating bonds with the counter-ions of the salt used as a ligand structure (SO4−2). The suspension was centrifuged after each washing step to separate the precipitates without significant loss of synthesized particles and to remove the counter-ions and ligand structure within the remaining solution. Finally, the precipitates were dried at 80 °C, and a white powder was obtained (Simakov and Tsur, 2007).
The baseline and synthesized branched titania particle size was measured using two methods; (i) static light scattering technique by Coulter LS 13 320 and, (ii) using an atomic force microscope (AFM). Commercially obtained anatase powder and the laboratory synthesized branched titania particles were prepared as dilute suspensions in DIW and finishing solution separately at 0.1 wt % by mixing 0.01 g powder in 100 ml DIW or solution. Since the powders are prone to agglomeration (Simakov and Tsur, 2007) suspensions were ultrasonicated multiple times until all the agglomerated particles were suspended in the solution. Consecutive particle size distribution analyses were performed by Coulter LS 13 320 until a single peak was observed on the size distribution curve, verifying that the stabilization of the suspension was achieved. Furthermore, AFM based size analyses were also conducted by further dilution of the particles in DI water and drying a drop of sample on a freshly cleaved mica surface. After drying the suspensions on the cleaved mica substrate, samples were analyzed by AFM (Nanomagnetics Ambient AFM) in contact mode by scanning 5 × 11 μm and 3 × 5 μm size images to determine the individual particle size. Particle size measurements obtained from the two techniques are compared to evaluate stability as well as the relationship to the performance on the photocatalytic activity.
2.2.2 Evaluation of photocatalytic cleaning 2.2.2.1 Optimization of photocatalytic activity of particle suspensionsIn order to evaluate the photocatalytic efficiency of various particulate systems, initial experiments were conducted by preparing suspensions and measuring their stain bleaching ability under the UV light. 10 ml solutions of photocatalytic components were prepared and mixed with 2 ml methylene blue dye (MB+) solution to make a 2 × 10−4 M concentration of the active dye. The prepared suspensions were exposed to 365 nm UV light for 5 minutes to observe the discoloration of methylene blue by the degradation of the methylene blue as a function of time. The solutions were compared for the change in color by taking pictures before and after the UV light exposure at 365 nm. The absorbance spectrums of the solutions were also attained as a function of time by using a Shimadzu UV1280 UV-visible spectrometer as methylene blue degradation occurs.
2.2.2.2 Evaluation of photocatalytic activity on textilesISO 10678:2010 standard “Determination of the photocatalytic activity of surfaces in an aqueous medium by the degradation of methylene blue” was used to determine the photocatalytic particle concentration efficiency on cleaning (Mills, 2012). This standard method requires the measurement of the rate of photocatalytic bleaching of MB+ in aqueous solution via UV/VIS spectrometry (Mills and McFarlane, 2007). The ISO 10678 standard was used to evaluate the absorbance performance of the coated textiles by measuring the efficacy methylene blue degradation in the presence of the coated textiles in solutions as they are exposed to UV light. The textiles samples treated with different coatings were cut into 10 cm2 and dipped into a beaker filled with a 2 × 10−4 M MB+ solution followed by UV exposure. A sample of the solution was collected every 20 minutes, and the absorbance values were measured to evaluate the efficiency of photocatalytic degradation as a function of time.
In addition, stain tests were performed on the coated textiles by cutting them into 10 cm2 pieces and dipping into a 2 × 10−5 M methylene blue solution for 20 seconds. The stained textiles were dried in an oven at 36 °C and exposed to UV light at 365 nm wavelength for a week. The stain bleaching was evaluated by taking pictures on daily basis. The UV light at 365 nm wavelength (Philips Fluorescent Light Bulb TL-D F15T8) was utilized during the analyses.
