2021 Volume 38 Pages 155-167
2021 Volume 38 Pages 155-167
With the economic development, environmental pollution is getting worse in many developing countries including China. Traditional processing as CaO neutralization of acidic solutions is still a burden to many local governments of China and development of new process with lower cost is highly required. Mining exploitation produces large amounts of tailings in which industrial minerals such as carbonates, silicates and others have been left without proper utilization. Based on the intensive investigations in the field of mechanochemistry, we propose to use these mineral samples to deal with the purification of heavy metal polluted water by enhancing their reactivity through mechanochemical activation. The industrial minerals such as silicates (serpentine and kaolinite), carbonates (calcite) have been studied for the purposes on removal of contaminants from solution. In addition, the synergistic effects from mixtures of calcite with other material have been investigated to give their excellent adsorbent performance for purification of the wastewater. This review summarizes the recent progresses for mechanochemical preparation of mineral based adsorbent and its effective purification ability for toxic matter-containing wastewater.
Industrialization of the human society may begin with the exploitation of various kinds of minerals. Mineral processing has been an important technique to support the development of civilization. The applicable minerals can be categorized mainly into two groups: the first one is metallic mineral resource such as hematite, magnetite, chalcopyrite and galena, which are used to produce metals such as iron, copper and lead, and another one is non-metallic mineral resource, so called as industrial minerals, such as silica, gibbsite, clay, calcite, mica and talc, which are used particularly as additives and fillers in the productions of paper, plastic, paint, as well as raw materials in the productions of cement, ceramic, glass and so on. Metallurgical processing of metallic minerals is also a main source to emit heavy metals to pollute the environment, but strange though it may seem, the abandoned mines are actually the other source, seeping pollutant into the environment with underground water or raining.
A key technology enabling to develop new applications for processing these industrial minerals is mechanochemical activation, mainly triggered by a simple operation of ball milling, which is widely used in mineral processing for concentration and enrichment of target minerals. When powder particles are trapped between balls colliding inside a mill pot, then the particles deform elastically and/or plastically, and are finally fractured into fine fragments, which are easily agglomerated due to the mechanical activation. The force of the impact during the grinding acts on the powder particles, leading to crystallographic bonds broken and new surface is produced. The new surfaces created enable the particles to aggregate and agglomerate each other easily and this leads to an increase in the degree in the activation of solid material. Profound changes affecting the surface as well as the chemical, physicochemical and structural properties may also take place. This is manifested by the presence of a variety of crystal defects such as increased number of grain boundaries, dislocations, vacancies and interstitial atoms, stacking faults, and deformation with rupture of chemical bonds. The presence of such crystal defects enhances the diffusivity of chemical components in the solid.
We have been doing investigations from the viewpoint of mechanochemistry, involving a wide range from mineral processing, materials synthesis, metallurgical extraction to detoxification of hazardous materials. Accumulations of our obtained knowledge and understanding of the nature of mechanochemistry allow the development of new mechanochemical route for preparing mineral based adsorbent and its effective purification ability for toxic matter-containing wastewater. This review gives brief points reflecting our recent issues in this field, with related publications listed.
At the early stage of economic growth, developing countries may face serious environmental problems, and one of them is the heavy metal pollution. The large amounts of metal sulfate wastewater produced in the mining and other industries are the major cause of heavy metal pollution to the environment. The solution to such problem requires imminent action. In this regard, the action would be important to work out it by two approaches; one is a positive one improving the processing efficiency, reducing the amount of waste emissions, and the second is a passive one purifying and decontaminating wastewater. Lime precipitation is currently the main method for purification purpose, but it has brought us obvious disadvantages such as high pH of water, high water percentage to the sediment, difficult filtration and so on. The use of non-metallic minerals for the purification purpose does not cause normally significant negative problem, so that it would be recommended in practical application, if such disadvantages are improved. One of the methods for improving them would be chemical modification of adsorbents.
Heavy metal contamination is a growing concern in the world, inadequate polluted water and/or wastewater treatments, coupled with increased industrial activity, have brought about serious heavy metal contamination in rivers, lakes, water reservoirs and so on, spreading over the vast area. However, popular technological methods for removing heavy metals from water sources such as membrane filtration, activated carbon adsorption and electro-coagulation are not always feasible for developing countries due to the viewpoints of economy and infrastructure. As a result, many researches have been attempted for the applicability of low-cost adsorbents to remove heavy metals in the solution. Several scientific papers discussed on the use of alternative adsorbents such as agricultural waste, soil, mineral deposits, aquatic and terrestrial biomass and waste materials.
On the other hand, clay minerals, which are typical non-metallic minerals, exhibiting the adsorbent characteristic, and have been excavated as sedimentary or residual deposit from the earth. This implies that the minerals are normally cheap and abundant natural resources found easily in the world. The minerals have been examined as adsorbents of many contaminants in mainly aqueous solutions. Clay minerals are very reliable and an environmentally friendly remediation material for heavy metal contaminated media. Also, several methods are available for the modification of clay minerals in order to increase their adsorption capacity. However, in order to take first preference in the use of clay minerals as heavy metal remediation materials compared to other methods, further technological investigations are required, leading to determine the best modification type for clay minerals as well as their least dosage required for the adsorption of heavy metals.
Similar to the clay minerals, carbonate minerals such as calcite and dolomite are also widely studied as economical adsorbents to dispose in heavy metal polluted water by neutralizing typical acidic wastewater. Due to the relatively low capacity in these raw minerals as absorbents, chemical modification is, in general, required to enhance the adsorption ability as well as its regeneration and reduce the waste sludge amount after the disposal.
