2022 Volume 39 Pages 119-129
2022 Volume 39 Pages 119-129
Recently, various types of functionalized metal oxide nanoparticles have been used for many applications because of their unique chemical and physical properties. To synthesize metal oxide nanoparticles, liquid-phase synthesis techniques have been developed. The production process of metal oxide nanoparticles in aqueous media is extremely complex because the formation, crystal structure, crystallinity, chemical composition, and morphology of the particles are considerably dependent on the preparation conditions (e.g., anion and cation concentrations and species, additives, solution pH, reaction temperature, and reaction time). Accordingly, clarifying these effects is fundamental to accurately understand the formation mechanism of metal oxide nanoparticles to further develop new functionalized nanoparticles. In this review, the influence of anions (Cl−, SO42−, and NO3−) and cations (Ni2+, Cu2+, and Cr3+) on the formation and structure of iron oxide nanoparticles in aqueous media is described.
In industries, various types of functionalized metal oxide nanoparticles, such as iron oxide, silica gel, alumina, zinc oxide, and titania, have been developed and used for many applications (e.g., catalysis, adsorbent, biomedicine, magnetic data storage, agriculture, fuel cell, pigment, and sensor material) because of their unique chemical and physical properties (Iler R.K., 1979; Cornell R.M. and Schwertmann U., 1996; 2000; Fujishima A. et al., 1999; Rodríguez J.A. and Fernández-García M., 2006; Chavali M.S. and Nikolova M.P., 2019; Korotcenkov G. et al., 2019). To further improve the particle properties, controlling the particle shape and size, monodispersity, and crystallinity is necessary. However, in general, the synthesis of high-quality nanoparticles, such as monodisperse single-crystal nanoparticles, is considerably difficult (Matijević E., 1985; Sugimoto T., 1987; 2001; Pelizzetti E., 2011). Metal oxide nanoparticles can be produced using solid, liquid, and gas-phase synthesis techniques. Among these, the liquid-phase synthesis affords several advantages (Gutch A. et al., 2004; Feng J. et al., 2015; Karatutlu A. et al., 2018): (1) low-cost particle production can be performed; (2) surface active nanoparticles can be obtained; (3) particles from a homogeneous solution can be prepared; (4) preparation conditions (e.g., reaction temperature and time, anion and cation concentrations, additives, solution pH, and raw materials) can be easily controlled; (5) particle shape and size can be regulated by the abovementioned preparation conditions and method. Based on these benefits, various liquid-phase synthesis techniques for the production of metal oxide nanoparticles have been developed, and approximately 45 % of functionalized nanoparticles are synthesized through these methods in the industry (Charitidis C.A. et al., 2014). The liquid-phase synthesis can be classified into two approaches: “Top-down approach” and “Bottom-up approach.” In the “Top-down approach,” nanoparticles are synthesized by etching the macro-sized or micro-sized bulk materials in aqueous media (e.g., wet milling, lithography, laser ablation, and chemical etching). In the “Bottom-up approach,” nanoparticles are prepared by assembling small blocks, such as atoms and molecules, in aqueous media (e.g., sol-gel, precipitation, pyrolysis, and chemical reduction methods).
The formation and growth processes of metal oxide nanoparticles in aqueous media have been investigated (Ostwald W.Z., 1900; LaMer V.K. and Dinegar R.H., 1950; Reiss H., 1951; LaMer V.K., 1952; Feitknecht W., 1959; Lifshitz I.M. and Slyozov V.V., 1961; Hsu P.M., 1972; Hsu P.M. and Ragone S.E., 1972; Misawa T. et al., 1974; Dousma J. et al., 1976; 1978; 1979; Murphy P.J. et al., 1976; Blesa M.A. and Matijević E., 1989; Cornell R.M. and Schwertmann U., 1996; 2000; Ishikawa T., 1997; Schultz M. et al., 1999; Jolivet J-P., 2000; Kandori K., 2002; Tamura H., 2008; Thanh N.T.K. et al., 2014; Plote J., 2015). Fig. 1 shows the formation and growth processes of iron oxide nanoparticles from aqueous Fe3+ solution.

