2017 Volume 34 Pages 183-196
2017 Volume 34 Pages 183-196
This study reports on the synthesis and microstructural evaluation of ZrB2/ZrO2 ceramic powders prepared by milling-assisted magnesiothermic reduction of oxide raw materials. Powder blends containing ZrO2, B2O3 and Mg reactants were milled in different type of mills at different durations and were subsequently annealed in a tube furnace under Ar atmosphere. An additional purification step (HCl leaching) was conducted on the milled and annealed samples to obtain only Zr-based products. FactSage™ thermochemical software was used in order to show a preliminary route for the experiments. The effects of milling duration (up to 100 h), milling type (a SPEX™ 8000D Mixer/Mill and a planetary ball mill), excess Mg amount (5–20 wt.%) and annealing duration (6 and 12 h) on the formation and microstructure of the products were examined. The milled, annealed and leached products were characterized using an X-ray diffractometer (XRD), stereomicroscope (SM), scanning electron microscope (SEM) and differential scanning calorimeter (DSC). Pure ZrB2/ZrO2 ceramic powders having particles in size range of 200 nm – 1 μm and containing two different crystal structures of ZrO2 phase (monoclinic and tetragonal) were obtained after milling in the SPEX™ 8000D Mixer/Mill for 30 h, annealing at 1200 °C for 12 h and leaching with 5 M HCl.
There has been recently a growing interest in ZrB2-based ceramics due to their outstanding properties such as high melting point, high electrical and thermal conductivity, as well as excellent chemical inertness, high strength and high thermal shock resistance (Fahrenholtz and Hilmas, 2007; Monteverde et al., 2003). Based on these properties, ZrB2-based ceramics are attracting more and more attention in ultrahigh temperature applications where resistance to corrosion, wear and oxidation is demanded (Fahrenholtz and Hilmas, 2007; Zhu et al., 2009a, 2009b). They have been found to be suitable materials in various usage areas such as electrodes, thermowells, molten metal crucibles, armors, thermal protection systems for hypersonic flight, atmospheric re-entry vehicles, rocket propulsion and nose caps (Melendez-Martinez et al., 2002; Chamberlain et al., 2004; Monteverde et al., 2008; Brouchu et al., 2009). Furthermore, they have been used as wear parts, nozzles, coating on cutting tools and cathodes for electrochemical processing of aluminium (Upadhya et al., 1997; Opeka et al., 1999). Many of the composite materials have been developed to improve the sinterability and mechanical properties of the ZrB2-based materials (Monteverde, 2005; Guo, 2009). Amongst them, ZrB2-ZrO2 ceramics have raised significant concern due to the high contribution of ZrO2 phase on the densification, oxidation resistance and fracture toughness of the ZrB2-based composites (Zhang et al., 2008; Li W. et al., 2009; Zhu et al., 2009a, 2009b; Li et al., 2010).
Many processes are available for the preparation of ZrB2 or ZrB2-based ceramic powders: They have been traditionally produced by high temperature methods using solid-state reactions, borothermal and carbothermal reductions (Zhang et al., 2009; Qiu et al., 2012). Powders and coatings of ZrB2 have been also produced by electrometallurgy processes including molten salt electrolysis (Frazer et al., 1975; Malyshev and Gab, 2009). Chemical vapor deposition (CVD) technique has been applied to obtain ZrB2 coatings by using ZrCl4, BCl3 and H2 gas mixtures (Gebhardt and Cree, 1965; Greenwood et al., 1966; Deng et al., 2012). In recent years, low-temperature synthesis in an autoclave (Chen et al., 2004; Chen et al., 2012) and self-propagating high-temperature synthesis (SHS) (Radev and Klissurski, 2001; Khanra et al., 2008) methods have been developed to achieve ZrB2 or ZrB2-based ceramic powders. Synthesis of ZrB2 powders by high-energy ball milling derived mechanochemical reaction has been examined by a group of researchers using the starting materials of ZrO2, B2O3 and Mg (Setoudeh and Welham, 2006; Guo et al., 2011; Akgün et al., 2011; Jalaly et al., 2013, 2014). The preparation of ZrB2/ZrO2 powders was also reported by using high-energy ball milling/annealing processes and/or boro/carbothermal reduction methods (Balcı et al., 2012; Qiu et al., 2012). Review in the archival literature showed that mechanochemical route or milling-assisted synthesis processes have been more interesting due to its low-cost, simplicity, high throughput and product quality.
On the other hand, ZrB2-based dense bodies have been fabricated by spark plasma sintering (SPS), hot pressing (HP) and pressureless sintering (PS) from the starting powders containing ZrB2 and some required additives such as SiC, ZrC, ZrO2, MoSi2, AlN, B4C, Al2O3, etc (Guo et al., 2009; Monteverde et al., 2008; Chen et al., 2009; Li B. et al., 2009). Preparation of ZrB2/ZrO2 ceramics with these methods have also been investigated using ZrB2 and ZrO2 as raw materials (Basu et al., 2002; Zhang et al., 2008; Hong et al., 2008; Zhu et al., 2009b; Li W. et al., 2009). Li W. et al (2009) has reported the fabrication of ZrB2/ZrO2 ceramics with various ZrO2 content by using hot pressing and provided a considerable increase in their fracture toughness values. Zhang et al (2008) has reported the fabrication of ZrB2/ZrO2 ceramics by utilizing spark plasma sintering of ZrB2 and ZrO2 powders and the increase in their toughness from ZrB2-rich layer to ZrO2-rich layer due to the phase transformation of ZrO2. Thus, ZrB2/ZrO2 ceramic powders with fine and homogeneous microstructure can be a potential precursor for the development of ZrB2-based dense bodies.