Grinding operation of the raw mineral samples has widely been used to reduce the particle size, to increase the specific surface area together with mechanical activation sites on the surfaces. This activation leads therefore to enhancement in the reactivity with heavy metals when it is suspended in the polluted water and its removal efficiency from the solutions. Besides the physical changes in particle size, structural deformation and so on, intense grinding a solid can induce its physicochemical changes, namely mechanochemical effects, by which high reactivity of the ground solid toward heavy metal removals in the solution may be expected. This result is, therefore, linked with considerable reduction in the amounts of the mineral samples with keeping efficient performance for environmental remediation. This special modifying method based on mechanochemical effects particularly resulting from intense ball milling is fully investigated and discussed in our group and serves as the main contents of this review paper.
Serpentine, (Mg, Fe)3Si2O5(OH)4, is a generic term for a typical class of hydrous magnesium-rich phyllosilicate minerals. At present, serpentine is generally associated with some metal deposits in nature and often abandoned as a gangue mineral, which is not only a waste of serpentine resource, but also a serious impact on the environment. Serpentine has unique characteristics of phyllosilicate minerals on the adsorption of heavy metal ions, however the raw sample gives limited capacity on heavy metal removal from environmental pollution. When serpentine is modified and activated mechanically by milling, structural damage to this mineral would be a drawback, obstructing the use of its unique characteristics. However, if milling this mineral makes it possible to maintain its adsorption ability with keeping increment in the specific surface area, there would be possible to increase its reactivity to other species such as heavy metal ions and contaminants in solution.
One typical feature of serpentine milled is the gradual transformation of its crystalline structure to amorphous state, which is commonly observed with hydrated phyllosilicates by intense dry grinding. Investigations into further changes with the ground serpentine sample have revealed interesting phenomena. We have ground serpentine sample by a stirred mill, followed by a dissolution test on the sample into a distilled water. Typical results on this work are shown in Fig. 1 (Huang et al., 2017). The serpentine samples milled for 2 h at different speed were dispersed into distilled water for 4 h without any other chemicals, as seen in Fig. 1(a), Mg2+ ion content in the water increases rapidly with increase in mill speed and level off over 400 rpm milling operation. This means that the serpentine activated is dissolved into the water with releasing Mg2+, while the raw sample without activation has not shown such obvious dissolution behavior of Mg2+ in the water. This suggests that the serpentine milled has transformed into activated state even more than brucite of which an industrial mineral form is magnesium hydroxide, the chemical formula Mg(OH)2. Furthermore, Fig. 1(b) show the pH of the water when the serpentine samples activated are dispersed in as a function of mill speed. As seen, the pH is changed from 6.25 in the initial stage to 10.8 at 500 rpm in mill speed, and it increases drastically in the rage from 200 to 400 rpm. According to the data on XRD analysis of the sample, the structural change in the sample is remarkable in the same mill speed range as described above. Both changes in specific surface area and crystalline structure may affect the Mg ions release and pH increase. It is understood that at mild milling with speed lower than 300 rpm, increase in specific surface area may occur as the main factor and structural changes occur mainly at milling speed around 600 rpm, respectively.

Changes in dissolved Mg2+ concentration (a) and pH value (b) in the solution with the rotational speed of the stirred mill when the serpentine activated was dispersed in it (Huang et al., 2017).
We have extended the investigation on the reactivity of serpentine sample activated by the mill at different conditions to find out its adsorption ability in several polluted solution with Cu and Ni ions. The dosage, initial concentration of the solution and other factors were changed and tested in the experiments. A typical result on the adsorption of Cu2+ in solution by the serpentine sample activated under different mill speeds in the range from 200 to 400 rpm is shown in Fig. 2. At first, it is recognized from the figure that the activated serpentine sample exhibits the adsorption ability to Cu2+ in the solution. In addition, the residual Cu2+ ion content in the solution is decreased with an increase in the dosage of the sample, depending on the mill speed. The residual Cu2+ content becomes extremely low as the mill speed is increased, especially in the range from 300 rpm or more. In all cases for the sample activated, the content is much lower than that for the raw sample without activation. The residual Cu2+ content in the solution for the sample activated at 400 rpm in mill speed and 0.1 g/L in dosage is about 0.5 mg/L or less, which reaches the Environmental Protection Agency (EPA) the copper discharge limit (1.00 mg/L). We also found that the sample activated by the milling under the condition of 400 rpm or higher brings about a remarkable effect on its adsorption capacity, while serpentine raw material itself has not obvious adsorption ability to Ni or Cu ions. To be concrete, we have confirmed that the capacity of the activated sample at the rotational speed of 600 rpm in the Ni and Cu solution reaches 226.04 mg/g and 538.84 mg/g, respectively.

Changes in remaining copper concentration with the dosage of the activated serpentine at different milling speed (rpm) of the mill with 10 mg/L initial Cu2+ concentration (Huang et al., 2017).