Formation and growth processes of iron oxide particles from aqueous Fe3+ solution.
| (1) |
The Fe(H2O)63+ ions are hydrolyzed to generate Fe(OH)n(H2O)6–n3+ ions (n = 1–6) via protolysis reaction (2).
| (2) |
Further, the Fe(OH)n(H2O)6–n3+ ions are condensed via oxolation reaction (3).
| (3) |
Then, the >Fe–O–Fe< groups are hydrolyzed via olation reaction (4).
| (4) |
Reactions (1)–(4) continuously proceed to yield multi-nuclear Fe3+ complex ions. These ions further grow to produce an embryo through ion collisions in which the reaction continuously breaks down and reforms during the reversible reaction stage. When the embryo exceeds a certain critical size, it becomes a nucleus, which is considerably more stable. The generated nucleus is further grown to form fine primary particles, which transform into crystals via two possible routes: “aggregation mechanism” and “ion diffusion growth mechanism”. In the former route, aggregates are generated by the agglomeration of primary particles. During aging, the primary particles in the aggregates solidify into crystalline particles. In the latter mechanism, solute ions are diffused and deposited on the primary particles to form crystalline particles. The growth process of α-FeOOH particles is depicted in Fig. 2. In this case, the primary particles composed of fine crystals are regularly combined by –O– and –OH– bonds to crystallize as α-FeOOH. Accordingly, the formation process of metal oxide nanoparticles is considerably complex and is not fully understood. The formation, crystal structure, crystallinity, chemical composition, and particle morphology are extremely dependent on the preparation conditions, such as anion and cation concentrations and species, additive, solution pH, and reaction temperature and time. Among these, anion and cation were observed to markedly affect the formation and structure of iron oxides. The phase stability of γ-FeOOH was lowered by Cl− and SO42− (Sudakar S. et al., 2003). Moreover, the increase of [SO42−]/[Cl−] ratio in the aqueous Fe2(SO4)3–FeCl3 solution accelerated the α-FeOOH formation (Oh S.J. et al., 2002). Further, the addition of SO42− and SO32− significantly impeded the formation and crystallization of β-FeOOH (Ishikawa T. et al., 2005; Kamimura T. et al., 2005; Tanaka H. et al., 2016a; b). The crystallization and growth of α-FeOOH and β-FeOOH particles were inhibited by adding Cu2+, Ni2+, Cr3+, and Ti4+ (Inouye K. et al., 1968; 1972; Ishikawa T. et al., 2000; 2001; 2002; 2003; 2009; Nakayama T. et al., 2005; Tanaka H. et al., 2013a; b). Accordingly, elucidating these effects is fundamental to accurately understand the formation mechanism of metal oxide nanoparticles.

Growth process of α-FeOOH crystal.
In this review, the influence of anions and cations on the formation and structure of iron oxide nanoparticles is described based on our research. The iron oxide nanoparticles were synthesized in the presence of anions (Cl−, SO42−, and NO3−) and cations (Ni2+, Cu2+, and Cr3+) (Tanaka H. et al., 2013a; b; 2015; 2016a; 2018). The results can aid in developing new functionalized nanomaterials using the liquid-phase synthesis technique.
The influence of anions on the formation of iron oxide nanoparticles was examined by synthesizing the particles from Fe3+ solutions containing Cl−, NO3−, and SO42−. A mixture of aqueous FeCl3, Fe2(SO4)3, and Fe(NO3)3 solutions were aged at 50 °C for 48 h. Prior to aging, the solution pH was approximately 2. The generated precipitates were filtered out, thoroughly washed with deionized-distilled water, and air-dried at 50 °C.
The amount of products formed is shown in Fig. 3. This amount is increased by incrementing the quantity of FeCl3 added to the FeCl3–Fe(NO3)3 system. In the Fe(NO3)3–Fe2(SO4)3, Fe2(SO4)3–FeCl3, and FeCl3–Fe(NO3)3–Fe2(SO4)3 systems, increasing the quantity of Fe2(SO4)3 addition reduces the amount of formed products. The amount of these products eventually become zero when higher amounts of SO42− are added. These results indicate that the formation of iron oxides is inhibited by the addition of SO42−.