An efficient method should be developed for the synthesis of submicron-sized ZrB2/ZrO2 precursors with high purity. Already, it came out as a need for the preparation of their dense bodies since consolidation of ZrB2/ZrO2 precursors obtained from oxide raw materials is cheaper than that of their commercial ones. High-energy ball milling which is the main driving force of the displacement or redox/reduction reactions in the mechanical alloying and mechanochemical synthesis processes, has the advantageous of enabling rapid preparation for oxide dispersion strengthened alloys, amorphous materials, solid solution alloys, non-equilibrium alloys, intermetallics, compounds, nanocomposites, ceramics and advanced materials that are difficult or impossible to be obtained by conventional production techniques (Suryanarayana, 2001; El-Eskandarany, 2001). It can be controlled by several parameters such as type of mill, milling speed, milling duration, ball-to-powder weight ratio, milling container, milling atmosphere, milling environment, type, size and size distribution of milling media, etc (Suryanarayana, 2001; El-Eskandarany, 2001). It offers a wide range of options by changing the desired parameters for leading the reactants towards to the size reduction and reaction. So, in the present study, milling-assisted magnesiothermic reduction process was used for the synthesis of the ZrB2/ZrO2 ceramic powders from oxide raw materials. Two different types of mills present in the laboratory facilities were employed for the milling process in order to monitor the reaction products of the ZrO2-B2O3-Mg powder system. The differences of these mills are speed of rotation, material/diameter of milling media and material/size of milling container, meaning that the efficiency of milling is different for this non-equilibrium processing technique. Subsequently, milled powders were annealed and leached to achieve pure and fine-grained ZrB2/ZrO2 ceramic powders.
Oxide raw materials such as ZrO2 powders (Alfa Aesar™, in purity of 99.7 %, with an average particle size of 44 μm) and B2O3 powders (ETI Mine, in purity of 98 %, with an average particle size of 467 μm) were used in the experiments, respectively as zirconium source and as native boron source for the synthesis of ZrB2/ZrO2 ceramic powders. Mg powders (Alfa Aesar™, in purity of 99.7 %, with an average particle size of 145 μm) were utilized as reducing agent in order to yield magnesiothermic reduction of the oxide reactants.
Powder blends containing stoichiometric amounts of reactants were prepared according to the theoretical reduction reaction given in Eq. (1). However, it is already known that the complete reduction of ZrO2 to ZrB2 can not be possible considering its high chemical stability (Balcı et al., 2012). Thus, the rationale of preferring stoichiometric amount in the preparation of the powder blends is impossibility in the complete reduction and conversion of ZrO2 to ZrB2.
| (1) |
For each run, powder batches were weighed in a Precisa™ X B320M sensitive balance. The prepared ZrO2-B2O3-Mg powder batches were homogenized in a WAB™ T2C Turbula blender for 1 h and they are hereafter referred to as-blended (ab) ZrO2-B2O3-Mg powders.
As-blended powders were separately milled both in a SPEX™ 8000D Mixer/Mill (6 g powder batch) and in a Fritsch™ Pulverisette 5 classic line planetary ball mill (12 g powder batch), with a rotation speed of 1200 and 400 rpm, respectively. Commonly, SPEX™ 8000D Mixer/Mill is employed for exploratory purposes and planetary ball mill is utilized to produce larger quantities of milled powders. In the milling experiments, ball-to-powder weight ratio (BPR) was chosen as 10:1. Milling containers changed according to the type of mills: a hardened steel vial with a capacity of 50 ml was used for the SPEX™ 8000D Mixer/Mill and a zirconia vial with a capacity of 500 ml was used for the planetary ball mill. Hardened steel balls with diameters of 6 mm and zirconia balls with diameters of 10 mm were employed as milling media, respectively. Extent of filling the vial is about 45 % for the utilized mills, knowing that adequate space should be given to the balls and to the particles to move around freely in the vial and to collide each other for getting enough impact energy (Suryanarayana, 2001; El-Eskandarany, 2001). Ar gas (Linde™, in purity of 99.999 %) was preferred as milling atmosphere. Different milling durations up to 100 h were conducted during the experiments. The milling vials were sealed by evacuating to about 10−2 Pa and by back filling with Ar gas in a Plaslabs™ glove box. The milled powders were unloaded again under Ar atmosphere in the same glove-box.
Annealing experiments of the milled ZrO2-B2O3-Mg powders were carried out in quartz boats which were inserted in a Protherm™ tube furnace, at 1200 °C for 6 and 12 h with a heating and cooling rate of 10 °C/min under Ar gas flow.
Milled and annealed ZrO2-B2O3-Mg powders were leached with HCl (Merck™, in concentration of 37 %) under the effect of ultrasonic stirring using a Bandelin Sonorex™ RK-100 H ultrasonic bath in order to remove unwanted MgO phase. HCl solutions in concentrations of 3.6 and 5 M were used for the leaching treatments. Leaching parameters such as solid-to-liquid ratio of the solution and duration were selected respectively as 1 g/10 cm3 and 30 min. The solutions containing insoluble solids were subjected to repeated centrifuging in a Hettich™ Rotofix 32A centrifuge with a rotation speed of 3500 rpm for 30 min, to repeated decanting and to repeated washing with distilled water. Finally, remained solids were dried under air in a FN 500 stove at 120 °C for 24 h.