We have examined adsorption behavior of the serpentine sample activated to alkali metals such as alkali cesium (Cs) ions in the solution. Normally fixing alkali Cs ions is a difficult task but of significant importance in radioactive wastewater treatment at nuclear power stations. Our approach started with the active Mg in the ground serpentine with an aid of phosphate anions, for example, (PO4)3−, incorporating alkali Cs in solution base to form a complex phosphate as a struvite structure of (CsMgPO4). It is noted that the struvite, NH4MgPO4·6H2O, itself is sparingly soluble in neutral and alkaline conditions, but readily soluble in acid. A solution with three alkali ions of Na, K and Cs of phosphates to provide PO43−, was prepared in the experiment, and the serpentine samples activated for 2 h for different speeds were dispersed in it. Fig. 3 (Lei et al., 2019) is a result demonstrating changes in removal rate of three alkali ions in the solution with milling speed in the mill. The removal ions means their adsorption by the sample. As seen, the removal rate of only the Cs ion by the sample is increased marginally in the slow speed mill range, bit it increased rapidly in the speed range from 300 to 400 rpm, and reaches 95 % or more. This implies that Cs ion is transferred to precipitates which are adsorbed into the serpentine sample or settled down in the vial. According to the analysis by XRD, it was found that the serpentine structure is broken at the speed over 400 rpm, suggesting release of active Mg in the solution. In fact, most of Cs ions in the solution is removed by the activated serpentine sample to form precipitation. The precipitation sample was checked by XRD analysis, then it was conformed to be CsMgPO4 which is the struvite phase. Therefore, this activation technique to serpentine sample would be a promising and alternative one for adsorbing Cs in radioactive wastewater. Of course, the raw serpentine sample does not show such high adsorption behavior for Cs ions. It is interesting to note that both Na and K ions remain in the solution. The properties of alkali elements are very similar, and methods for Cs precipitation usually cause a similar effect to others like K and Na ions. This means that quite a large amount of serpentine sample has to consume for K or Na removal. It is known that alkali elements have different radius size and exhibit some slight difference in the stability when forming struvite phase. If such small difference is applied for selective precipitation of Cs ion on the ground serpentine, both disposing of radioactive Cs source and enrichment of it are achieved. The characteristics of K and Cs ions to the mechanical activated serpentine were not found in conventional chemical reactions. The key point is that mechanochemical processing may offer special solutions to those difficult cases and we are looking for the chances to solve particularly the environmental issues.

Removal rates of Cs, Na and K ions in the solution with the serpentine sample activated by the stirred mill as a function of milling speed (Lei et al., 2019).
Coagulant has been widely used in the pretreatment of wastewater, and a simple and environment-friendly process to prepare high-efficiency coagulants is highly desired with increasing the amount of wastewater treatment. Kaolinite Al2Si2O5(OH)4, as one of the industrial minerals of kaolin group or clay minerals is a popular one, having 1:1 type layered silicate consisting of Si-O tetrahedron and Al-O octahedron. This mineral has been offered to industries of pottery products, additives in medicines and foods, daily necessities, agricultural soil, fertilizer reagent and so on. We have attempted to develop a novel process to prepare aluminum-based coagulant by the use of mechanochemical activation of kaolinite, followed by co-grinding with concentrated sulfuric acid (H2SO4) solution. Fig. 4 shows briefly the flowsheet for preparation process for such coagulant sample. The sample is composed of aluminum sulphate, Al2(SO4)3, and it was then tested for removal of pollutant in solution.

Flowsheet for preparation process for kaolinite base coagulant sample (Zhao et al., 2019).
The composition, structure and morphology of the coagulant samples were characterized using X-ray Diffraction (XRD), Fourier Transform Infrared (FT-IR), Spectrometer and Scanning Electron Microscopy (SEM) and so on. Capacity of the prepared product as coagulant, compared with pure aluminum sulfate, was evaluated by the removal efficiency of turbidity, phosphate (PO43−), arsenate (AsO43−) and humic acid (Zhao et al., 2019). The final values of these efficiencies are 99.9 % for turbidity clearing up, 91.2 % for phosphate, 89.6 % for arsenate and 92.3 % for humic acid, respectively. Moreover, during the preparation process, the formation of alum, was observed over a wide pH range from 4.3 to 9.0. XRD analysis has demonstrated that a reaction between kaolinite and sulfuric acid occurs during the milling operation and Al sulfate is formed to work as coagulant for the effectiveness confirmed as above.
Instead of the sulfuric acid addition, phosphoric acid (H3PO4) was added to the kaolinite sample activated in order to modify kaolinite. In this case, the final product sample was formed as aluminum phosphate, Al-PO4, and was also tested for removal of pollution in the solution. Since Al phosphate is not water soluble, the sample keeps phosphate anions inside even in solution for adsorbing some target substances. In the case of modifying kaolinite by phosphoric acid, part of the free hydrogen ion of phosphoric acid is combined with the hydroxyl groups in the kaolinite structure, leading to the production of water molecules, so that phosphoric acid is fixed onto amorphous kaolinite to form Si-O-Al-P binding, which offers good adsorption sites for potassium ion (K+) (Lei et al., 2018).
The adsorbent through phosphoric acid modification of kaolinite provides a new option for recovering potassium from seawater and tailing wastewater. Further improvements may be obtained by together use with Mg-Al layered double hydroxide or the Mg silicate of serpentine activated as an example and the detailed information may be referred to other publications. The coagulant sample would be a promising one for pre-treatment process of sewage as well as a new route to rich tailings of coal mining gangue etc.
3.3 Calcium carbonate: CalciteAs described, the conventional precipitation method is not enough to solve the problems, due to large amount of chemical reagents needed in order to neutralize wastewater, besides difficulty in separation and recovery of precipitated metals and poor record for processing wastewater with low-concentration of heavy metals. One of the important issues would be to develop a sample exhibiting excellent capability of removal amount for pollutants such as heavy metal ions.