Plots of amount of products formed in FeCl3–Fe(NO3)3–Fe2(SO4)3 system. Adapted with permission from Ref. (Tanaka H. et al., 2013a). Copyright: (2013) Elsevier B.V.
The major formation species of iron oxides is shown in Fig. 4. Iron-oxyhydroxysulfate (Fe8O8(OH)6(SO4)·nH2O: Schwertmannite) is formed by adding SO42−, however, the addition of a large amount of SO42− prevents the generation of iron oxides. In contrast, β-FeOOH is mainly yielded in the FeCl3–Fe(NO3)3 system, and α-FeOOH is only generated in the Fe(NO3)3 system, indicating that the FeCl3–Fe(NO3)3 system favors the formation of β-FeOOH rather than α-FeOOH.

M ajor iron oxides formed in FeCl3–Fe(NO3)3–Fe2(SO4)3 system. (
 ) α-FeOOH, (
) α-FeOOH, (
 ) β-FeOOH, (
) β-FeOOH, (
 ) Schwertmannite, (
) Schwertmannite, ( ) no precipitates. Adapted with permission from Ref. (Tanaka H. et al., 2013a). Copyright: (2013) Elsevier B.V.
) no precipitates. Adapted with permission from Ref. (Tanaka H. et al., 2013a). Copyright: (2013) Elsevier B.V.
Transmission electron microscopy (TEM) images of the formed particles are shown in Fig. 5. The spherical α-FeOOH particles with an average diameter of 358 nm are generated in the Fe(NO3)3 system. In the FeCl3–Fe(NO3)3 system, the spherical α-FeOOH particles virtually diminish, and rod-shaped β-FeOOH particles are observed. Further, as the amount of added Cl− increases, the mean length and width of β-FeOOH particles increase from 677 nm to 1.48 μm and from 130 to 238 nm, respectively. Hence, the incorporation of NO3− slightly suppresses the growth of β-FeOOH particles. In the Fe(NO3)3–Fe2(SO4)3 system, needle-like Schwertmannite particles with a mean length and width of approximately 260 and 15 nm, respectively, are mainly formed. By adding NO3−, the Schwertmannite particles disappear, and irregular-shaped α-FeOOH particles with an average size of 68 nm are generated. In the Fe2(SO4)3–FeCl3 system, increasing the amount of added SO42− decreases the particle size of β-FeOOH and forms Schwertmannite particles (mean length = 136 nm, mean width = 49 nm). In the FeCl3–Fe(NO3)3–Fe2(SO4)3 system, due to the Schwertmannite, only needle-like particles with a length and width of approximately 310 and 40 nm are recognizable. These facts reveal that the presence of SO42− in the aforementioned system remarkably precludes the formation of α-FeOOH and β-FeOOH particles and produces needle-like Schwertmannite particles. Note that a higher SO42− content in the solution inhibits the formation of particles. Thus, the effect of SO42− on the formation of iron oxide particles is greater than that of Cl− and NO3−.

TEM images of the particles formed in FeCl3–Fe(NO3)3–Fe2(SO4)3 system. Adapted with permission from Ref. (Tanaka H. et al., 2013a). Copyright: (2013) Elsevier B.V.
These results indicate that anions, such as SO42−, Cl−, and NO3−, affect the formation, crystallization, and particle growth of iron oxides, and the effect is in the order SO42− >> Cl− > NO3−. The affinity of anions to Fe3+ can be evaluated through the stability constant (log K) of Fe3+ complexes with anions. The stability constant of Fe3+ complexes with SO42− is 4.1 and is considerably larger than that with NO3− (1.0) and Cl− (0.6), indicating the strong coordination of SO42− with Fe3+ compared with NO3− and Cl− (Sillen L.G. and Martell A.E., 1964). Hence, the SO42−-containing aqua Fe3+ complex [Fe(SO4)m(H2O)6–m]3+ is formed in the Fe(NO3)3–Fe2(SO4)3, Fe2(SO4)3–FeCl3 and FeCl3–Fe(NO3)3–Fe2(SO4)3 systems via hydrolysis reaction (5).
| (5) |
Moreover, [Fe(SO4)m(H2O)6–m]3+ is hydrolyzed via protolysis reaction (6).