The phase compositions of the milled, leached and annealed powders were identified using a Bruker™ D8 Advanced Series X-ray diffractometer (XRD) with CuKα (1.54060 Å) radiation in the 2θ range of 10–80° incremented at a step size of 0.02° at a rate of 2°/min. International Center for Diffraction Data® (ICDD) powder diffraction files were utilized for the identification of crystalline phases. Thermal property of the milled powders was examined in a TA™ Instruments SDT Q600 differential scanning calorimeter (DSC)/thermogravimetric analyser (TGA). DSC/TGA experiments were conducted in alumina crucibles up to a heating temperature of 1200 °C at a rate of 10 °C/min under Ar atmosphere. In order to identify the phases at the exothermic and endothermic peak points, milled powders were heated to 500, 600, 700, 800 and 900 °C in a Thermoscientific™ tube furnace under Ar atmosphere and they were subjected to additional XRD analyses at the same conditions. Microstructural characterizations of the milled, annealed and leached powders were carried out using a Zeiss™ Discovery. V12 stereomicroscope (SM) coupled with a Zeiss™ Axiocam ERc5s high resolution digital camera and a Hitachi™ TM-1000 scanning electron microscope (SEM) operated at 15 kV. For SEM analysis, the specimens were prepared by following the procedure of dissolving the powders in C2H5OH (Merck™, in purity of 99.9 %), syringing them onto a base plate, drying them in air and coating their surfaces with a thin layer of gold using a Polaron™ SC7620 Sputter Coater to enhance their conductivities. Several different regions of the samples were monitored during the SEM analyses: homogeneous distributions of the particles were observed thankful to the milling process conducted for different durations. Thus, representative SEM images of the samples were shown in order to determine the size range of the particles instead of their average size. Lastly, Zr element and also probable Fe impurity, which could be formed as a result of the wear and tear from the hardened steel milling vial and balls, were analyzed in the supernatant liquids by using a Perkin Elmer™ 1100B atomic absorption spectrometer (AAS).
FactSage™ 6.2 thermochemical software was used for the determination of reaction possibility in terms of standard Gibbs free energy change versus temperature relation and for the interpretation of the probable reaction products, in ZrO2-B2O3-Mg system. The standard Gibbs free energy change versus temperature curve of the ZrO2-B2O3-Mg system was plotted up to 2000 °C and illustrated in Fig. 1(a).
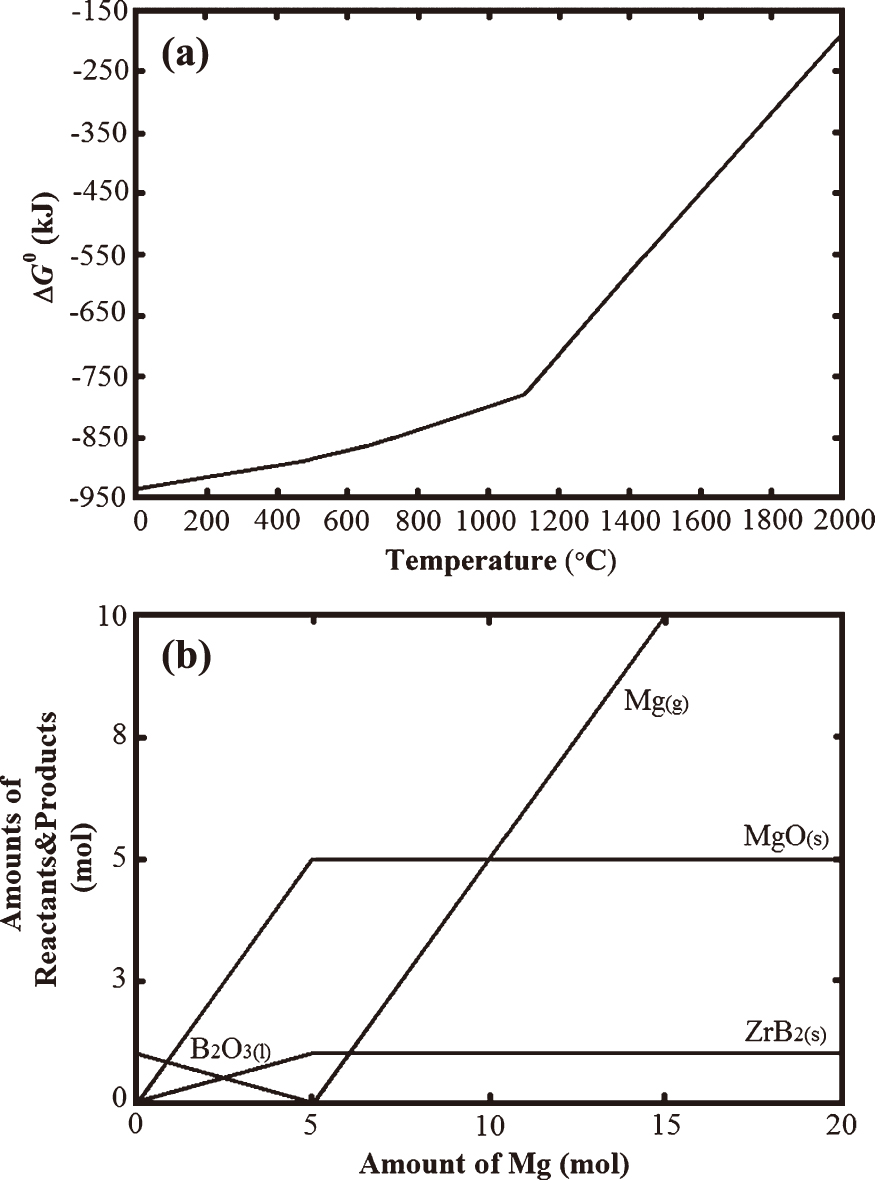
Thermodynamic calculations of the ZrO2-B2O3-Mg system obtained from FactSage™ 6.2 thermochemical software for the reaction given in Eq.(1): (a) The standard Gibbs free energy change versus temperature curve, and (b) Molar amounts of reactants/products varying according to the molar amount of Mg at 1200 °C.