We have proposed a new method for preparation of such samples by using wet stirred ball milling a calcite sample (CaCO3) with solutions containing Pb2+, Cu2+. Since precipitations of heavy metal ions are expected, a wetting grinding rather than dry grinding was used for such purpose. A systematic exploration was done to investigate effects of several factors such as calcite dosage, milling time and ball volume ratio in the milling on adsorbent capacity of calcite activated for Pb2+ and Cu2+ in the wastewater. It is not easy to analyze in situ the samples in the solutions directly, calcite activated by the milling as well as the precipitates of heavy metals from the water were characterized by XRD, TG-DSC, FTIR and other analytical methods. Fig. 5 shows the lead (Pb) removal rate (%) in the solution on the added ball volume to the solution (a) and the copper (Cu) removal rate (%) on the Ca/Cu molar ratio (b), respectively. The initial concentrations were 0.5 mM for Pb solution, and 1 mM for Cu solution, respectively, and the pH was fixed at 6.51. In the experimental work on Pb removal, shown in Fig. 5(a), the conditions were as follows; CaCO3/Pb2+ = 1 in moral ratio, 120 min in milling time, 10 % in ball material ratio, and 200 rpm in rotation speed of the stirred mill. As shown in Fig. 5(a), the initial Pb2+ removal rate in the solution is 23.3 % at the agitation test (0 % in ball volume ratio), but it increased rapidly with an increase in the ball volume ratio, and reaches to almost 100 % is at 8 in the ball volume ratio, then levelled off. This suggests that the precipitation in the solution happens during the wet milling of calcite and Pb ions in its mill pot, even under low ball volume ratios in the range of 1 to 2 %. This precipitation happens more and more in the higher ball volume range from 5 to 13 %. The precipitates were confirmed as PbCO3, according to the XRD analysis. Therefore, the remarkable increment range in Pb removal rate seems to be effective period to form such PbCO3 precipitates and the milling with proper ball volume ratio facilitates this precipitation of Pb ion on the calcite sample surface in the solution. It may be noted that this milling period would activate the calcite sample adsorbing the Pb ion in the solution, and that no activation happens on the calcite sample, resulting in only the physical adsorption of Pb2+, which is insufficient to remove it, in the absence of balls in the milling (Wen et al., 2020; Xiong et al., 2020; Hu et al., 2019).

Pb removal rate (a) on the added ball volume ratio in the stirred milling and Cu removal rate (b) on Ca/Cu ratio using calcite activated by the stirred mill (Modified from Hu et al., 2017; 2019).
Similar trend in the Cu removal in the solution was also observed, as shown in Fig. 5(b). Specific experimental conditions were as follows: 1 mM in initial concentration of Cu2+ solution, CaCO3/Cu2+ = 1 in moral ratio, 90 min in milling time, 10 % in ball material ratio, and 200 rpm in rotation speed in the stirred milling. It is found that the Cu2+ in the solution can be precipitated from the solution, and the precipitates were confirmed as a solid of Cu4(SO4) (OH)6·2H2O. The removal rate is increased up to 99.62 %, at which the pH in the solution was 8.25. Then, the concentration of the solution with Cu2+ after the treatment is 0.35 mg/L, which is much lower than the National Emission Standards (Hu et al., 2017).
We have further extended the activated calcite sample to other metal ions such as iron (Fe), zinc (Zn) and cadmium (Cd). In the experiment, the calcite sample was milled and activated by a planetary ball mill, which can give the milling strength higher than that by the stirred mill. This may cause easily mechanochemical reactions between calcite and coexistent species. We have made a milling experiment using the calcite sample and heavy metal sulfates. It is very interesting to note that, depending on the type of element, the reaction behavior between metal sulfate and calcite were different: some were quite easy to form precipitation for efficient removals of the ions from the solution, but other metal ions remained in the solution, due to no reaction with calcite. The differences were mainly caused by two aspects: One is the characteristics of different metal ions, and another is the different conditions of reactions. Within the scope our research experiment, the reactivity of ions to calcite has been put in the order of Pb2+ > Fe2+ > Cu2+ > Zn2+ > Cd2+, Ni2+. When the intensity of mechanical activation is very high, the coprecipitation of multiple metals would occur. One plausible reason for such differences in the reactivity may be attributed to the thermodynamic stability of hydrated ion, M(H2O)n2+, where M denotes metal and n is the water coordination number. When the coordination number of around water is six for Fe2+, Ni2+, Cd2+, the spatial configuration of hydrated ion is octahedron. The spatial configuration corresponding to four for Pb2+, Cu2+, Zn2+ is a regular tetrahedron. The water molecules in the form of octahedron are more difficult to be removed away than those of the regular tetrahedron. It is understood that Pb2+, Cu2+ and Zn2+ are easier to react with CaCO3 than Fe2+, Ni2+ and Cd2+ (Li et al., 2020). The reaction mechanism between Fe2+ and CaCO3 seems to be a special case and will discuss in detail in the section 3.4, which will introduce synergistic effects of a composites to some anions of phosphate and arsenate.
Hydroxide precipitations of heavy metal ions take place with traditional alkaline neutralization, and calcium hydroxide, Ca(OH)2, sample has been used as a chemical reagent, and its amount may be a lot, depending on the scale of pollution site. As described, it is possible to use calcite sample, CaCO3, activated by wet stirred ball milling to treat heavy metals. Due to the high reactivity with heavy metal ions in the solution, it may be replaced with Ca(OH)2 used in traditional alkali precipitation, and also realizing the chemical reaction of CaCO3 and heavy metal ions even in equivalent molar and its solid concentration range lower than the current process. When the milling speed is high enough to allow calcite enabling to react with almost all the metal ions in solution to form precipitates (solid phase), the purpose of purification is environmental-friendly achieved. When the milling speed is controlled at mild condition, some metal ions may precipitate on the calcite surface, but others may remain in the solution. In this case, a mutual separation for metal ions would be possible. More interestingly, it is possible to change the activation strength on the industrial minerals like calcite (CaCO3) by a mill operated under different conditions, the CaCO3 sample activated shows different reactivity against the heavy metals in the solution. By applying the difference in the reactivity, we designed a new process to conduct a mutual separation of these metals by the use of calcite sample activated by a mill with solution containing the ions.