| (6) |
Consequently, when a small amount of SO42− is added, the major product becomes Schwertmannite containing SO42−. Upon increasing the amount of added SO42−, the SO42− content in the [Fe(SO4)m(H2O)6–m]3+ ions increases and the number of active >Fe–OH groups is reduced, resulting in the suppression of olation reaction (3). Accordingly, no precipitates are generated at higher SO42− contents in the solution. In contrast, β-FeOOH is mainly produced in the FeCl3–Fe(NO3)3 system. The stability constants of Fe3+ complexes with Cl− and NO3− are considerably lower than that with SO42−. Hence, the addition of Cl− and NO3− prevents the progress of reactions (1)–(4), leading to the formation of the amorphous precursor of α-FeOOH and β-FeOOH particles during aging. It has been established that the isoelectric point (iep) of iron oxides and oxyhydroxides, such as α-FeOOH, β-FeOOH, γ-FeOOH, Fe3O4, and α-Fe2O3, is a neutral pH, and aforementioned materials have a positive surface charge in the acidic solution (Cornell R.M. and Schwertmann U., 1996; 2000). Moreover, the ionization energy of NO3− is larger than that of Cl− (Lang P.F. and Smith B.C., 2003). Therefore, the added Cl− preferentially adsorbs on the surface of the amorphous precursor to form Cl−-containing β-FeOOH particles. However, the decrease in the amount of added Cl− reduces the size of β-FeOOH particles, indicating the inhibition of their growth as a result of the adsorption of NO3− on the precursors.
To clarify the influence of anion and solution pH on the formation of iron oxide nanoparticles, the particles were synthesized from the FeCl3–Fe2(SO4)3–Fe(NO3)3 system at different OH−/3Fe3+ molar ratios. The OH−/3Fe3+ ratio ranges 0–1.5 through the addition of NaOH solutions. The pH titration curves of aqueous FeCl3, Fe2(SO4)3, and Fe(NO3)3 solutions are shown in Fig. 6. All the curves are practically identical, indicating that the solution pH is independent of the anions. At OH−/3Fe3+ = 0–1.0, the solution pH becomes practically constant (i.e., 2–3) because of the reaction of Fe3+ with the added OH− to precipitate Fe(OH)3. At OH−/3Fe3+ > 1.0, the pH level of the solution suddenly increases because all the Fe3+ transforms into Fe(OH)3. The major products synthesized at OH−/3Fe3+ = 0–1.5 are shown in Fig. 7. At OH−/3Fe3+ = 0.5, the hydrated iron oxide Fe2O3•nH2O particles are mainly produced in the FeCl3–Fe(NO3)3 system. In contrast, the Fe2(SO4)3–FeCl3, Fe(NO3)3–Fe2(SO4)3, and FeCl3–Fe2(SO4)3–Fe(NO3)3 systems enhance the α-FeOOH formation. This is caused by the transformation of Schwertmannite during aging because this material is among the precursors of α-FeOOH (Cornell R.M. and Schwertmann U., 1996; 2000; Tanaka H. et al., 2013a; 2015; 2016a; Muguruma et al., 2015). The major products at OH−/3Fe3+ = 1.0 and 1.5 (pH levels are approximately 7 and 14, respectively) are ferrihydrites (Fe5HO8·4H2O) and α-FeOOH, respectively. The foregoing means that raising the solution pH before aging lowers the influence of anions on the structure and composition of iron oxide nanoparticles.

pH titration curves of a queous FeCl3, Fe(NO3)3 and Fe2(SO4)3 solutions. Adapted with permission from Ref. (Tanaka H., 2015). Copyright: (2015) The Society of Powder Technology, Japan.

Major product of the iron oxides formed at OH−/3Fe3+ = 0–1.5. (
 ) α-FeOOH, (
) α-FeOOH, (
 ) β-FeOOH, (
) β-FeOOH, (
 ) Schwertmannite, (
) Schwertmannite, ( ) no precipitates, (
) no precipitates, (
 ) Fe2O3·nH2O, (
) Fe2O3·nH2O, (
 ) Ferrihydrite. Adapted with permission from Ref. (Tanaka H., 2015). Copyright: (2015) The Society of Powder Technology, Japan.
