2022 Volume 39 Pages 29-44
2022 Volume 39 Pages 29-44
Understanding the flowability of cohesive powders at high temperature is of great importance for many industrial applications where these materials are handled at harsh thermal conditions. For instance, the Calcium-Looping (CaL) process, involving the transport, storage and fluidization of limestone powders at high temperature, is being considered nowadays as a promising technology for thermochemical energy storage (TCES) in concentrated solar power plants (CSP). In this context, the High Temperature Seville Powder Tester (HTSPT) is presented in this work as a useful tool to analyze how the flow behavior of cohesive powders changes with temperature. The manuscript reviews the main results obtained so far using this novel apparatus. The change of powder cohesiveness and therefore of powder flowability as depending on temperature, particle size, material properties and nanosilica surface coating is illustrated.
The calcium looping (CaL) process has been widely investigated as a 2nd generation technology to capture CO2 from fossil fuel power plants with already proven high efficiency in large scale pilot plants (1–2 MWh) (Barker, 1974; Blamey et al., 2010; Chacartegui et al., 2016; Kierzkowska et al., 2013; Prieto et al., 2016; Valverde, 2013). The CaL technology benefits from the abundance, low price and lack of toxicity of natural CaO precursors such as limestone and dolomite that can be used as raw material. Basically, the CaL process relies on the reversible calcination/carbonation reaction
| (1) |
During the exothermic carbonation reaction, solid CaO particles react in a fluidized bed reactor at approximately 650 °C with the flue gas containing a small concentration of CO2 (around 15 % vol). The carbonated particles are then taken to a fluidized bed calciner where the endothermic decomposition of CaCO3 takes place to regenerate the CaO at temperatures above 900 °C in a high CO2 concentration environment (Valverde, 2013; 2015). More recently, the CaL process has been the subject of several studies to store energy in concentrated solar power (CSP) plants (Chacartegui et al., 2016; Paksoy, 2007; Pardo et al., 2014). Currently, small scale pilot plants are under development showing promising results (Cordis, 2020).
Due to the growing need to employ green renewable energy, the massive deployment of CSP plants has come up as an affordable and highly efficient solution for electricity generation at large scale. However, the efficiency of CSP plants is hindered by the intermittence of solar direct radiation which leads to an unbalance between electricity production and demand. Thermal energy storage (TES) technologies allow releasing thermal energy to generate electricity when is required by means of heat storage materials, thus solving shortcomings and discontinuity issues. A major challenge to minimize the cost of CSP with storage and enhance its efficiency is to select adequate thermal energy storage system. Based on different factors, energy storage technologies are classified as sensible heat storage (SHS), latent heat storage (LHS), and thermochemical energy storage (TCES) (Hall and Hausz, 1979). Sensible heat storage stores and releases sensible heat by means of a change in the temperature of the material used. Synthetic oils, molten salts, liquid metals and concretes are the main sensible heat storage materials employable in CSP. Characterized by their high specific heat capacity, relatively low cost and acceptable safety, molten salts are being currently employed in commercial CSP plants (Fernández et al., 2014; Sang et al., 2015; Wu et al., 2017; Zhang et al., 2013). However, below temperatures around 200 °C, molten salts solidify, which requires keeping the whole circuit above this melting temperature even during prolonged periods of absence of solar direct solar radiation. In addition, above temperatures ~560 °C, molten salts degrade whereas the temperatures achievable at CSP plants with tower technology are around 1000 °C. Thus, the use of molten salts for power generation burdens the potential efficiency of CSP plants for power production. Moreover, molten salts are corrosive particularly at high temperature which requires the use of special equipment and therefore leads to high maintenance costs (Dunn et al., 2012; Ortega et al., 2008).
Latent heat storage or phase-change energy storage is based on the heat stored and released when the material experiences a phase change (evaporation, melting, crystallization etc.). The choice of phase change materials (PCMs) intended for TES applications needs to meet some suitable features: kinetic, physical, chemical, thermodynamic and, of course, economic affordability. Solid-liquid PCMs are the most used ones in research projects under development. Yet, these materials release low latent heat and only in the limited range of temperatures wherein phase transition occurs. In addition some significant drawbacks are their generally low thermal conductivity, phase separation, expensive maintenance costs, and also low operating temperature ranges (up to ~90 °C) (Herrmann and Kearney, 2002; Pintaldi et al., 2015; Sharma et al., 2009).
In this context, thermochemical energy storage (TCES) is considered as a promising technology for energy storage in CSP systems (Abedin, 2011; Cabeza et al., 2012; Paksoy, 2007; Tatsidjodoung et al., 2013), as demonstrated by the growing number of reviews published in the last years on this technology which is currently under development (André et al., 2016; Barker, 1974; Carrillo et al., 2019; Gil et al., 2010; Mahlia et al., 2014; Pardo et al., 2014). TCES relies on the storage/release of heat via reversible chemical reactions (Carrillo et al., 2019; Dinçer and Rosen, 2010). The most relevant advantages of TCES include the possibility of long term heat storage, high stored energy density, small energy loss and the suitability to operate at very high temperatures (up to 1000 °C or above (Abedin, 2011; Cabeza et al., 2012; Tatsidjodoung et al., 2013). Among the diverse options investigated for TCES a major candidate is the CaL process. Besides of the low cost, abundance and lack of toxicity of limestone used as raw material, a particularly relevant advantage of the integration of the CaL process in CSP plants is that the CaCO3 calcination temperature fits in the range of temperatures attainable at the receiver of CSP plants with tower technology (Pinel et al., 2011).