Fig. 6 shows the experimental data on mutual separation of different ions in the solutions with Cu-Ni (a), Cu-Co (b), Cu-Cd (c), Pb-Zn (d), Fe-Cu (e) and Fe-Mn (f) systems, respectively. In the case of (a) Cu-Ni separation, a mixture of CaCO3, CuSO4, NiSO4 and few amount of distilled water (3 mL) was put into a pot of a planetary mill, and milled for 30 min. The milling speed, Ca/Metal molar ratio and so on were changed, and the products after milling was taken out for evaluation. CuSO4 and NiSO4 are the component of the solution, therefore no use of other chemicals is an advantage of our method, and the same procedure was done for other systems such as Cu-Co (b), Cu-Cd (c), Pb-Zn (d), Fe-Cu (e) and Fe-Mn (f). As seen in Fig. 6(a), with wide range of Ca/Cu molar ratio, the residual ratio in Cu2+ is almost zero, implying that they are completely precipitated, while Ni2+ remains at 90 % or more in the solution over the wide range of Ca/Cu ratio. Therefore, our sample prepared by wet milling of calcite and phosphates is possible to separate mutually Cu2+ and Ni2+ in the solution. If one would try this mutual separation by the traditional alkaline neutralization process using a chemical reagent, Ca(OH)2, it would not be achieved the purpose, and more than this, enormous amount of reagent would need to precipitate these ions (Li et al., 2017a).

Summarized six groups of mutual separations between heavy metals by the use of CaCO3 sample activated (Modified form Hu et al., 2020; Li et al., 2016, 2017a, 2017b; Wang et al., 2019; Zeng et al., 2020).
Similar results are seen in Fig. 6(b) and (c). As for the case of selective precipitation of Cu from the solutions containing Co and Cu, the separation efficiency is not high in comparison with that in Cu-Ni system shown in Fig. 6(a), but the residual ratio in Cu is very low over the whole range of Ca/Cu ratio. This means most of Cu2+ is precipitated, while most of Co2+ ion is still remained in the solution (Li et al., 2016). In the case of Cd-Cu-Fe ions in the solution, Cu and Fe ions are precipitated, while large amount of Cd ion is remained in the solution, as seen in Fig. 6(c) (Li et al., 2017b). It is, therefore, recognized that the proposed method could achieve mutual separation among Cd, Cu and Fe ions at fairly high possibility, although the separation efficiency is not so high as the case of Cu-Ni system, as shown in Fig. 6(a).
Selective lead (Pb) removal from metal-containing wastewater such as acid mine drainage (AMD) is an important issue in environmental purification and secondary resources recovery. Similar test on copper (Cu) or iron (Fe) removal was done using CaCO3 activated by wet ball milling with the solution containing Pb2+. The Pb2+ in the solution is precipitated to form PbCO3, and this is achieved based on the reaction between Pb2+ and CO32− in the solution, as shown in Fig. 6(d) (Zeng et al., 2020). The removal efficiency of Pb2+ from the solution is increased with an increase in the molar ratio of CaCO3/Pb2+, and it reaches over 99 % at 1.0 in the molar ratio of CaCO3/Pb2+, while more than 99 % Zn2+ is remaining in the solutions. During this removal change, the pH in the solution is changed from about 5.0 to 6.3. This result implies that the high selective precipitation in Pb2+ is attained in the range over 1.0 in the molar ratio of CaCO3/Pb2+, without Zn as a contaminant. Looking at the solution after separation of Pb, it is possible to recover Zn as zinc carbonate, as an alternative precipitant to CaCO3 in the separation process.
Iron (Fe) impurity in manganese sulfate (MnSO4) solution will seriously affect the quality of manganese products if it is not purified. Ferric ions (Fe3+) will precipitate even in weak acidic solution, so that it is possible to remove Fe3+ from the manganese sulfate solution by controlling and regulating the pH value in the solution. However, it is not easy to remove Fe3+, and oxidization of ferrous ions (Fe2+) into Fe3+ is normally conducted for iron impurity removal. Our study was focused on removal of ferrous iron from manganese sulfate solution by the use of calcite (CaCO3) activated mechanically with manganese sulfate solution. This sample preparation was also wet milling used by a stirred ball mill. One of the results at 350 rpm in mill speed are shown in Fig. 6(f), and the removal rate of ferrous ions depends on the milling time as well as milling time, and it reaches approximately 100 % under the condition at 180 min in the milling time, 15 % in the ball/liquid volume ratio and 3/1 in CaCO3/Fe2+ (Wang et al., 2019). Thus, selective ferrous iron (Fe2+) removal from manganese sulfate solution can be achieved by the sample proposed. Thus our sample preparation is to use calcite and the polluted solution(s), and no use any chemicals. This method would be useful for removing iron ion from manganese sulfate solution. The iron impurity removal can be applied also for purifying other solutions containing copper, nickel, cobalt or zinc.
Thus, the phenomena on the reactivity of CaCO3 with heavy metals in the solution can be controlled by altering milling operation, and it may not be easy to manage this removal results by the traditional process using alkaline neutralization.