) Ferrihydrite. Adapted with permission from Ref. (Tanaka H., 2015). Copyright: (2015) The Society of Powder Technology, Japan.
The influence of cations on the formation of iron oxide nanoparticles was examined by synthesizing the α-FeOOH particles with Ni2+, Cu2+, and Cr3+. Metal salts, such as NiSO4, CuSO4, or Cr(NO3)3, and FeSO4 were dissolved in O2-free deionized-distilled water, of which the metal/Fe atomic ratio of the solution was 0–0.1. The aqueous NaOH solution was added to the solution until the OH−/2Fe2+ ratio reached 0.05. The generated suspension was aged in bubbling air at 50 °C for 24 h. The resulting precipitate was filtered out, washed with deionized distilled water, and air-dried at 50 °C.
X-ray diffraction (XRD) patterns of the products synthesized with Ni2+, Cu2+, and Cr3+ are shown in Fig. 8. As shown in Fig. 8(A), with the addition of Ni2+, the intensity and position of α-FeOOH peaks remain virtually unchanged. As shown in Fig. 8(B), with Cu2+ addition, the α-FeOOH peaks are weakened and broadened by increasing the Cu/Fe ratio. A similar tendency is observed with the addition of Cr3+ as shown in Fig. 8(C). Besides, the α-FeOOH peaks vanish at Cr/Fe ≥ 0.04 and broad peaks ascribed to Schwertmannite are developed. Furthermore, sharp peaks due to Natrojarosite NaFe3(SO4)2(OH)6 appear at Cr/Fe ≥ 0.08. Hence, the addition of Cu2+ and Cr3+ inhibits the crystallization of α-FeOOH and a considerable amount of added Cr3+ impedes the transformation of Schwertmannite into α-FeOOH. The crystallite size of α-FeOOH, which is calculated using the Scherrer equation, is plotted against the metal/Fe ratio in Fig. 9. The crystallite size is 24.1 nm when metal/Fe = 0 and it decreases as the Cu/Fe ratio increases up to 0.02, indicating that the crystallization of α-FeOOH is inhibited. The Cr3+ addition also reduces the crystallite size and its effect is slightly higher than that of Cu2+ addition. Nonetheless, no considerable influence is observed by Ni2+ addition. These results indicate that the addition of Cu2+ and Cr3+ inhibits the crystallization of α-FeOOH and the effect is in the order Cr3+ > Cu2+ >> Ni2+.

XRD patterns of the products synthesized in the presence of (A) Ni2+, (B) Cu2+ and (C) Cr3+. metal/Fe: (a) 0, (b) 0.005, (c) 0.01, (d) 0.02, (e) 0.04, (f) 0.06, (g) 0.08, (h) 0.1. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.

Crystallite size of α-FeOOH vs. atomic ratio metal/Fe. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.
In Fig. 10 is plotted the metal/Fe atomic ratio of the particles estimated from the Ni, Cu, Cr, and Fe contents against the metal/Fe ratio of the solution. It is clearly observed that the Cr/Fe ratio of the particles is considerably larger than that of the solution. However, the Cu/Fe and Ni/Fe ratios of the particles are lower than those of the starting solutions. These indicate that the added Cr3+ is more easily incorporated in the particles than Fe3+, in contrast, the uptake of Ni2+ and Cu2+ is difficult.

Plots of atomic ratio metal/Fe in particles against atomic ratio metal/Fe in solution. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.
TEM images of the particles generated in the presence of Ni2+, Cu2+, and Cr3+ are shown in Fig. 11. When metal/Fe = 0, rod-shaped α-FeOOH particles with a length and width of approximately 270 and 40 nm, respectively, are recognized. The morphology of α-FeOOH particles is unchanged by Ni2+ addition, indicating that the added Ni2+ has no considerable influence on the growth of α-FeOOH particles. At Cu/Fe = 0–0.01, no significant change in the morphology of α-FeOOH particles is observed. At Cu/Fe ≥ 0.02, the rod-shaped α-FeOOH particles transform into irregular α-FeOOH particles with a size of approximately 65 nm. Similar to Cu2+ addition, with Cr3+ addition, increasing the Cr/Fe ratio up to 0.01 substantially reduces the size of α-FeOOH particles to approximately 50 nm and alters the rod-shaped particles into irregular ones. These results indicate that the growth of α-FeOOH particles is considerably suppressed by Cu2+ and Cr3+ addition. At Cr/Fe ≥ 0.04, irregular-shaped Schwertmannite particles with a size of approximately 25 nm are observed. Furthermore, hexagonal Natrojarosite particles with a size of approximately 0.8 μm appear when Cr/Fe ≥ 0.06.

