Notes
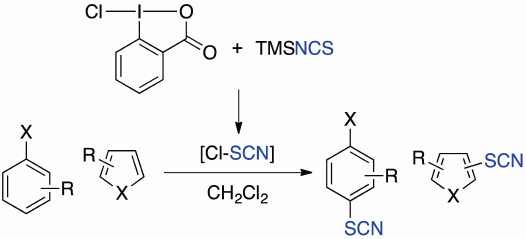
Thiocyanation of Aromatic and Heteroaromatic Compounds with 1-Chloro-1,2-benziodoxol-3-(1H)-one and (Trimethylsilyl)isothiocyanate
Keywords:
thiocyanation,
thiocyanogen chloride,
aromatic electrophilic substitution,
hypervalent iodine
2019 Volume 67 Issue 9 Pages 1015-1018
Details