2.2.3 Zeta potential measurement of coated textilesThe zeta potential of the baseline and coated textile samples were measured by using Electro-kinetic Analyzer (Anton Paar SurPASS) as a function of pH of the circulating electrolyte. The textile samples were placed in a cylindrical cell with supporting disks, which have holes to allow the liquid flow. After the placement of the textile sample into the cylindrical cell, the cylindrical cell was placed in between the electrodes to bind to mobile pistons to allow variation in the distance between the electrodes and adjusting pressure applied to the sample specimen. The liquid flow was provided with a 1 mM KCl solution and the pH titration was performed by adding 50 mM HCl solution at each time interval, as directed by the Attract 2.0 software data analyzer of the SurPASS instrument. The isoelectric points of the coated textiles, which is the pH value where the zeta potential of the materials is zero, was determined based on the data attained from Attract 2.0 software. The data is plotted as a scatter graph of zeta potential as a function of pH is fitted to a polynomial correlation by utilizing MS Excel software. The isoelectric points were determined through placement of zero value for pH in the equations for each individual textile sample.
2.2.4 Bandgap energy determinationTo determine the bandgap of the photocatalytically active samples, solutions prepared with anatase and branched titania concentrations in finishing solution were deposited on the glass slides. The glass slide was used in place of textiles to benefit from transparency of the glass for spectroscopy as the textiles cannot be analyzed as substrates due to their inhomogeneous opaque structure. The chemical bath deposition method was used to deposit the prepared solutions on the ultrasonically cleaned glass substrates, and the glass substrates were dried in glass Petri dishes at room temperature. The transmittance and absorbance measurements of the deposited thin films were obtained by Shimadzu UV1280 UV-visible spectrometer. The graphs were evaluated by using OriginLab software for the thickness and bandgap energy of the films.
The light energy which initiates a photocatalytic reaction must be at least equal, or higher, than the energy bandgap. The band energy plays a role as the threshold energy for the photocatalytic reaction. The absorption method is used for the identification of forbidden band energy, simply known as the bandgap energy. In this method, specification of the forbidden band energy is evaluated from the relation between the absorption coefficient and the forbidden band energy, as given in Eq. (1).
| (1) |
where, h, v and α represent the Planck constant, the frequency, and the absorption coefficient, respectively. In this method, a graph of (αhv)n versus hv is drawn. The energy value, [(αhv)n = 0], is determined by Tauc plot through plotting to the linear portion of hv representing the bandgap energy of examined material against (αhv)n (Godet et al., 1991). When the n value in the Eq. (1) is chosen to be 2, direct bandgap energy of material can be determined. If the n value is 1/2, the determined value is defined as the indirect bandgap value of the material (Akaltun et al., 2011; Astam, 2016; Cerhan-Haink and Basim, 2020).
| (2) |
| (3) |
The maximum or minimum peak of the transmittance graph was obtained from the UV-VIS spectrometer and was used for the analysis of thin film thickness evaluation according to Eq. (2) and Eq. (3). Tm, λ, t are the maximum peak parameters of transmittance, wavelength corresponding to maximum peak center, and thickness of the film, respectively. The thickness of the thin films was calculated by substituting amplitude of the first maximum peak, Tm1, and amplitude of the second maximum peak, Tm2, values into the Eq. (2) and replacing the values in the Eq. (3). The ns represents the refractive index of the glass used to coat the nanoparticle solutions (Swanepoel, 1983).
2.2.5 Water vapor transmission rate analysis on textilesThe methods of measuring water vapor transmission rate (WVTR) represents the permeability of the coated textiles to water vapor (Hu et al., 2001). The highly permeable films are generally preferred in products for daily use such as in self-cleaning textiles. In order to provide human skin respiration continuity after the coating procedure, the water vapor transmission rate is measured through a modification of the wet cup method described by the ASTM E 96–95 standard (Hu et al., 2001). In this method, the textile sample is used as a cover on a cup filled with DI-water and the change in mass is recorded as a function of time. The weight change is due to the evaporation of water through the textile and Eq. (4) is used to determine the water vapor transmission rate.
| (4) |
The textiles were prepared for water transmittance tests by cutting 10 cm square samples to cover the top of the beakers after adding 100 ml DI-water in each beaker. The system was weighed to follow the change in mass in weekly periods under ambient conditions (22 °C and 20 % humidity-winter and the 35 °C and 50 % humidity-summer). The system mass change was recorded for 700 hours (~30 days).