The reaction has negative free energy change between −930 and −200 kJ in the temperature range of 0–2000 °C, which means that the reduction reaction in Eq. (1) is possible and takes place spontaneously at room temperature and above. However, there is a change in the slope of the curve in Fig. 1(a). The standard Gibbs free energy change of the reaction is between −930 and −775 kJ for the temperatures up to 1095 °C but the slope of the curve increases after this temperature at which Mg boils. Thus, boiling of Mg causes a significant change in the free energy value and hence slows the reaction proceeding. Fig. 1(b) shows the FactSage™ plot for the molar amounts of reactants/products varying according to the molar amount of Mg at 1200 °C. Under the stoichiometric amount of Mg (< 5 moles), the molar amounts of ZrB2 and MgO increase with decreasing amount of unreacted B2O3. When stoichiometric amount of Mg (5 moles) is used in the reaction, B2O3 reactant vanishes and 1 mole of ZrB2 and 5 moles of MgO are obtained as the products. Increasing the molar amount of Mg up to 20 moles does not contribute to the further formation of ZrB2 and MgO phases because excess amounts of Mg convert into gas phase and leave the system without participating in the reduction reaction. Thus, maximum amounts of ZrB2 and MgO reaction products can be achieved in the case of using stoichiometric amount of Mg, according to the thermodynamic calculations in Fig. 1(b).
3.2 Milling of the as-blended powdersFig. 2(a)–(h) displays the XRD patterns of the as-blended powders and those milled in the SPEX™ 8000D Mixer/Mill for different durations (3, 5, 7, 9, 15, 20 and 30 h). As clearly seen from Fig. 2, there are no reactions between ZrO2, B2O3 and Mg even after milling for 30 h because the ZrO2 and Mg phases are still present in the XRD patterns of the powders. No peaks belonging to the B2O3 phase are observed in the XRD pattern of the as-blended ZrO2-B2O3-Mg powders, probably due to its amorphous nature. Furthermore, any Fe impurity which could be released from the hardened steel vial/balls during milling did not detected in the XRD patterns in Fig. 2(b)–(h) since the diffractometer is not capable of analyzing phase amounts under 2 wt.% of the overall sample. Table 1 presents the details of the crystal structural parameters of the different phases encountered in this study.

XRD patterns of the as-blended powders and those milled in the SPEX™ 8000D Mixer/Mill for different durations: (a) ab, (b) 3 h, (c) 5 h, (d) 7 h, (e) 9 h, (f) 15 h, (g) 20 h and (h) 30 h.
The details of the crystal structural parameters of the different phases encountered in this study.
| Phase | ICDD Card # | Bravais Lattice | Lattice Parameters (nm) |
|---|---|---|---|
| ZrO2 | 89–9066 | Primitive Monoclinic | a = 0.531 b = 0.520 c = 0.514 |
| B2O3 | — | — | — |
| ZrO2 | 50–1089 | Primitive Tetragonal | a = b = 0.359 c = 0.515 |
| Mg | 35–0821 | Primitive Hexagonal | a = b = 0.329 c = 0.521 |
| ZrB2 | 34–0423 | Primitive Hexagonal | a = b = 0.317 c = 0.353 |
| MgO | 45–0946 | Face-centered Cubic | a = b = c = 0.421 |
Similarly, in a related study reported on the carbothermal reduction of ZrO2 and B2O3 powder blends, there exists no reaction even after milling for 6 h in the SPEX™ 8000D Mixer/Mill with a BPR of 10:1 (Balcı et al., 2012). Moreover, ZrO2 and Mg peaks broadened and their intensities gradually decreased upon increasing the milling time from 0 to 30 h, indicating a gradual decrease in their crystallite sizes and an increase in their lattice strains due to the continuous deformation during milling. There is a visible decrease in the intensities of the ZrO2 and Mg peaks from 0 to 3 h. However, this decrease is seen less from 3 to 30 h of milling duration. Thus, no change occurred in the phase composition of the powders after milling for 30 h, suggesting that prolonged milling did not contribute to the chemical reaction, but contributed only to the particle size reduction.
Representative SEM images of the powders milled in the SPEX™ 8000D Mixer/Mill for 15 and 30 h are given in Fig. 3(a) and (b). At first view, it is clearly seen from the SEM images that powders milled for 15 and 30 h do not consist of particles with definite and perfect morphologies throughout the structure. Fig. 3(a) reveals both the round-shaped particles having sizes smaller than 2 μm and the irregular agglomerates in sizes not larger than 5 μm. It is also easily observed from the microstructure in Fig. 3(a) that some smaller round-shaped particles are embedded in larger agglomerates. Extended milling to 30 h resulted in the particle size reduction (from 2 μm to 700 nm) and provided the breaking down of the larger agglomerates (from 5 to 2.5 μm). Moreover, it was reported in previous studies that repeated fracturing and welding mechanism took place in the vial during milling creates agglomeration and inhomogeneity in the shapes of the particles due to the contact of active and small fresh surfaces at each collision between vial walls/balls and particles (Suryanarayana, 2001; Ağaoğulları et al., 2012a, 2016; Ağaoğulları, 2014).