3.4 Composite of calcite with ferrous saltThe excessive use of pesticides, the discharge of municipal and industrial wastewater have led to a significant increase of phosphorus levels in water bodies and serious eutrophication pollution. For such high concentration eutrophic water and industrial/agricultural phosphorus wastewater, chemical precipitation treatment can immobilize the phosphate as solid compounds without the need for additional oxygenation or excessive post-treatment. This treatment involves the addition of soluble salts of aluminum and iron or even rare earth to wastewater. Ferric (Fe3+) salt is a kind of widely used reagent which is an economical and environmental-friendly reagent rather than aluminum and rare earth salts, and has an excellent precipitation ability, similar to the ferric orthophosphate (FePO4) with free of toxicity. Applications of Fe3+ generated via the oxidation of ferrous (Fe2+) salt have received much attention due to its cost performance rather than direct usage of Fe3+. The main reason is that the precipitation reaction between Fe2+ and PO43− occurs in the process of transformation from Fe2+ to Fe3+ hydroxyl oxides under aerobic condition, which avoids the formation of FeOOH by hydrolysis of Fe3+. At the same time, the oxidation process of Fe2+ is greatly affected by OH−. We have noticed recently that the surface of CaCO3 could provide a boundary for metal ions participation through the reaction with OH−, resulting from the hydrolysis of CO32−, a successful alternative to Ca(OH)2 with a clear advantage of final pH in neutral range of the wastewater for direct discharge after the treatment. When Fe2+ was used with CaCO3, the Fe(OH)2 formed on its surface could be easily oxidized into Fe3+ hydroxide even in air, without addition of specific oxidant. It is interesting to note that the slowly generated fresh ferric compositions exhibit tremendous removal capacity for phosphate, without evident formation of FeO(OH). Fig. 7(a), (b) shows the experimental results for the removals of both arsenite anions (for example, AsO33−) (a) and phosphate anions (for example, PO43−) (b) from the solutions. The sample of calcite was mixed with the Fe2+ salt and milled by a planetary mill for some time. The results show the dramatic change in each anion, AsO33− and PO43−, from the solution by the use of CaCO3 with Fe2+, which is oxidized into ferric (Fe3+) species (Zhang et al., 2019a; Zhang et al., 2019b; Li et al., 2018).
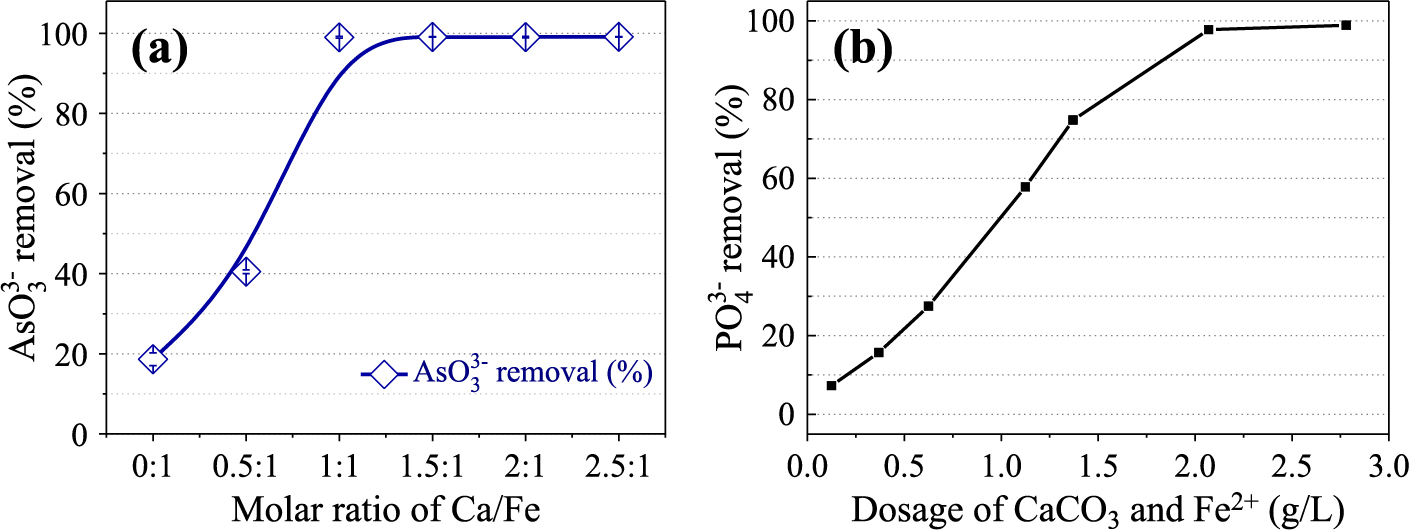
Removal efficiencies of arsenite anions (a) and phosphate anions (b) with calcite and ferrous combination (Modified from Zhang et al., 2019a; Li et al., 2018).
The mechanism is as follow: At first, CaCO3 is added to Fe2+ salt and it is agitated with phosphate (PO43−) solution. Then, these components transform into an amorphous Fe3+-P or Ca-Fe3+-P precipitates. During this change, phosphate removal proceeds in the solution and finally reaches almost 100 % in removal rate. The concentration of phosphorus (P) in the solution is changed from 100 mg/L in the initial stage down to 0.03 mg/L, which is definitely lower than the discharge permissible limit (the National Emission Standards) (USEPA Office of Wastewater Management, 2010). This can be achieved under the conditions as low as 9 in a stoichiometric ratio of CaCO3/PO43− and 1.5 in ratio of Fe2+/PO43−. It is amazing that the final pH of the solution does not change so much and remains around a neutral range from 5 to 8. Therefore, it is possible to discharge the water after treatment without any further additional treatment like acid neutralization used in the alkaline precipitation process. Mechanochemical ball milling could significantly reduce the size of calcite (CaCO3) and enhance its reactivity to the anions and solubility in the solution, hence it could promote the efficiency of simultaneous oxidation of Fe2+ during the process of phosphorus (P) removal from the solution. When the ball milling speed was 600 rpm, the phosphorus removal process could be completed within 4 hours. The increase of temperature in the precipitation process could also promote phosphorus removal efficiency due to simultaneous oxidation of Fe2+ with CaCO3. The percentage of P2O5 in the precipitate by agitation of Fe2+ and CaCO3 in the phosphate solution is as high as 19.4 %, which is enough grade as phosphate source in fertilizer production. Difference from the conventional alkaline neutralization process with high concentration of OH− group is the slow hydrolysis of CaCO3, resulting in low concentration of OH− group for the formation of Ferrous (Fe2+) in the solution or its compound (Fe(OH)2). The ferrous (Fe2+) is oxidized easily by air into trivalent Fe (Fe3+), achieving continuous formation of fresh ferric composition for phosphate precipitation and could avoid its rapid formation and subsequent transformation into stable FeO(OH) of large particle size to lose the activity. Our method introduced has significant advantage in the use of industrial mineral, CaCO3 and common material, ferrous (Fe2+) salt, so that it may be possible to apply easily to phosphate precipitation.