A possible technique for operating the CaL process in CSP plants is to use fluidized bed reactors albeit other type of reactors such as falling particle, entrained flow and centrifugal particle reactors that might be better integrated in solar receivers are being investigated (Cordis, 2020; Esence et al., 2020; Karasavvas et al., 2020; Lisbona et al., 2020; Sarrion et al., 2016; Tesio et al., 2019; 2020), CaCO3/CaO solids to be employed in the CaL-CSP integration would preferably be in the form of fine powders to mitigate reaction rate limiting mechanisms such as pore plugging (Benitez-Guerrero et al., 2017) that might hinder the process at the most favorable operating conditions for the CaL-CSP integration (Hanak et al., 2015). As it is well-known from experience, the lack of material flowability may be a serious issue in industrial applications where fine powders are employed due to their high cohesiveness, which can be enhanced even further at high temperatures (Chirone et al., 2020; Lettieri et al., 2000; Macrì et al., 2017; Tomasetta et al., 2011). The Sevilla Powder Tester (SPT) has proven itself as a reliable and accurate device for the characterization of cohesive powders (Barletta et al., 2019; Castellanos, 2005; Castellanos et al., 2004; Chen et al., 2009; Valverde et al., 2000; Zafar et al., 2015). Recently, it has been upgraded to be used at high temperatures. The present manuscript aims to present a detailed description of the so-called High Temperature Sevilla Powder Tester (HTSPT). We review the main results recently obtained by means of this novel setup, with a special focus on the effect of temperature on the tensile yield strength of fine powders as depending on particle size, surface coating and material properties.
Exhaustive research has been conducted with the aim of exploring relevant techniques to assess the ability of powders to flow (Schwedes, 2003). Here we summarize the main methods envisaged from a critical comparative analysis.
One of these methods, as described in the book of the American Society for Testing and Materials Standards (ASTM, 1978), is based on measuring the time required by a certain amount of powder to discharge through a hopper. Hall and Cutress (1960) showed that this technique can be useful for metallic powders. An external energy source should be provided to enhance the flow for very cohesive powders, which introduces uncertainty to the results. A rheometer composed of shear heads, blades and pistons is another tool to evaluate powder flowability. To measure the rotational and axial forces, the components of the setup are rotated and stirred in the axial direction. A detailed description of the setup can be found in (Freeman, 2004). The reliability of these testers is somewhat compromised due to the lack of reproducibility. For instance, the history of the powder is dependent on the filling procedure, the applied load, the device used and the working conditions (Castellanos et al., 2004).
At the microscopic level, interparticle contact forces are ultimately the main drive for powder flowability (Quintanilla et al., 2001a). Thus, the direct measurement of these forces is the best method to understand the physical mechanism that governs powder flow behavior. Shimada et al. (2002) conceived an instrument to directly measure adhesive contact forces between particles or between a plane surface and a powder sample. This study concerned organic powders (potato starch, lactose and cornstarch). An advantage of the device is that it can handle fine and very cohesive particles (even below 10 μm in size). Thereafter, by means of a camera or microscope, it analyses the adhesion force for a selected individual particle. They compared the obtained results with those found by another direct method (the centrifugal method) based also on a post-process image analysis of powder avalanches in a rotating drum (Lüddecke et al., 2021; Quintanilla et al., 2001b). The authors confirmed that both methods agree qualitatively. Unfortunately, these techniques do not allow to control the stress state of the powder that greatly impacts the contact forces (Quintanilla et al., 2001a). In this context, Quintanilla et al. (2001a) employed an atomic force microscope (AFM) to determine the adhesion force between irregularly shaped particles of xerographic toners. The load force previously applied on the individual particles can be accurately controlled through this technique. Comparing their findings with the interparticle forces estimated from measurements of the bulk stresses in the Seville Powder Tester (SPT) (Castellanos et al., 2004), they reported an acceptable agreement. However, it should be highlighted that the results on the forces directly measured using the AFM technique showed a large degree of dispersion, which was attributed to the critical dependence of the adhesion contact forces to the highly variable local surface properties (Castellanos, 2005; Castellanos et al., 2004).
Recently, Marchetti and Hulme-Smith (2021) investigated the flowability of 11 metal powders with different properties and particle size distributions employing six different testers. The ability of the powders to flow was determined by means of diverse indicators such as the flow rate, Hausner ratio, basic flowability energy (BFE), compressibility (CPS), major principal stress (MPS), angle of internal friction (AIF), flow factor (FF), angle of repose, specific energy (SE), and the unconfined yield stress (UYS). The interested reader could find further details about the powder testers and the different parameters in (Marchetti and Hulme-Smith, 2021; Schwedes, 2003; Castellanos et al., 2004; Lettieri and Macrì, 2016).
Marchetti and Hulme-Smith (2021) demonstrated the existence of correlations between these different parameters to characterize powder flow. They reported that, for cohesive powders, the Hall and Carney funnels are blocked, while the flowability indicator for these devices is the flow rate. Consequently, they are not appropriate in this case. Indeed, thanks to their simplicity, the tests based on fundamental properties (all the mentioned tests except for the rheometer with shear cell device) are widely employed in industrial and academic fields. However, the insufficient reproducibility represents a serious drawback (Ghadiri et al., 2020; Santomaso et al., 2003).