SEM images of the powders milled in the SPEX™ 8000D Mixer/Mill for different durations: (a) 15 h and (b) 30 h.
On the basis of XRD patterns in Fig. 2 and SEM images in Fig. 3, it was understood that milling of stoichiometric amount of ZrO2, B2O3 and Mg powders in the SPEX™ 8000D Mixer/Mill for 30 h yielded only smaller particles of the reactants without any formation of reduction reaction. Although FactSage™ plot in Fig. 1(b) showed that using 20 moles of Mg as starting material rather than 5 moles (stoichiometric amount) did not increase the molar amounts of ZrB2 and MgO products, milling with the addition of excess amounts of Mg (5, 10, 15 and 20 wt.%) was conducted considering that the thermodynamic calculations exhibit only the equilibrium phase compositions at room and elevated temperatures without taking into account synthesis history and reaction kinetics. As previously known, the impact energy accumulated in the system during milling converts into other types of energies (potential, kinetic, chemical, etc.): these energies are effective in the formation of new structural arrangements which can be deviated from thermodynamic equilibrium states (Suryanarayana, 2001; El-Eskandarany, 2001). In this case, the molar amount of Mg was increased to observe an indication of the reduction reaction. Taking into account that the peak intensities of the ZrO2 and MgO phases are similar after milling for 7 h with those after milling for 30 h (Fig. 2(d) and (h)), experiments with excess Mg amounts were carried out using 7 h of milling duration. Fig. 4(a)–(d) illustrates the XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 7 h with the use of excess amounts of Mg (5, 10, 15 and 20 wt.%), respectively. As seen from Fig. 4(a)–(d), there are still ZrO2 and Mg phases in the microstructure of the milled powders, definitely in the presence of amorphous B2O3 which could not be observed with a distinctive peak by XRD. Also, there are indications of a very small combined peak of ZrB2 and MgO phases at the 2θ range of 40–45° and very small ZrB2 peaks at the 2θ value of about 34, 66 and 73°. Amongst the XRD patterns in Fig. 4(a)–(d), the highest intensity of ZrB2 and MgO phases were obtained when 20 wt.% excess Mg was used in the powder blend. It is expected to detect ZrB2 and MgO phases in higher intensities than those in Fig. 4 since the addition of excess Mg could compensate the surface oxidation of the reactants and could trigger the reactants for the desired reaction. However, the temperature increase in the vial arose from the impact energy during milling is not sufficient for activating the ZrO2 material exhibiting a ceramic character and high chemical stability. If sufficient temperature increase was obtained and hence activation energy barrier was exceeded in the system under the effect of impact energy, there would be a sudden exothermic reaction following to the achievement of adequate particle size reduction. The driving force for this exothermic reaction is ignition occurred at the solid-solid interface of the reduced particles by the help of the high oxygen affinity of Mg. Thus, further ignition of the milled powders should be provided by applying external heat such as annealing.

XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 7 h with the use of excess amount of Mg: (a) 5 wt.%, (b) 10 wt.%, (c) 15 wt.% and (d) 20 wt.%.
After obtaining the experimental outputs from milling in the SPEX™ 8000D Mixer/Mill, the type of high-energy ball mill was changed to monitor its effects on the reaction time and product type, considering that the kinetics of the milling process depend on the stress conditions in the milling devices. Fig. 5(a)–(f) demonstrates the XRD patterns of the powders milled in the planetary ball mill for 20, 25, 40, 50, 70 and 100 h. According to the XRD patterns in Fig. 5(a) and (b), milled powders for 20 and 25 h contain ZrO2 and Mg crystalline phases certainly with the amorphous B2O3, revealing that any reaction did not take place till to the end of these durations.

XRD patterns of the powders milled in the planetary ball mill for different durations: (a) 20 h, (b) 25 h, (c) 40 h, (d) 50 h, (e) 70 h and (f) 100 h.
As milling time was increased to 40 h, either a significant decrease in the intensities of the ZrO2 and Mg phases or small incubations of ZrB2 and MgO phases were observed (Fig. 5(c)). Moreover, the intensity decreases of ZrO2 and Mg phases are seen sharper for 25 and 40 h milling durations (Fig. 5(b) and (c)). Prolonging milling time from 40 h to 50, 70 and 100 h enabled the further size reduction of ZrO2 and Mg particles but did not enable the formation of complete reduction: ZrB2 and MgO phases have still very small peak intensities in the presence of major ZrO2 phase. However, Mg peaks almost disappeared due to the continuous deformation. As the XRD analysis results in Fig. 2(b)–(h) and 5(a)–(f) are compared with each other, it is understood that the only difference emerged in the phase type as the incubations of ZrB2 and MgO. Roughly, these incubations could be expected to occur after milling in the SPEX™ 8000D Mixer/Mill because the rotation speed of this mill is three times higher than that of planetary one which corresponds to a higher impact energy and shorter reaction time. However, there is no indication of ZrB2 and MgO even after milling for 30 h in the SPEX™ 8000D Mixer/Mill (Fig. 2(b)–(h)). The emergence of ZrB2 and MgO after 40 h milling in the planetary ball mill may be explained by the strong impact forces occurred between ZrO2 reactant and vial/balls which were made from the same material. It should be also noted that processing parameters of the two mills such as material of milling vial/balls, diameter of the milling balls and milling energy did not match each other and they could not be evaluated in a comprehensive way. Already, the main aim of using two different mills was not to compare their corresponding products in the case of changing one milling parameter by fixing the others, but was to observe the reaction products of the as-blended ZrO2-B2O3-Mg powders in two different systems in the within the laboratory facilities.