The size distribution and shape of powders change the total surface area of the particles and affect the rate of reactions, stability as well as product appearance and texture. Hence, particle size measurement is one of the indicators of quality and performance of suspensions for a successful implementation of the nanoparticles on textiles. Fine particles with high total surface area speed up chemical reactions and dissolution rates while simultaneously becoming more prone to settling due to the loss of suspension stability. Unstable suspensions lead to inhomogeneous coatings since the photocatalytic particles settle out of liquid suspension and cannot hold on to the textile surface properly. Therefore, the particle size of anatase and branched titania were measured in DI-water as well as finishing solution by using static light scattering via Coulter LS 13 320 as well as through AFM scanning.
Table 1 summarizes the mean particle size measurements obtained by Coulter LS 13 320. It can be seen that the anatase particles were similar in size both in DI-water and finishing solution with 0.087 ∓ 0.005 μm and 0.084 ∓ 0.001 μm mean diameter, respectively. The consistency in the measured mean size with a controlled standard deviation indicates the stability of the anatase particles in both environments. We have demonstrated in the earlier studies that the titania particles are more stable in the finishing solution which has a pH of 3.52 as compared to the DI water which has a pH of ~6 (Cerhan-Haink and Basim, 2020). This is due to the fact that the isoelectric point of anatase is at pH 5.8 (McNamee et al., 2005) and hence retains more positive surface charge in the finishing solution with pH 3.52 as compared to the DI water with pH of 6. Stability of the suspensions is expected to reduce when the pH is close to the iso electric point (i.e.p) of the system due to the fact that the particles retain no surface charge at the i.e.p (Ney, 1973). If the suspension is to be stabilized, one of the ways to do this is to change the pH away from the i.e.p to increase the electrical repulsion in between the particles (Salopek et al., 1992). Alternatively, mechanical vibration can be induced through effective ultrasonication. In this case, titania particles were stabilized in both suspensions through rigorous ultrasonication as can be seen from the consistent mean size measurement values.
Change of material stability depending on zeta-potential size.
| Photocatalytic particle | Mean Particle size (μm) | Standard Deviation (μm) |
|---|---|---|
| Anatase in DIW | 0.087 | 0.005 |
| Anatase in F.S. | 0.084 | 0.001 |
| Branched titania in DIW | 0.541 | 0.003 |
| Branched titania in F.S. | 1.029 | 0.058 |
In the case of branched titania, measurements showed a mean size of 0.55 ∓ 0.003 μm with tight control on the standard deviation for the solutions prepared in the DI-water background. In the presence of the finishing solution, on the other hand, the mean size increased almost twice and measured to be 1.029 ∓ 0.058 μm. It is believed that in the presence of the ingredients of the finishing solution, such as the silicon particles, the branches created on the titania particles attach to these additives and measure larger in mean size as compared to the DI-water based measurements. The increase in the variance of the measurement also supports the proposed concept in the finishing solution environment.
In order to understand the stability and verify the proper size of the synthesized branched titania particles, morphological particle size analyses were conducted by AFM scanning. Particles deposited on mica substrates were characterized and summarized in Table 2. The scanning of the anatase versus the branched titania particles with the AFM technique allows the characterization of the particles in their dried form. It can be seen that the mean sizes of the anatase and the branched titania particles are much closer measured as 0.277 ∓ 0.035 μm for anatase and 0.244 ∓ 0.118 μm branched titania. This is an indication that the branched titania particles have similar size cores but measure larger due to the presence of polymeric branches in the solution background. Furthermore, the standard deviation of particle size measurements obtained with light scattering on branched silica particles is lower as compared to the AFM measurements, while the particle sizes measured with AFM are almost half of the mean size measured by the Coulter LS 13 320. The smaller size measurement by AFM can be attributed to the fact that the branches attached to the main particle are collapsed by drying, whereas they are extended effectively in the solution environment for the light scattering measurements. Furthermore, the presence of the silicon and the polymeric additives in the finishing solution also results in the measurement of a larger size which is more pronounced with the branched structure of titania.