The Jenike shear cell is one of the most commonly used testers. Firstly developed by Jenike in 1953 (Schwedes, 1976), the powder sample in this test is previously subjected to a controlled consolidation stress. The sample is contained in a cylindrical cell formed by two superposed steel rings. Once the normal load is withdrawn, the tester determines the minimum steady state shear stress that must be applied to initiate powder flow. However, the Jenike tester is not suitable to evaluate powder flowability at very small consolidations (typically below 1 kPa). Besides, this technique assumes that the powder shears in a horizontal slip plane homogeneously across the sample, which is uncertain (Svarovsky, 1987).
The Peschl shear cell (Barletta et al., 2005; 2007; Bruni et al., 2007a; 2007b) was presented as an alternative to circumvent some of the above mentioned problems. The tester is essentially based on the same technic as the previously cited cells albeit the shear stress is exerted by the rotation of the upper part of the annular shear cell containing the powder sample (Svarovsky, 1987). This tester allows to determine the powder flow for an unrestrained shear distance and for a constant shear surface. Nevertheless, non-negligible wall effects and non-uniformity of stress distribution may give rise to strong uncertainties (Castellanos et al., 2004).
The above reviewed shear testers are designed to be used only at ambient temperature. Early works to assess powder flowability at high temperature were carried out by Smith et al. (1997). These authors employed the Jenike shear cell to test CaSO4 and MgSO4 powder samples that were previously heated (up to 750 °C) although the sample temperature was not controlled during the tests. The authors demonstrated that the powder yield strength increased with temperature presumably due to the formation of agglomerates during heating. Kanaoka et al. (2001), Kamiya et al. (2002) and Tsukada et al. (2008) succeeded to control the temperature during the measurement by placing the shear cell inside a furnace. These authors mainly studied the adhesion behavior as represented by the variation of the shear yield stress of fly ash particles. Kanaoka et al. (2001) assumed that the samples behavior followed the Coulomb’s law to estimate powder cohesion and the angle of internal friction. In general, they observed an increase in the shear yield stress with temperature, and therefore of cohesion, which was attributed to the formation of solid bridges during heating. Similar observations are reported by Tsukada et al. (2008), who showed that this phenomenon can be attenuated by adding coarse silica particles.
More recently some works have modified the standard Schulze cell in order to study the effect of temperature (Chirone et al., 2015; 2020) (Macrì et al., 2017; Tomasetta et al., 2011). Further details about these studies will be discussed in section 5.
Although these tests have shown satisfactory results, assessing powder flowability at high temperature in fluidized beds would be desirable as this system stands as the most commonly used for an adequate temperature control, better efficiency of heat transfer and gas-solid contact (Ding et al., 2019). All in all, since interparticle forces are very sensitive to numerous parameters (humidity, temperature, particles’ size and shape, solid mechanical properties, etc.) there is still a long way to go to fully understand powder flow behavior. Our HTSPT based on the fluidized bed technique is presented in detail in the next section as a further contribution to further exploring this critical issue.
Fig. 1 shows a schematic view of the HTSPT used in the works reviewed in this manuscript. This setup is aimed to measure the tensile yield strength and porosity of fine powders as depending on temperature and consolidation stress. It is based on the original SPT originally conceived by Valverde et al. (2000). Essentially, the equipment is based on measuring the pressure drop of a controlled gas flow across the bed. The powder bed is held in a cylindrical quartz vessel of 4.5 cm diameter. A quartz porous plate is placed at the bottom of the vertical cylinder that serves as gas distributor. Before entering the bed reactor, the gas crosses a filter and dryer (model SMC IDFA3E) in order to get rid of pollutant particles or humidity that can modify particle surface properties and therefore powder cohesion (Schubert, 1984). Then a controlled gas flow is pumped through the bed by means of a digital mass flow controller (omega model FMA-2606A, 2000 sccm). The gas pressure drop across the powder is measured by a MKS differential pressure transducer that generates an analogue signal readable by the computer. The measured pressure drop has two contributions, the pressure drop across the powder bed and the pressure drop across the porous plate. Obtaining the pressure drop across the porous plate requires a calibration procedure which consists of measuring the pressure drop of the plate alone. This pressure is subtracted from the total pressure drop to obtain Δp, the actual pressure drop across the powder bed. The registered pressure drop has an accuracy of ±5 Pa. A loudspeaker is placed at the top of the vessel that yields an acoustical excitation of 150 dB at a frequency of 130 Hz. This high intensity sound field is held for 5 s during the initialization procedure as explained below to disrupt any plugs or channels that may form and hinder fluidization, particularly in the case of highly cohesive powders (Valverde, 2013) yields an acoustical excitation of 150 dB at a frequency of 130 Hz. This high intensity sound field is held for 5 s during the initialization procedure as explained below to disrupt any plugs or channels that may form and hinder fluidization, particularly in the case of highly cohesive powders (Valverde, 2013). A set of valves is used to control the gas flow direction, when valves 1 and 3 are open while 2 and 4 are closed the gas flows upward across the powder bed. This is the operating mode for breaking and fluidize the bed. On the other hand, in the inverse situation (valves 1 and 3 closed while 2 and 4 open) the flow is directed downward, which is used for bed consolidation. The bed is placed inside a furnace which serves to put the bed at high temperature.