Representative SEM images of the powders milled in the planetary ball mill for 20, 25, 50 and 100 h are given in Fig. 6(a) to (d), respectively. As seen from Fig. 6(a) and (b), milled powders for 20 and 25 h have irregular-shaped particles in sizes below 20 μm. There is not a very significant difference in the particle sizes of the powders milled for 20 and 25 h (Fig. 6(a) and (b)), as compatible with the similar intensities of ZrO2 and Mg phases in their XRD patterns (Fig. 5(a) and (b)).

SEM images of the powders milled in the planetary ball mill for different durations: (a) 20 h, (b) 25 h, (c) 50 h and (d) 100 h.
In other words, further milling for 5 h in planetary ball mill was not adequate to create a remarkable change in the particle size. Besides, the gradual decrease in the particle sizes of the milled powders can be obviously seen from Fig. 6(b) through (d). Fig. 6(c) shows agglomerates in sizes not larger than 5 μm and also irregular-shaped particles having sizes below 2 μm. As milling time was increased to 100 h, particle sizes decreased to 2 μm and below (Fig. 6(d)). Similar to the microstructures obtained after milling process in the SPEX™ 8000D Mixer/Mill (Fig. 3(a) and (b)), agglomeration of powder particles was encountered after milling in the planetary ball mill (Fig. 6(c) and (d)). This phenomenon generally prevents the observation of smaller particles throughout the microstructure. Furthermore, approximate particle sizes were obtained after milling for 30 h in the SPEX™ 8000D Mixer/Mill and for 100 h in the planetary ball mill even if milling parameters for two different equipment could not be comparable in regard of the material of milling vial/balls, the diameter of the milling balls and the rotation speed.
3.3 Annealing of the milled powdersAfter milling processes carried out using different type of mills for different durations, application of an external heat came out as a need for the reduction reaction and for the partial/complete conversion of ZrO2 to ZrB2 since milling did not yield the characteristic peaks of ZrB2 phase even after very long milling durations. Besides, milling the stoichiometric powder blends or those added with 20 wt.% excess Mg in the SPEX™ 8000D Mixer/Mill is considered as more advantageous than milling in the planetary ball mill because similar particle sizes could be achieved at shorter durations by taking into account their independent process parameters. Thus, powders milled for 30 h in the SPEX™ 8000D Mixer/Mill with/without excess Mg amounts were subjected to the subsequent annealing processes. Prior to the annealing, thermal behavior of the sample milled for 30 h was determined by DSC-TGA experiments in order to suggest an annealing temperature.
Fig. 7 shows the DSC-TG scans of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h. According to the DSC scan, milled powders have an exothermic peak at about 600 °C and a broad endothermic peak at about 900 °C. Although powders milled for 30 h have ZrO2, B2O3 and Mg phases (Fig. 2(h)), there are not any endotherms corresponding to the melting of B2O3 at 450 °C and melting of Mg at 650 °C in the DSC scan in Fig. 7. Instead of these endotherms, there is an exotherm with a sharp peaking point at 600 °C and with an onset temperature of about 400 °C. Homogeneous distribution of active ZrO2, B2O3 and Mg particles in small sizes enabled them to react with each other rather than their individual melting. Furthermore, the initial weight of the sample increases from 21.45 to 24.50 mg, as seen in the TG scan. In order to reveal the reasons of the exothermic and endothermic peaks, milled powders were just heated to 500, 600, 700, 800 and 900 °C without a holding time and they were cooled instantly down to the room temperature. The XRD patterns of these milled and heated powders are given in Fig. 8(a) to (e). After milled powders were heated to the temperatures of 500, 600, 700 and 800 °C, the microstructure contained ZrO2 and MgO phases. This means that the exotherm peaking at 600 °C belongs to the oxidation of Mg. This is in good agreement with the TG scan because it has sharp increase (from 21.45 to 23.62 mg) until 850 °C.

DSC-TG scans of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h.

XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h and subsequently heated to (a) 500 °C, (b) 600 °C, (c) 700 °C, (d) 800 °C and (e) 900 °C.
Heating of the milled powders to 900 °C resulted in the incubation of ZrB2 phase ( Fig. 8(e)), indicating that the endothermic peak is a sign of ZrB2 formation. As already known, monoclinic ZrO2 transforms into tetragonal structure at about 1170 °C (Nettleship and Stevens, 1987). However, there is no indication of this transformation in the DSC scan (Fig. 7). This phenomenon can be attributed to the non-isothermal fast DSC heating conditions without a holding time, carried out in an alumina crucible in which particles of the reactants placed in a very small volume. Considering the results in Fig. 7 and Fig. 8(a)–(e), the melting temperature of ZrO2 (about 2715 °C) and Tamman temperature (0.4–0.5 × Tmelting), the annealing temperature of the milled powders was selected as 1200 °C.