TEM images of the particles generated with Ni2+, Cu2+ and Cr3+. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.
The formation and crystallization of iron oxide particles considerably depend on the solution pH (Misawa T. et al., 1974; Blesa M.A. and Matijević E., 1989; Cornell R.M. and Schwertmann U., 1996; 2000; Tamura H., 2008). The solution pH before aging is plotted as a function of metal/Fe atomic ratio in Fig. 12. The solution pH before aging is 7.0 at metal/Fe = 0 and is essentially not altered by Ni2+ addition. Further, the solution pH before aging is reduced by adding Cu2+ and Cr3+ up to metal/Fe = 0.02 and remains practically constant at approximately 4.5 and 4.0, respectively. This is due to the formation of Cu(OH)2 and Cr(OH)3 through the addition of NaOH solution because the reactions of Cu2+ and Cr3+ with OH− progress at pH ≥ 4.2 and ≥ 4.0, respectively (Baes Jr C.F. and Mesmer R.E., 1986). Hence, lowering the solution pH before aging by adding Cu2+ and Cr3+ suppresses the crystallization and particle growth of α-FeOOH. To verify this, α-FeOOH particles were prepared at pH = 4.0 and 7.0 without metal ion addition. The XRD patterns and TEM images of the obtained particles are depicted in Fig. 13. It is evident that the reduction in solution pH before aging significantly weakens and broadens the α-FeOOH peaks. Moreover, the crystallite size of α-FeOOH obtained at pH = 4.0 is 7 nm less than that at pH = 7.0 (24 nm). Further, the rod-shaped particles become irregular with a size of ca. 30 nm. These results indicate that reducing the solution pH before aging by adding Cu2+ and Cr3+ considerably prevents the crystallization and particle growth of α-FeOOH. The crystal structure of α-FeOOH consists of double chains of edge-shared Fe3+ octahedron, as shown in Fig. 2 (Cornell R.M. and Schwertmann U., 1996; 2000). The ionic radii of octahedral Ni2+ (0.069 nm) and Cu2+ (0.072 nm) are larger than that of octahedral Fe3+ (0.064 nm), hence, the substitution of Ni2+ and Cu2+ with Fe3+ in the α-FeOOH crystal is difficult (Shannon R.D., 1976). The solution pH before aging is markedly decreased by addition of Cu2+ and Cr3+, consequently impeding the growth of α-FeOOH crystals and particles, nonetheless, Ni2+ addition exhibits no distinct influence. In contrast, the added Cr3+ is easily incorporated into the α-FeOOH crystal by substitution with Fe3+ to form α-CrxFe1–xOOH solid solution because the ionic radius of Cr3+ in the octahedron (0.063 nm) is the same as that of Fe3+ (0.064 nm) (Shannon R.D., 1976). Further, increasing the amount of added Cr3+ induces the formation of Cr3+-doped Schwertmannite particles. As mentioned above, Schwertmannite is one of the precursors of α-FeOOH. Therefore, the incorporation of Cr3+ into Schwertmannite stabilizes its crystal structure, thus inhibiting its transformation into α-FeOOH. Consequently, the formation, crystallization, and particle growth of α-FeOOH are markedly impeded by Cr3+, and the effect is in the order Cr3+ > Cu2+ >> Ni2+.