HTSPT: schematic of the experimental set-up. Reprinted with permission from Ref. (Espin et al., 2020). Copyright: (2020) Elsevier.
The fully automated setup starts by a standard initialization procedure during which a sufficiently high gas flow rate drives the bed into the bubbling fluidization regime for 30 s. Fluidization is assisted for the first 5 s by the sound excitation. Then the gas flow is turned off and the bed is left to settle for 30 s. This first step is important in order to ensure reproducibility of the initial state of the sample. In the second step the bed is heated by using a PID controller (Eurotherm 3216). When the target temperature is reached, a one-hour thermal stabilization period is set, aiming to guarantee a homogenous temperature distribution within the sample. Thereafter, the bed is subjected to a gradually increasing downward gas flow rate (by increments of 5 cm3/min each 3 s) up to reach a target consolidation stress (ranging from the weight per unit area of the bed up to σc~2 kPa). When the target consolidation stress is met, the downward directed gas flow rate is maintained for 10 s. Then it is decreased progressively until it is stopped. In the last stage, a gradually increasing upward gas flow is imposed to put the bed under tension which allows to derive the tensile yield strength of the powder as a function of temperature and the previously imposed consolidation stress as explained below.
Importantly, in all the tests the height of the bed is kept below the bed diameter which allows us to dismiss wall retention effects to calculate the tensile yield stress. This critical issue is discussed in further detail in Castellanos et al. (2004).
The gas pressure drop across the powder bed Δp is made non-dimensional by the weight per unit area (W) in order to better assess when fluidization is reached (
| (2) |

Dimensionless pressure drop Δp/W vs the gas flow rate qm for CaCO3 powder samples (average particle size dp = 88.02 ± 0.7 μm, T = 500 °C) previously subjected to different consolidation stresses σc as indicated. Reprinted with permission from Ref. (Espin et al., 2020). Copyright: (2020) Elsevier.
The pressure drop balances the powder weight per unit area at the so-called minimum fluidization gas flow rate qmf. At this point, a non-cohesive powder would be fluidized. However, when interparticle adhesive forces are significant (compared to particle weight), the powder remains in a solid-like state as the gas flow is further increased over qmf.
Then, the gas flow puts the bed under tension while the pressure drop continues to increase linearly. At a critical gas flow, interparticle adhesive forces are overcome and a sudden break of the powder occurs at the bottom of the bed while the pressure drop falls abruptly to a level around the powder weight per unit area. The tensile yield strength of the powder at the bottom of the bed is obtained from the overshoot of the gas pressure drop over the weight per unit area σt = Δpmax − W. As the gas flow rate is further increased over the breaking point, the pressure drop oscillates around the weight per unit area while the powder enters a heterogeneous fluidization state (see Fig. 2).
Studying the powder behavior at high temperatures is very important not only for the CaL process but also for many other industrial applications (hydrocarbons cracking, nuclear and metallurgical industries etc.) (Lettieri P. and Macrì D., 2016) where operating conditions involve the handling of powders at temperatures much higher than room temperature (Kunii D. et al., 1991). Several works have addressed the effect of temperature on the fluidization of powders. However, the reported observations on the minimum fluidization velocity are somewhat controversial. Different correlations have been proposed to predict the effect of temperature on the minimum fluidization velocity (Lettieri P. and Macrì D., 2016). However, these correlations are not reliable at high temperature. Furthermore, an extrapolation of the results to the tested conditions is required (Valverde et al., 2000; Yang et al., 1985). Some alternatives trying to solve this problem have been suggested (Wen and Yu, 1966), yet the evolution of the minimum fluidization velocity and bed voidage ɛmf with temperature is still considered as uncertain despite the several correlations reported in the literature (Botterill et al., 1982).
In order to check the efficiency and agreement between the different powder shear testers, Salehi et al. (2017) compared the results obtained with the Schulze ring shear tester, the Jenike shear tester and the Brookfield powder tester (PFT) using three different materials that belong to different Jenike powder classes (calcium lactate, calcium carbonate and dolomitic lime). Their results had shown that the degree of agreement between the different setups depends on the nature of powder tested. For instance, for dolomitic lime, the PFT and Schulze tester show a good agreement while the Jenike tester yielded higher cohesion values. For calcium lactate material, the Jenike and Schulze testers yield a considerable increase of the powder cohesion with consolidation stress, however the PFT measures do not uphold this observation. In all the cases, the Jenike tester give the higher cohesion values. In what concerns particularly CaCO3 powders, the cohesion determined by the Schulze tester is typically higher than the estimated by the other testing devices. It is noteworthy that all the mentioned tests have been performed at ambient temperature due to technical limitations.
In regards to the assessment of powder flow properties as affected by temperature, Tomasetta et al. (2011) made some modifications to the standard annular Schulze shear cell. However, for small consolidations (typically below ~1 kPa), the results are not fully reliable. They attributed this issue to design problems. For this reason, only tests at higher consolidation stresses were realized. At high consolidations they confirmed that temperature (in the range up to 500 °C) does not have a significant impact on the powder flowability for all the materials tested (FCC powder, Corundum and fly ashes). Using the same setup, Chirone et al. (2015) investigated the flow characteristics at room temperature and at 500 °C of five different ceramic powders. Their findings show that the powder cohesiveness considerably increased with temperature and consolidation stress. However, for the samples with the smallest size, the powders were already very cohesive, and the effect of temperature was not significant. For powders of 20–38 μm particle size, there is an increase of cohesion of about 35 %. For samples of 63–88 μm particle size and over 88 μm particle size, a cohesion increase of 35 and 45 % at 500 °C was noticed.