AFM scanning results for mean particle size evaluations of anatase and branched titania.
| Photocatalytic particle | Mean Particle size (μm) | Standard Deviation (μm) |
|---|---|---|
| Anatase | 0.277 | 0.035 |
| Branched titania | 0.244 | 0.118 |
Optimization of photocatalytic activity initially studied by using MB+ and the experimental setup adopting ISO 10678 standards. Fig. 1 illustrates the stain bleaching on suspensions prepared by 0.001, 0.01 and 0.1 wt % anatase in DI water and finishing solution for optimization of photocatalytic activity as a function of titania particle addition. It can be seen that in DI-water, anatase did not show any photocatalytic activity even under the UV light. When we conducted the same tests in finishing solution, on the other hand, 0.1 wt % anatase concentration showed significant degradation of methylene blue under the UV light changing the color of the suspension to white. It can be concluded that the presence of finishing solution helps activate the anatase under the UV light, which can be attributed to the additives like silicon in the active ingredients of the finishing solution.
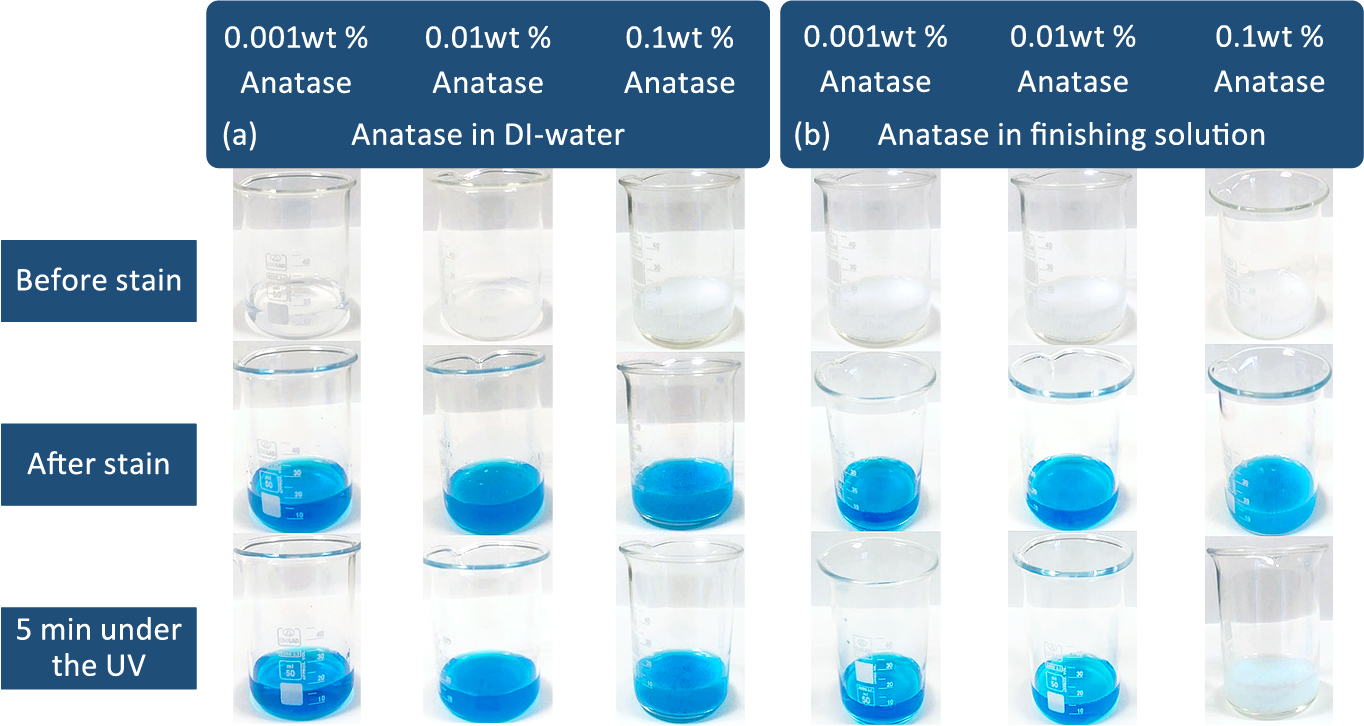
Methylene blue stain bleaching on suspensions prepared by 0.001, 0.01 and 0.1 wt % anatase in (a) DI water and (b) finishing solution for optimization of photocatalytic activity as a function of titania particle addition.