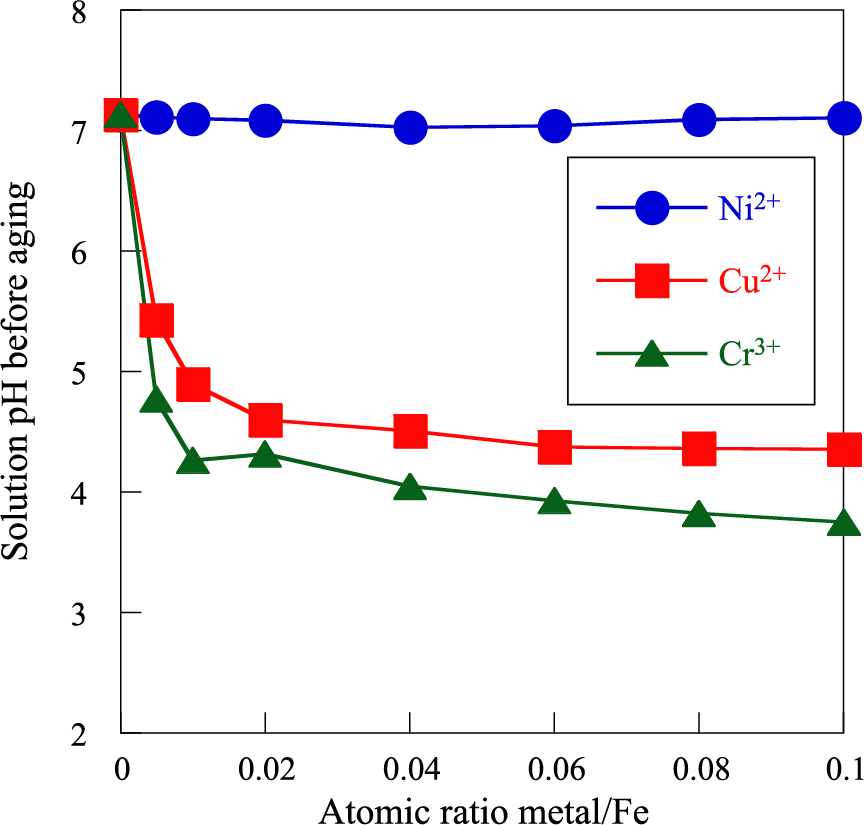
Plots of the solution pH before aging as a function of atomic ratio metal/Fe. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.

XRD patterns and TEM images of α-FeOOH particles prepared at pH = 4.0 and 7.0. Adapted with permission from Ref. (Tanaka H. et al., 2018). Copyright: (2018) Elsevier B.V.
Recently, various functionalized metal oxide nanoparticles with unique chemical and physical properties have been developed and applied in the fields of chemical engineering, medicine, food, cosmetics, environment, etc. The particle properties are extremely dependent on the crystal structure, crystallinity, chemical composition, and particle morphology. Therefore, nanoparticles with high crystallinity, homogeneous chemical composition, uniform shape and size, and narrow particle size distribution possess excellent chemical and physical properties. However, the synthesis of such particles is extremely difficult. Hence, many researchers have investigated the synthesis of high-quality nanoparticles using the liquid-phase synthesis technique. The formation of nanoparticles is considerably affected by a number of factors, such as the anion and cation concentrations and species, additive, solution pH, and reaction temperature and time. To derive high-quality nanoparticles, gaining insight on the fundamental principles of the formation mechanism and process of nanoparticles is necessary. Accordingly, a systematic study of the particle formation is absolutely essential for the development of new functionalized nanoparticles.
The author profoundly expresses gratitude to Professor Emeritus Tatsuo Ishikawa of Osaka University of Education and Dr. Takenori Nakayama of Kobe Steel Co., Ltd., for supporting this research. This study was partly supported by Grants-in-Aid for Scientific Research (C) (20K05156 and 17K06862) the Next Generation Tatara Co-Creation Centre (NEXTA), Shimane University. Here, NEXTA is established with Grant-in-aid for the Promotion of Regional Industries and Universities from Cabinet Office, Japan.
Hidekazu Tanaka
Hidekazu Tanaka received his Ph.D. from the Tokyo Metropolitan University in 1999. He worked as a JSPS Research Fellow PD at the Osaka University of Education from 1999 to 2000. He joined at the Shimane University as an Assistant Professor in 2000 and an Associate Professor in 2006. In 2014, he became a Professor of Graduate school of Natural Science and Technology at Shimane University. His research interest is wet-synthesis of highly functionalized inorganic particles by control of the particle morphology and modification of the surface structure focusing on the surface functional groups.