The results demonstrated also that free flowing powders (63–88 μm and more than 88 μm) turned into easy flowing at 500 °C as a consequence of the increase in cohesion by 35 and 45 % respectively. In this context, for the same temperature range and using the same setup, Macrì et al. (2017) confirmed these observations but for two different types of TiO2 powders (natural and synthetic).
Chirone et al. (2020) studied the flow behavior of powder samples sieved to obtain a range of particle sizes covering Group B, A and C of Geldart’s classification at temperatures in the range between 25 °C and 500 °C in a heated fluidized bed. They found that the tensile strength of the powders was increased with temperature. They claimed that this behavior was not attributable only to the variation of interparticle forces with temperature but also to other factors such as the difference in particle size and bed expansion.
Durán-Olivencia et al. (2020) studied the effect of temperature on the tensile strength measured for limestone samples having an average particle size of about 50 μm (details on the powder used are illustrated in Table A1) by means of the HTSPT. Their results indicated a considerable increase of powder cohesiveness as the temperature was increased (Figs. 3 and 4). Moreover, the effect of temperature was further enhanced as the consolidation stress to which the sample was previously subjected was increased. The obtained results fitted well to the empirical correlation (Fig. 5).
| (3) |

Tensile strength measured for a limestone powder as a function of the temperature for different values of the previously applied consolidation stress σc: the gray highlighted area stands for σc < 1000 Pa, the red colored curve area represents the tensile strength values for σc ≥ 1000 Pa. Reprinted with permission from Ref. (Durán-Olivencia et al., 2020). Copyright: (2020) Elsevier.

Tensile strength of a limestone powder measured as a function of the previously applied consolidation stress for temperatures ranging between 25 °C and 500 °C. The solid lines represent the best fit to a power law σt ~ σc 1/3. Reprinted with permission from Ref. (Durán-Olivencia et al., 2020). Copyright: (2020) Elsevier.

3D representation of the tensile strength measured for a limestone powder as a function of consolidation stress and temperature. The plotted surface shows the cross effect existing between these parameters governed by the expression
Particle size is a major physical property that impacts the cohesive behavior of powders (Macrì et al., 2017; Chirone et al., 2020; Espin et al., 2019; 2020). As particle size is reduced, the gas-solid contact efficiency is enhanced, which promotes mass and heat transfer in solid-gas reactors. However, powder cohesiveness is also promoted, which hinders powder flowability and fluidizability (Castellanos, 2005). Using the SPT, Espin et al. (2019) showed that the tensile strength of limestone powders is notably enhanced as the average particle size is decreased. Importantly, the limestone powder samples used in these tests were obtained by aerodynamic classification yielding a narrow particle size distribution, which made possible to better analyze the relevant effect of temperature on powder cohesiveness as depending on particle size. The experiments revealed that there is a synergetic effect between the consolidation stress to which the powder was previously subjected and particle size on the increase of the tensile strength. The impact of the consolidation stress on the powder tensile strength becomes more marked for smaller particle sizes (Fig. 6). As seen in a previous work, the increase of temperature promotes further powder cohesiveness. This phenomenon may turn free flowing powders at ambient conditions into cohesive as temperature is increased whereas low cohesive powders may show a very cohesive behavior at high temperatures (Chirone et al., 2020). The effect of temperature and consolidation stress could be well fitted by a power law
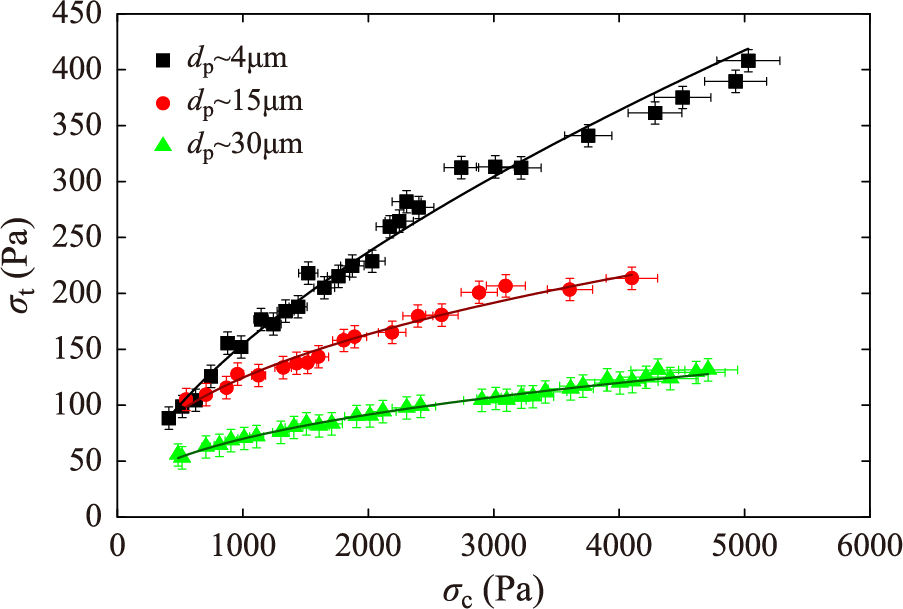
Powder tensile strength σt as a function of the consolidation stress σc previously applied to the powder bed for CaCO3 powder samples of particle size between 4 μm and 30 μm at room temperature. Reprinted with permission from Ref. (Espin et al., 2019). Copyright: (2019) Elsevier.