Further improvement in the photocatalytic activity was obtained with the addition of branched titania to the 0.1 wt % anatase solutions. Fig. 2 illustrates the change in the color of methylene blue solutions in finishing solution as a function of the branch titania doping at 0, 0.001, 0.01, and 0.1 wt % concentrations. As it can be seen in Fig. 2, finishing solution by itself did not show photocatalytic activity as observed by no change in the methylene blue color. This is consistent with the observation of no stain bleaching on the textiles which were coated only with the finishing solution in our earlier studies (Cerhan Haink and Basim, 2020). The optimal photocatalytic performance was obtained with 0.1 wt % anatase with 0.001 wt % branched titania doping as per the visual observations. Nevertheless, the determination of the photocatalytic activity according to the color change can be misleading a better quantification is needed. As an example, appearances of 0.1 wt % anatase doped with 0.001 wt % 0.01 wt % branched titania after UV exposure are similar. Therefore, the methylene blue degradation for these solutions was also evaluated by UV spectrophotometer absorbance tests as a function of time, as can be seen in Fig. 3. The decrease in the absorption is an indication of better transmittance in the solution and hence indicates the cleaning of the solution by the photocatalytic activity. Comparing the absorption peaks of the 0.1 wt % anatase (Fig. 3a), 0.1 wt % anatase doped with 0.001 wt % branched titania (Fig. 3b) and 0.1 wt % anatase doped with 0.01 wt % branched titania (Fig. 3c) show that the UV absorption peaks representing methylene blue degrades fastest for the combination of 0.001 wt % branched titania with anatase. Consequently, better photocatalytic cleaning efficiency is observed in the presence of 0.001 wt % branched titania in 0.1 wt % anatase. The speed of photocatalytic degradation can also be seen in Table 3, where the absorption delta is tabulated as a function of time for the selected three treatments. This observation highlights the fact that the ingredients in the finishing solution can enhance the kinetics of the photocatalytic activity when the polymeric branches interact with the silicon in the environment. It can be suggested that the polymeric branches on the titania particles can induce the synergistic effect of better particle attachment on the textile surfaces while simultaneously enhancing the photocatalysis.

Optimization of anatase/branched titania mix ratio in both finishing solution by methylene blue degradation analyses.

Absorbance versus wavelength representing the MB+ degradation under the cool light as a function of time for, (a) 0.1 wt % anatase, (b) 0.1 wt % anatase doped with 0.001 wt % branched titania and (c) 0.1 wt % anatase doped with 0.01 wt % branched titania in finishing solution.
Absorption delta for methylene blue degradation in finishing solution with 0.1 wt % anatase, 0.1 wt % anatase doped with 0.001 wt % branched titania and 0.1 wt % anatase doped with 0.01 wt % branched titania as a function of time.
| Solutions | Time (minute) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 4 | 6 | 8 | 9 | 10 | 12 | |
| 0.1 wt % Anatase 0.01 wt % Branched titania |
3.985 | 3.929 | 3.142 | 2.703 | |||||
| 0.1 wt % Anatase 0.001 wt % Branched titania |
3.863 | 3.801 | 3.100 | 2.497 | |||||
| 0.1 wt % Anatase | 3.898 | 3.789 | 3.313 | 2.857 | 2.571 | 2.504 | |||
In order to observe the photocatalytic performance of the textiles coated with the optimized particulate systems, absorbance tests were also conducted on the textiles coated with the selected optimal nano-particle combinations and compared to the industrial standards. Fig. 4 illustrates the absorbance tests performed for textile samples coated with 0.1 wt % anatase, 0.1 wt % anatase doped with 0.001 wt % branched titania, and 0.1 wt % P25 (used as a benchmark to compare photocatalytic activeness) in the finishing solution. It can be seen that the textile coated with the optimized solution, which is 0.1 wt % anatase doped with 0.001 wt % branched titania, degraded the methylene blue solution most effectively with a high drop in the absorbance value indicating efficient photocatalytic activity. The optimized combination of the nanoparticle system resulted in a significantly better performance relative to the standard P25 and even anatase coated by themselves.

Absorbance values of textiles coated with 0.1 wt % Degussa P25, 0.1 wt % anatase and, 0.1 wt % anatase doped with 0.001 wt % branched titania dipped into methylene blue solutions under exposure to 665 nm wavelength light as a function of time.