Fig. 9(a) and (b) represent the XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h and annealed at 1200 °C for 6 and 12 h, respectively. After annealing for 6 h (Fig. 9(a)), ZrB2 and MgO were obtained with their characteristic peaks in the presence of unreacted ZrO2 phase. However, an amount of monoclinic ZrO2 phase transformed into tetragonal (ZrO2-t) structure. This arose by the help of the holding time for 6 h at 1200 °C because annealing temperature is just a little higher than the transformation temperature of ZrO2 (1170 °C). When the annealing time was increased from 6 to 12 h by fixing the temperature, the peak intensities of the ZrB2, MgO and ZrO2-t remarkably increased (Fig. 9(b) in the presence of monoclinic ZrO2 phase which is almost in the approximate amount with that of in Fig. 9(a). Moreover, Fe impurity which could be incorporate into the powders from the hardened steel vial/balls during the milling process did not detected in the XRD patterns even after annealing, meaning that its probable amount is under the detection limit of XRD (< 2 wt.%).

XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h and annealed at 1200 °C for different durations: (a) 6 h and (b) 12 h.
Additionally, any emergence of Mg borate compounds such as MgB4O7, Mg2B2O5 and Mg3B2O6 did not observed in Fig. 9(a) and (b) at the end of the annealing processes. It was thought as a probability because Mg/MgO and B2O3 can react and form Mg borates in different compositions between the temperatures of 700 and 1000 °C (Ağaoğulları et al., 2012b). Thus, 1200 °C and 12 h can be respectively evaluated as an optimum annealing temperature and annealing time for yielding ZrB2/ZrO2 ceramic powders with an amount of MgO which will be easily leached out using HCl as well as probable Fe impurity. Due to the fact that the higher annealing temperature with a longer annealing duration may cause excess grain growth in the resultant powder particles, annealing parameters were not altered for the following experiments.
Fig. 10(a) and (b) exhibit the XRD patterns of the 20 wt.% excess amount of Mg added powders after milling in the SPEX™ 8000D Mixer/Mill for 30 h and those after annealing at 1200 °C for 12 h, respectively. In comparison with the XRD pattern of the powders milled for 7 h with the same amount of excess Mg (Fig. 4(d)), it can be clearly seen that the peak intensities of the ZrO2 and Mg phases decreased and the incubations of the ZrB2 and MgO phases increased in very small amounts after milling for 30 h (Fig. 10(a)). Thus, there is still no reaction between reactants even after milling for 30 h with the use of 20 wt.% excess amount of Mg.

XRD patterns of the powders: (a) milled in the SPEX™ 8000D Mixer/Mill for 30 h with the use of 20 wt.% excess amount of Mg, and (b) those of annealed at 1200 °C for 12 h.
After annealing of the powders milled for 30 h with the use of 20 wt.% excess amount of Mg at 1200 °C for 12 h (Fig. 10(b)), ZrB2 phase in high peak intensities and MgO occurred in the microstructure in addition to the unreacted monoclinic ZrO2 and its transformed form (ZrO2-t). The utilization of 20 wt.% excess amount of Mg seems to increase the ZrB2 peak intensities as compared with Fig. 9(b). However, the intensities of the ZrO2 peaks also increased and hence the ZrB2/ZrO2 intensity ratio decreased with the use of excess Mg. Besides, MgO phase formed in lower amount than that of in Fig. 9(b). The peak intensities of ZrO2-t in Fig. 10(b) are significantly lower than those of in Fig. 9(b) because excess amount of Mg (20 wt.%) up to a limited level provides ZrO2 to contribute in the formation of ZrB2 instead of its transformation to ZrO2-t during annealing at 1200 °C. However, remained excess amount of Mg evaporated from the system without further participating into the reduction reaction, as compatible with the FactSage™ plot in Fig. 1(b). Thus, annealing of the milled powders containing stoichiometric amounts of reactants can give the ideal route for the production of ZrB2/ZrO2 ceramic powders.
It should be also stated that the thermochemical calculations in Fig. 1(a) and (b) performed using FactSage™ 6.2 software for the theoretical reduction reaction given in Eq. (1) conflicted with the experimental outputs of the annealing process, when the obtained products were taken into account. Already, the starting stoichiometric powder blends were prepared in regard of Eq. (1) considering the high stability of ZrO2 and hence its complexity in the reduction. According to the thermochemical calculations, the reaction products obtained from the stoichiometric amounts of raw materials are only ZrB2 and MgO. Though, ZrB2 and MgO in the presence of both monoclinic and tetragonal ZrO2 were obtained as the reaction products after milling and annealing of the stoichiometric powder blends. So, the reduction reaction of ZrO2-B2O3-Mg system at 1200 °C should be conformed to the experimental results. The actual reduction reaction rewritten in regard of achieved outputs is given in Eq. (2).
| (2) |
Following to milling and annealing processes, the reaction products with undesired MgO phase should be purified to achieve fine-grained ceramic powders without any contamination. Thus, leaching treatment was performed using different HCl concentrations (3.6 and 5 M) on the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h and annealed at 1200 °C for 12 h, with the intention of removing the whole MgO present in the microstructure and also probable Fe impurity which was not previously detected by XRD. Fig. 11(a) and (b) illustrate the XRD patterns of the leached powders. According to Fig. 11(a), 3.6 M HCl provided the dissolution of MgO but it is still not strong enough for the complete elimination of the undesired phase. As HCl concentration was raised to 5 M, the peak intensities of both monoclinic and tetragonal ZrO2 and ZrB2 phases increased without any appearance of MgO (Fig. 11(b)).

XRD patterns of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h, annealed at 1200 °C for 12 h and leached in HCl solution at different concentrations: (a) 3.6 M and (b) 5 M.