Tensile strength σt versus the consolidation stress σc for CaCO3 powders for different temperatures and particle size: (a) dp = 88.2 ± 0.7 μm; (b) dp = 59.3 ± 0.8 μm; (c) dp = 42.5 ± 0.8 μm; and (d) dp = 32 ± 1 μm. The solid lines are the best fits of the equation
Durán-Olivencia et al. (2021) tested the effect of the increase of temperature on the tensile yield strength for powders of similar particle size (between ~42 μm and ~72 μm) but of different materials (limestone, soda lime and SiC). The main results obtained are summarized in Fig. 8. These measurements revealed that, regardless of the powder nature, cohesiveness is augmented with temperature. This effect is especially appreciable when the temperature is T ≥ 200 °C for limestone and soda-lime powders, with a negligible difference in the measured σt up to 300 °C for both materials as it can be clearly observed in Fig. 9. On the other hand, cohesiveness was promoted further for the soda-lime powder at higher temperatures. Powder beds could not be broken when the temperature was above 300 °C, even by the highest gas flow rates achievable in the test (qmmax = 0.8 g/min), which indicates a great increase of the powder tensile strength near this temperature. Indeed, at temperatures above 300 °C, soda lime is close to its glass-transition temperature Tg (Tg ~520 °C) (Gerhard, 2017; Varshneya and Mauro, 2019). At this temperature, the powder transits from pure-solid to rubbery-like. This change is accompanied by an appreciable decrease of the material hardness and, therefore, a marked increase of the tensile strength according to contact mechanics predictions (Castellanos, 2005). In addition, high consolidation stresses promote further material softening as T approaches Tg. Fig. 10 shows SEM picture of soda-lime powder samples after being heated up to 500 °C and subjected to a consolidation stress σc = 2 kPa. As may be seen, the particles have experienced a visible local deformation presumably due to material softening. Dimples are clearly seen at the surface of the glass beads which were arguably created at interparticle contact points during consolidation at high temperature. In contrast, powder samples of a much harder material (SiC) show values of the tensile strength up to four times smaller than those measured for the CaCO3 powder of similar particle size for given values of the consolidation stress and temperature (Fig. 8). In the case of limestone, no deformations of the surface of the particles were appreciable after the samples (either silica coated or uncoated) were subjected to the high temperature fluidization cycles (Espin et al., 2020). These findings can be qualitatively explained by the change in the mechanical properties of the material as affected by temperature, particularly its hardness, which decreases significantly with temperature (Michel et al., 2004; Wheeler and Michler, 2013; Zhang et al., 2017) yielding an increase of the tensile strength as predicted by contact mechanics (Castellanos, 2005) when the particles deform plastically at contact points. Another important parameter to consider in the case of non-glass solids is the Tamman temperature TTam which is estimated as half of the melting point and indicates the temperature at which material deformation starts to be appreciable (Tammann, 1932).

Tensile yield strength σt of diverse powders measured as a function of the previously applied consolidation stress σc and temperature T. (a) CaCO3, (b) soda-lime glass beads and (c) SiC powder samples. The solid lines represent the best fit of a power law to the data σt = aσcb, with b close to unity. Reprinted with permission from Ref. (Durán-Olivencia et al., 2021).

Comparison of Tensile yield strength σt as a function of the previously applied consolidation stress σc for the different powders tested at T = 300 °C. Reprinted with permission from Ref. (Durán-Olivencia et al., 2021). Copyright: (2021) Elsevier.

SEM pictures of soda-lime glass beads after undergoing a consolidation stress of σc ≈ 2000 Pa at a temperature of 500 °C (magnifications: 2500×). The arrow indicates permanent deformations on the beads surface suffered during consolidation at high T (not observed in the fresh sample). Reprinted with permission from Ref. (Durán-Olivencia et al., 2021).
For limestone, this temperature is approximately in the same order of the highest temperature tested in the works reviewed in this manuscript (TTam (CaCO3) = 565 °C (Xu et al., 2016)), which would explain the appreciable increase in the tensile strength seen in this case as the material would soften considerably at temperatures close to TTam (Fig. 8 (soda-lime particles)). However, in the case of SiC, the Tamman temperature is very high (TTam ~1238 °C (Xu et al., 2016) ). Thus, the tensile strength measured for this powder in the range of tested temperatures (below 500 °C) is increased only slightly and powder flowability is not seriously compromised as is the case for the soda-lime and limestone powders.