To better observe the stain removal efficiency of the coated textiles, the stain tests were also performed, as can be seen in Fig. 5. The textiles coated with the specified particle combinations were stained in one half and compared to the unstained half as they are exposed to UV light and pictured daily basis. Branched titania particles were synthesized by following the procedure described in the literature (Simakov and Tsur, 2007). It was illustrated by SEM micrographs that the diethylene glycol monomethyl ether attached on the titania surface and extended out as branches. It was also reported by XPS measurements that the binding energy of the carbon atoms and the oxygen atoms was detected to be around 250 eV and 530 eV, respectively (Simakov and Tsur, 2007). In this study, the UV light energy applied on the textile samples coated with the branched titania was around 3.4 eV and the textiles were exposed to UV source which was 15 cm away from the textile surface in the CAMAG UV Cabinet. Therefore, the polymer degradation is not expected to occur under the experimental set-up. Furthermore, no particular color change was observed on the branched titania coated textiles within the duration of the experimental evaluations. Furthermore, the stain test results also demonstrated that the textile sample coated with 0.1 wt % anatase and 0.001 wt % branched titania in the finishing solution showed the maximum level of stain discoloration based on the visual judgment of stain bleaching by the human eye. This observation is in an agreement with the absorbance measurements performed on the textiles coated with the same particle combinations.

Discoloration scale of textiles coated with 0.1 wt % Degussa P-25, 0.1 wt % anatase, and 0.1 wt % anatase doped with 0.001 wt % branched titania in finishing solution.
The textiles coated with photocatalytic particles were compared to the baseline uncoated textile to analyze any changes in the surface charge as a function of the solution pH. The zeta potential analyses were carried out for the uncoated textiles and the textile samples coated with 0.1 wt % anatase, 0.001 wt % branched titania and the optimal photocatalytic concentration of 0.1 wt % anatase doped with 0.001 wt % branched titania in the finishing solution. Fig. 6 illustrates the measured surface potentials as a function of pH to be very similar regardless of the surface treatment. It can be seen that the i.e.p of all the textile samples was measured between pH of 2.5 and 3, indicating that the procedure of photocatalytic enhancement by coating with various particle systems maintain a consistent surface finish while improving the photocatalytic efficiency.

The zeta potential as function of pH of un-coated textile and textiles coated with anatase concentrations doped with 0.001 wt % branched titania in finishing solution.
To quantify the photocatalytic efficiency of the optimized particulate system, band gap energy measurements were conducted as described in the experimental evaluations. The transmittance and absorbance of the thin films prepared from the solutions composed of 0.1 wt % anatase and 0.1 wt % anatase doped with 0.001 wt % branched titania in finishing solution were measured to determine the thickness and bandgap energy as shown in Fig. 7 and Fig. 8.

Thickness and transmittance graphs of thin films coated with (a) 0.1 wt % anatase and (b) 0.1 wt % anatase doped with 0.001 wt % branched titania in finishing solution.

Band gap energy and absorbance graphs of thin films coated with (a) 0.1 wt % anatase and (b) 0.1 wt % anatase doped with 0.001 wt % branched titania in finishing solution.
The film thicknesses (t) were attained by placing peak values of transmittance into Eq. (2) and Eq. (3), respectively. Furthermore, the thickness values were used to determine the energy bandgap for the thin films generated. The bandgap value of the 0.1 wt % anatase doped with 0.001 wt % branched titania was calculated to be 2.8 eV. This bandgap corresponds to the visible light range of 443 nm, according to the Plank-Einstein relationship (Landsberg, 1978). The band-gap energy, or in other words, the initial energy for photocatalysis being in the visible light range, means that the photocatalytic activity is plausible under the visible light exposure. Whereas the band gap energy of the pure anatase at 0.1 wt % concentration in the finishing solution was determined to be 3.31 eV, which is close to the value reported in the literature (3.26 eV) but still corresponding to the UV light region (400 nm). Thus, the enhanced performance with the optimized anatase, branched titania combination can be attributed to the energy bandgap shift into the visible light range in the presence of the polymeric additive doping. This observation also aligns with the earlier findings in the literature suggesting the core-shell particle decorated with polymeric branches can actively control the binding event by pairing ion, metal complex recognition, and hydrogen-bond recognition (Randazzo et al., 2012). Furthermore, textiles have an inherently rough surface and they are not conductive and hence the photocatalytic reaction should be considered as independent from any change in the surface area of the textiles by the addition of particles. The surface charge evaluations also do not indicate a significant change to infer that an increase in the surface area would make the observed photocatalytic activity improvement.