In the meantime, arsenic pollution in water is also considerable and serious environmental problems in the world, and the damage of its toxicity to human health has brought a menace to our society. We have challenged to develop an efficient method for removal of arsenic ion, As(V), from polluted solution by using active iron species from ferrous-calcium carbonate system (FeSO4-CaCO3). Fig. 8 shows a schematic diagram of the formation of ferric compound from ferrous sulfate (FeSO4) with CaCO3 for arsenic removal. The basic idea of the ferric compound is similar to the case of phosphate anion removal, but the small difference is that the relatively slow hydrolysis of CaCO3 would release gradually OH− which played a role to regulate the generation of active iron species and ensured the sufficient reaction time for adequately As5+ removal (Zhang et al., 2019c). At the same time, the reaction avoids the conversion of new ecological iron to low-reactive FeO(OH). Moreover, as the content of the arsenic-removing sediment can be significantly reduced by using CaCO3, the filtration speed is increased by nearly 6 times, and the volume of sediment is also reduced by about 2/3. The treating system in this work shows superior properties such as large sediment size, small sediment volume and high filtration rate, resulting in remarkable record in the processing of arsenic removal. Non-oxidative direct removal of As3+ in solution is possible by calcium carbonate with Fe3+, as shown in Fig. 7(a), and the Fe3+ hydrated oxide (Fe(OH)3) would be formed on the surface of CaCO3 under the action of OH− released by the slow hydrolysis, which is able to maintain high reactivity to As3+. When the Fe/As molar ratio is 4.5, it can achieve directly precipitation of As3+ of about 100 % removal efficiency without pre-oxidizing from As3+ to As5+ by forming basic ferric arsenite of low solubility instead of high-soluble ferric arsenite. This would open a new approach for removal of arsenite anions in the solution without preoxidation.

Schematic diagram of the formation of ferric compound from ferrous (FeSO4) with CaCO3 for arsenic removal (Zhang et al., 2019c).
As an efficient functional material to deal with environmental issues, zero-valent iron (ZVI) (Fe0) is widely used from the viewpoint of high reduction potential and environmental-friendly property. In order to prepare ZVI with high reactivity, many studies have been conducted with the focuses on sample preparation, modification or protection processes. To prevent the rapid oxidation of ZVI by oxygen dissolved in the process of the aqueous phase reaction, it is often necessary to pass through the inert gas such as helium, or to use organic solvent such as ethanol and acetone, to clean and preserve the ZVI product during the purification. Furthermore, bare ZVI tends to agglomerate due to its fine particles and magnetic property, which will hinder the adsorption and reduction capabilities. There has been investigation on coating the surface of ZVI with stable materials such as clay, graphene, carbon nanotubes (CNT), activated carbon (AC) and so on. The methods proposed are centered on protecting the fresh surface of the resultant ZVI and have achieved the desired results. However, most of them are the stage of lab research, and it would be better to enhance more, looking at the large-scale application of ZVI to the environmental use, for example.
We have attempted to prepare adsorbent of ZVI coated with CaCO3 activated mechanochemically. Our expectation is to apply this ZVI-CaCO3 composite to the removal of As3+ in solution, and its idea is shown in Fig. 9. The sample preparation starts from milling a mixture of CaCO3 and ZVI in air, which remains as mixture after milling operation. As shown in the figure, the change in adsorption capacity of the product for As3+ is found to vary with the CaCO3 content in the composite of (ZVI-CaCO3) prepared by the milling operation. Of course, neither ZVI nor CaCO3 show the adsorption capacity for the arsenic anions, but the composite activated by the milling indicates clearly increment in the adsorption capacity with the content of CaCO3 in it and reaches the maximum value at about 50 % in CaCO3 content of the composite. The value, 50 %, in CaCO3 content means the mass ratio of CaCO3 to ZVI as 1:1, enabling us to produce this composite in large scale production.

The adsorption capacity of the ZVI-CaCO3 samples to As3+ anions in the solution as a function of the content of CaCO3 in ZVI-CaCO3 system.
As to the reaction mechanism of ZVI toward arsenic, both oxidization and reduction may happen depending on the atmosphere. With an oxidative atmosphere of air, oxidization mechanism proceeds to generate more high active oxidative intermediates. When the oxidation from As3+ and Fe2+ to As5+ and Fe3+, respectively, is accelerated by some reason, it results in the removal of As3+ from the solution. However, in general, ZVI itself is very active substance and the passivation layer formed on the surface of ZVI will prevent from its reaction with other substance. Milling operation to ZVI may cause some mechanical change like breakage in the passivation layer, and this may trigger the reaction, causing the oxidization of the fresh surface. In addition, the milling ZVI with CaCO3 makes it possible to form coated layer like the passivation one over the fresh surface of ZVI without any agglomerates. This may be the reason why the surface of ZVI in the composite is kept detaching during the removal process of As3+ by the composite, ZVI-CaCO3. Thus, the milling the mixture of ZVI and CaCO3 plays a big role to continuous exposure of fresh ZVI surface promoting the excellent adsorption ability for arsenic anions.