The CaL process involves storing and handling CaCO3/CaO powders at high temperatures and consolidations (Paksoy, 2007). As it has been shown in the works reviewed above (Durán-Olivencia et al., 2021; Espin et al., 2020), the increase of temperature rises the cohesiveness of the powders which could be a serious restraint to their flowability. The results shown in Figs. 11 and 12 demonstrate that using nanosilica as additive at small concentrations can be a suitable solution to improve the flowability of diverse fine cohesive powders at ambient temperature (Chen et al., 2008; Quintanilla et al., 2006; Ramlakhan et al., 2000; Watson et al., 2001; Yang et al., 2005). Espin et al. (2019) demonstrated that for fine limestone powders the addition of nanosilica to cohesive limestone powders contributes to significantly reduce their tensile strength at ambient temperature (see Fig. 11). SEM pictures (Fig. 13) showed that CaCO3 particles were uniformly coated with nanosilica when both powders were physically dry mixed by a simple procedure in a rotating drum. More recently Durán-Olivencia et al. (2021) extended this observation to a range of temperatures up to 500 °C. At 500 °C, nanosilica coating leaded to a reduction of the powder tensile strength up to half of the value measured for the pure limestone powder (Fig. 12). Moreover, it was shown that the effect of nanosilica persisted when the sample was subjected to repeated fluidization cycles (see Fig. 13).

Tensile yield strength as a function of the previously applied consolidation stress for limestone powder samples (dp ~30 μm) mixed with nanosilica at different weight concentrations and ambient temperature. The experimental data are fitted to the equation
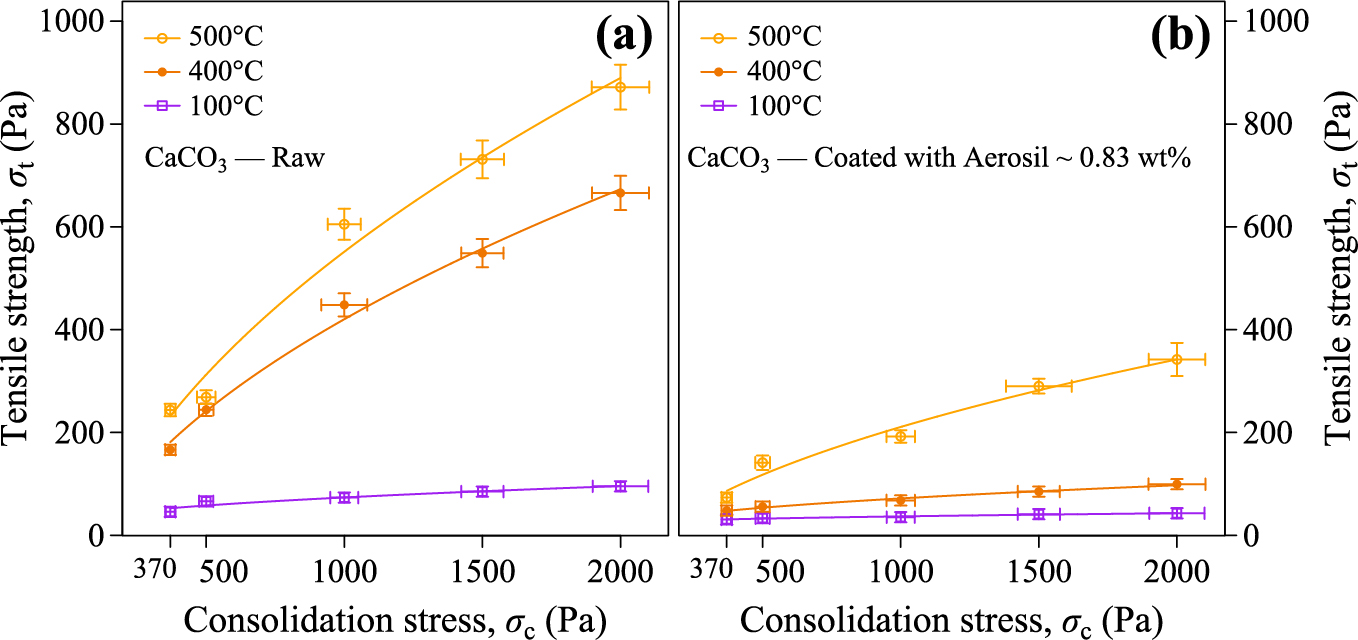
Measured tensile yield strength σt as a function of the applied consolidation stress σc at different temperatures T for powder samples of (a) pure limestone and (b) limestone mixed with nanosilica (0.83 % by weight). Reprinted with permission from Ref. (Durán-Olivencia et al., 2021).

SEM images of different size limestone particles coated with diffrent ratios of nanosilica : (a) dp ≈ 4 μm, 0.38 wt% SiO2 ; (b) dp ≈ 4 μm, 1.88 wt% SiO2 ; (c) dp ≈ 15 μm, 0.1 wt% SiO2 ; (d) dp ≈ 15 μm, 0.5 wt% SiO2 ; (e) dp ≈ 30 μm, 0.07 wt% SiO2 ; (f) dp ≈ 30 μm, 0.25 wt% SiO2. Reprinted with permission from Ref (Espin et al., 2019). Copyright : (2019) Elsevier.
Thus, a practical solution for CaL applications where fine limestone powders are to be employed would be the addition of nanosilica by small concentrations. Arguably, the physical mechanism behind this behavior is that the coating of limestone particles with nanosilica aggregates would effectively increase the hardness of interparticle contacts since silica is a much harder material than limestone thus mitigating contact plastic deformation and therefore reducing the increase of interparticle adhesion forces with consolidation and temperature, which would serve to mitigate the undesirable effect of high temperatures on powder flowability.
Nowadays, the deployment at large scale of power plants based on renewable energies is considered as a worldwide priority. Several technologies aiming to develop new alternatives based on non-polluting energy resources and using abundant, cheap and non-toxic materials that allow for a massive deployment of these technologies are conceived. In this regard CSP plants with energy storage are gaining an increasing interest.