3.6 Water vapor transmission evaluation for daily usage evaluation of the textilesThe water vapor transmission through the textiles is a vital factor affecting the thermo-physiological comfort of people wearing the textiles by allowing respiration of human skin (Arabuli et al., 2010). After coating the textiles with the selected particulate systems, the permeability could change due to the reduction of the textile porosity, which may limit the breathability of the garment.
Textiles coated with 0.1 wt % anatase and 0.1 wt % anatase doped with 0.001 wt % branched titania in the finishing solution were exposed to the WVTR analyses for detecting water vapor transmission rates. The weight change of the full experimental set-up as a function of time is summarized in Table 4 as evaluated for winter and summer conditions. It can be seen that the WVTR is around 35 % for each textile sample for winter set up under 22 °C and 20 % moisture environment. The WVTR values measured in winter were lower than the values measured in summer 35 °C and 50 % moisture, which were measured to be around 75 %. The results were statistically the same other that the trend of slightly lower values observed with the textiles coated with the 0.1 wt % anatase doped with 0.001 wt % branched titania. It can be concluded that the photocatalytically more active coating combinations are equality suitable for the daily use.
Water vapor transmission rate for uncoated and coated textiles under the winter and summer conditions.
| Type of Textile | WVTR (%) (20 °C-20 %RH) | WVTR (%) (35 °C-50 %RH) |
|---|---|---|
| Uncoated | 34.12 ± 0.37 | 77.26 ± 0.36 |
| 0.1 wt % Anatase in finishing solution | 34.61 ± 0.48 | 75.61 ± 0.59 |
| 0.1 wt % Anatase and 0.001 %w Branched titania in F.S. | 33.80 ± 1.43 | 72.52 ± 0.99 |
Extension of photocatalytic activity into the visible light range through modification of the bandgap energy was accomplished by polymeric branches decorated on titania particles. Branched titania synthesis was performed chemically, and the particle size measurements conducted in the solution and post drying suggested the free elongation of the polymeric branches from the core particle in the solution environment. An optimal concentration was established with 0.1 wt % anatase doped with 0.001 wt % branched titania in a hydrophobic finishing solution. The composite branched titania particles, in combination with the anatase in finishing solution, achieved a 2.8 eV bandgap corresponding to the visible light range with 443 nm wavelength. The synergistic effect of the branched titania particles in the presence of anatase was further confirmed by measuring the bandgap of 0.1 wt % anatase in finishing solution, which was found to be 3.1 eV. It was further demonstrated that the implementation of the optimized particle combination to the textile finishing process enhanced the self-cleaning efficiency in addition to better attachment of the particles on the textile surface through the polymeric branches. In summary, the new polymeric branched particles have the synergy of better coating capability on textiles with an enhanced photocatalytic activity that can be extended into the visible light range.
The authors acknowledge the financial support from the Eureka PhotoCat project E!8080 and Kivanc Tekstil Company in Adana Turkey.
Asena Cerhan Haink
Asena Cerhan Haink is a second-year PhD student in Electrical and Computer Engineering at the Technical University of Munich. She holds two masters degrees in Physics and Mechanical Engineering. She has a MSc Thesis, from Physics, on the characterization of the photovoltaic semiconductor. For her second MSc Thesis, she wanted to have a better understanding of the photocatalytic efficiency at the textiles. To do so, she focused on the photocatalysis mechanism in terms of optical characterization besides to self-cleaning textiles quality characterization. For her PhD Thesis, her research focusses on the morphological characterization of organic thin film systems. She can be contacted at: asena.cerhan-haink@tum.de
G. Bahar Basim
Dr. Bahar Basim is a Professor of Practice at the University of Florida Department of Materials Science and Engineering. She is an expert on electronic materials and particle science and technology. She has received the European Commission Marie Curie People Award in 2010 for her research on nanoscale protective oxide film. She has numerous international patents and applications on nanotechnology, microelectronics, coatings, and surface science in addition to many technical papers, books, and presentations. Her research continues on chemical mechanical planarization, thin films, interfaces, novel nanoparticle development and nano-structuring of biomaterials.