Besides the oxidative effect of ZVI on arsenite with an aid of oxygen in air, another example for the composite of ZVI and CaCO3 can be seen as follow: This phenomenon is attributed to reductive ability of this composite, indicating the reduction precipitation of hexavalent chromium (CrO42−) in solution. A tremendous improvement in the removal reaction by using the composite of ZVI and CaCO3 was obtained, in comparison with the adsorption results for the samples of raw ZVI, ground ZVI without CaCO3 addition. Fig. 10 shows SEM image of a mixture of the raw Fe0 sample, (a) ground Fe0 sample at 500 rpm for 8 h (b), ZVI and CaCO3 milled at 500 rpm for 8 h (c), and CrO42- removal from the solution as a function of the dosage for the four kinds of samples (d) ground by the stirred mill at the same condition (500 rpm for 8 h). Fine CaCO3 particles in nanometer size coated on the ZVI particles in micron-meter size are clearly observed from the SEM image, demonstrating the protective effect of CaCO3 fine particles to the ZVI ones. Three kinds of samples, raw ZVI, ZVI ground and CaCO3 ground, did not exhibit observable performance for removing CrO42−. This may be due to the passivation layer on the ZVI. However, the rate is increased drastically with an increase in the dosage of the sample for the ZVI-CaCO3 composite, and the value reaches 99.68 % at 1.0 in the dosage. The milling time required to this highest value is 30 min, and the solution containing CrO42− (10 mg/L) is obtained, and the ZVI mass ratio in the ZVI-CaCO3 composite is 30 wt.%. Addition of CaCO3 to the ZVI and its milling for some time plays significant role in both removal ability of CrO42− in the solution and protection of the fresh surface of active ZVI, enabling us to give such superior performance as ZVI. Besides the environment-friendliness of the preparation process, waste solid materials containing iron particles may be one of the candidates as the starting sample in order to prepare such adsorbent of ZVI-CaCO3 composite for practical use (Gu et al., 2019).

SEM images of different samples, (a) the raw Fe0 sample, (b) ground Fe0 sample at 500 rpm for 8 h, (c) mixture of Fe0 and CaCO3 milled at 500 rpm for 8 h and (d) removal (%) as a function of the dosage for four kinds of samples under the same conditions (Gu et al., 2019).
A simple milling operation can be used as a typical modifying tool for preparing and modifying non-metallic (industrial) minerals to increase the capacity as absorbents for environmental remediation. Depending on the purposes, either wet or dry milling operations is selected to achieve excellent performance of the adsorbents for pollutant removals in the solution. Intenseness in milling operation is depending on the milling conditions such as milling speed and time, mill vial size, ball charge, sample charge and so on, but it is also important to find out the most effective factor to trigger chemical changes of the target sample. Milling a material causes mechanochemical activation and/ or reaction to form the product which has unique properties and they are not the same one prepared by different milling conditions. In the precipitation of certain ions in solution, it is important to analyze its mechanism chemically in the solution, finding out the crucial aspects to remove a target ion, for example effectively and selectively. This means the both science and engineering knowledge and experience are the key to develop new futuristic methods and materials.
In this review, we have introduced current situation in wastewater treatment, and need adsorbents which would be environmental-friendly ones as much as possible. Under such circumstance, we have challenged and developed several new mechanochemical routes for preparing mineral based adsorbent with effective purification ability for toxic matter-containing wastewater. The minerals chosen in the experiments are non-metallic minerals such as hydrated magnesium silicate (serpentine), hydrated aluminum silicate (kaolinite), carbonate (calcite), calcite with ferrous compound and calcite with zero-valent iron. The mills used are mainly stirred mill and planetary mill in laboratory scale. The direction to prepare the adsorbent sample activated by the mill was shown in each section, and the main results are also introduced.
We have applied some adsorbents prepared by our methods to real and actual spots where solutions are contaminated with toxic substances and heavy metal ions in China. We have also obtained good data as a practical use so far, and in the near future, other spots will be selected for the removal of contaminants in solution by the use of the adsorbents. In the meanwhile, based on the requirement, it would be possible to prepare the adsorbent samples in large scale.
The authors are grateful to Emeritus Prof. Fumio Saito, Tohoku University, for his invaluable comments and suggestion on this paper.
Huimin Hu
Ph.D student, Wuhan University of Technology, China.
She received her Master’s degree (2015) in Environmental Science and Engineering from Wuhan University of Technology, where she specialized in purification of heavy metal wastewater through mechanically activated non-metallic minerals. She is now pursuing her Ph.D. in Environmental Science and Engineering under the supervision of Prof. Qiwu Zhang at the Wuhan University of Technology. Her research is currently focused on the development of perovskite-type oxide for wet catalytic oxidation of organic wastewater.
Qiwu Zhang
Professor, Wuhan University of Technology, China.
He obtained his bachelor and master degrees in 1984 from Wuhan University of Technology and 1987 from the graduate school in Beijing of Wuhan University of Technology, China. He worked as an engineer in Zhengzhou Institute of Multipurpose Utilization of Mineral Resources, China. He obtained his Ph.D degree in 1997 from Tohoku University, Japan. He worked as a faculty member in Tohoku University from 1997 to 2014. In 2004 he visited Ames Laboratory, Iowa State University as a research fellow. From 2015, he joined the Wuhan University of Technology. His research interest is in the field of mechanochemistry, covering various applications of mineral processing, environmental purification, material science and energy transformation by applying the discovered phenomena from mechanical activation.