However, a high efficiency and low-cost energy storage technology is still required for CSP plants to be competitive against fossil fuel-based plants. TCES is one of the most promising technologies for this goal, and the CaL process is a potential candidate to integrate TCES in CSP plants. Yet, the CaL process requires the handling and transport of CaCO3 and CaO powders at high temperatures. This may be an issue as in many other powder processing industrial applications where high temperatures are involved. Thus, understanding the powder behavior at high temperatures is crucial to improve the efficiency of these processes. In this manuscript the HTSPT device designed for this purpose is presented and the main results so far obtained have been reviewed.
The HTSPT can measure the tensile yield strength of fine powders as a function of temperature and the previously applied consolidation stress as depending on temperature. Generally, the results obtained have shown that powder cohesiveness is adversely affected at high temperatures as the consolidation stress is increased. Moreover, particle size is a critical parameter that determines the effect of temperature. As particle size is decreased, limestone powders become significantly more cohesive for temperatures just above 100 °C. Furthermore, for limestone powders with an average particle size of ~50 μm and below, the tensile yield strength increases significantly because of a synergistic effect of temperature and consolidation stress. Consolidation of the powders greatly enhances the detrimental effect of temperature on the tensile strength since plastic deformation at interparticle contacts is promoted by a reduction of the material hardness. For example, the tensile yield stress raises by one order of magnitude at 500 °C when a limestone powder of particle size around 50 μm is subjected to a consolidation stress of σc ~2 kPa compared to the case when no consolidation stress is applied at ambient temperatures. Thus, relative free flowing powders at ambient temperature may become highly cohesive at high temperatures, which may pose a serious problem in industrial applications. Some material properties play a relevant role on the effect of temperature on powder cohesiveness. Thus, the negative effect of temperature is mitigated in materials with a high Tammann temperature and mechanical hardness as, for example, SiC powders whose flowability is not seriously compromised with temperature and would be therefore in this regard an excellent candidate for thermal energy storage. On the other hand, it has been shown that surface coating with nanosilica by means of dry physical mixing, is a practical and efficient solution to lessen the increase of powder cohesiveness with temperature for limestone powders.
This research was supported by the Spanish Government Agency “Ministerio de Economía y Competitividad” (grant number CTQ2017-83602-C2-2-R).
| Material | Particle Density ρp (kg/m3) | Average Diameter dp (μm) | Young’s Modulus E (GPa) | Mechanical Hardness H (GPa) | Poisson ratio ν (-) | Surface Energy γ (J/m2) |
|---|---|---|---|---|---|---|
| CaCO3 | 2700a | 51a | 25–88.19b | 0.75–5.11c | 0.21–0.34d | 0.32–0.347e |
References: a (data provided by the supplier),
b (Crystran, 2020; Daniel et al., 1982; Ghadami Jadval Ghadam, 2015; Gunda and Volinsky, 2011; Gupta, 2010; Lee et al., 2016; Shi et al., 2018; Surfacenet, 2020)
c (Gunda and Volinsky, 2011; Hubermaterials, 2020; Lee et al., 2016; Matweb, 2020; Shi et al., 2018)
d (Daniel et al., 1982; Gunda and Volinsky, 2011; Gupta, 2010; Lomboy et al., 2011; Materialsproject, 2020; Ramlakhan et al., 2000; Upadhyay, 2013)
e (Røyne et al., 2011; Santhanam. and Gupta, 1968)
Rahma Gannoun
Holds a PhD (2019) in process-chemical engineering from the national school of engineers of Gabes in collaboration with laboratory of metrology and thermal measurement of the national school of engineers of Monastir. She was undergraduated as a chemical-process engineer in 2014 from the same school. Actually she has a post-doc fellowship with the faculty of physics (university of Seville). Her areas of interest include electrohydrodynamics, Computational fluid dynamics (CFD), heat transfer, renewable energy, fine powder flow.
José Manuel Pérez Ebrí
A member of the Electrohydrodynamics and Cohesive Granular Materials (EHD-CGM) research group at the Universidad de Sevilla (Seville, Spain). PhD in Science and Technology of New Materials (2016). He began his career as an industrial technical engineer in EHD-CGM group in 2005, where he took part in developing several experimental setups, one of his areas of interest. He worked four years in aeronautical industry as a materials engineer. He is developing his research activity in Ca-Looping process for CO2 capture and mechanical properties of granular materials.
Alberto T. Pérez
Was born in El Puerto de Santa María (Spain) in 1962. He received his PhD degree from the Universidad de Sevilla in 1989, where he has developed his academic career. Since 2009 he is Full Professor of Electromagnetism in this university. His interests are primarily in electrohydrodynamics, powder flow, and electric behavior of colloids. He has authored more than 90 papers in indexed journals on these subjects. He is also a popular science writer. Professor Pérez is currently the head of the group of Electrohydrodynamics and Cohesive Granular Materials of the Universidad de Sevilla
José Manuel Valverde
Professor of electromagnetism and materials engineering. PhD in physics (1997). His research activity has been focused on the study of fluidization and the mechanical properties of granular materials. A main subject of current interest is development of novel techniques to enhance the CO2 capture efficiency of CaO-based materials by means of the Ca-looping technology based on carbonation/calcination of natural limestone in fluidized beds. He has published over 100 papers mostly in Q1 journals with over 1000 citations.