Subsequent AAS analyses were conducted on the leach solutions decanted from the residual solids in order to detect the dissolved Zr and Fe elements. Since 5 M HCl is highly concentrated, 22 ppm Zr arose from the slight dissolution of ZrO2 phase was detected in the solution. However, trace amount of Zr was found in the leach solution in the case of using 3.6 M HCl. Furthermore, increasing the HCl concentration from 3.6 to 5 M increased the dissolution of Fe but not in a significant amount: the detected amounts were about 117 ppm for 3.6 M HCl and 125 ppm for 5 M HCl. In our previous studies about the synthesis of LaB6 and SmB6 from La2O3-B2O3-Mg and Sm2O3-B2O3-Mg powder blends by mechanochemical reactions in the SPEX™ 8000D Mixer/Mill for 5 h (using the same conditions such as BPR, milling container, media and atmosphere), the supernatant liquids were respectively found to have about 11 and 7.81 ppm Fe after leaching with 3.6 and 4 M HCl (Ağaoğulları et al., 2012a, 2015). In this case, the amount of Fe impurity released from the leached ZrB2/ZrO2 ceramic powders is at least ten times higher than those released from the purified LaB6 and SmB6 powders due to the higher hardness of ZrO2 and ZrB2 compounds. Besides, leaching treatment using 3.6 M was repeated for the obtained ZrB2/ZrO2 powders but Fe element was determined as trace amount which was under the detection limit of AAS. Thus, HCl concentrations above 5 M were not applied for leaching of the annealed powders and 5 M HCl was found strong enough to obtain pure ZrB2/ZrO2 ceramic powders.
Fig. 12(a)–(d) shows the SM images of the as-blended, milled (in the SPEX™ 8000D Mixer/Mill for 30 h), annealed (at 1200 °C for 12 h) and leached (in 5 M HCl) powders, respectively. Monitoring SM images are beneficial to see the general appearances and colors of the powders and to follow the process in a simple way. As the SM image of the as-blended powders in Fig. 12(a) is compared with those in Fig. 12(b)–(d), the change in the colors of the starting materials can be obviously seen and the effect of different treatments can be observed step-by-step.

SM images of the (a) as-blended powders, (b) powders milled in the SPEX™ 8000D Mixer/Mill for 30 h, (c) those annealed at 1200 °C for 12 h and (d) those leached in 5 M HCl.
After milling for 30 h in the SPEX™ 8000D Mixer/Mill, the particles of the reactants were homogeneously distributed throughout the microstructure, Mg particles covered the surfaces of the other reactants and it resulted in a color change in the overall volume (Fig. 12(b)). The morphology of the annealed powders (Fig. 12(c)) is different than that of milled one (Fig. 12(b)). The microstructure turned to a more incorporated and continuous form by the influence of heating with a long holding time. However, the color differences of the Zr-based (ZrO2, ZrO2-t and ZrB2) and MgO particles are clear in Fig. 12(c) because Zr-based particles are seen as embedded in the MgO phase. After leaching treatment, the microstructure became a homogeneous form by eliminating the color difference via removing the MgO contamination (Fig. 12(d)).
Fig. 13(a) and (b) are the representative SEM images of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h, annealed at 1200 °C for 12 h and leached in 5 M HCl, at magnifications of 2000 and 8000X, respectively. The general view of the resultant powders containing both monoclinic and tetragonal ZrO2 and ZrB2 phases in Fig. 13(a) exhibits irregular agglomerates which prevent the observation of smaller particles in the microstructure. Fig. 13(b) in higher magnification than Fig. 13(a) clearly shows the rounded-shaped particles in sizes ranging between 200 nm and 1 μm. On the basis of Fig. 13, it can be said that the final product is in the form of a ceramic powder in which individual particles of the components can be observed, rather than that of a composite powder in which each particle has the similar composition of resultant phases.

SEM images of the powders milled in the SPEX™ 8000D Mixer/Mill for 30 h, annealed at 1200 °C for 12 h and leached in 5 M HCl, at different magnifications: (a) 2000X and (b) 8000X.
Consequently, submicron-sized and pure ZrB2/ZrO2 ceramic powders containing two different crystal structures of ZrO2 phase (monoclinic and tetragonal) were prepared by milling, annealing and leaching processes from ZrO2-B2O3-Mg powder blends. The synthesized precursor material can be utilized as intermediate or final products in different application areas and can lead the way for the further advanced processing techniques to obtain special designated bulk forms of composites.
In this study, submicron-sized ZrB2/ZrO2 ceramic powders were successfully synthesized via magnesiothermic reduction of oxide raw materials using a combined process of milling and annealing. An additional HCl leaching step was conducted on the annealed powders for their purification. Based on the results reported in the present study, the following necessary conclusions can be drawn:
This study was supported by “Istanbul Technical University Scientific Research Projects” with the project title of “The Production of IVB Group and Lanthanide Group Metal Borides in Mechanochemical Reaction Medium” and with the project number of 32743. It was also funded by “The Scientific and Technological Research Council of Turkey (TÜBİTAK)” with the project title of “Synthesis of Refractory Metal Borides via Three Different Production Methods from Solid, Liquid and Gas Raw Materials for Various Application Areas; Sintering, Characterization, Comparison of Process and Final Products” and with the project number of 112M470.
Duygu Ağaoğulları
Duygu Ağaoğulları received her M.Sc. degree in 2007 and Ph.D. degree in 2014 in Materials Science and Engineering Department from Istanbul Technical University. She worked as a research assistant between 2005 and 2013, and has been affiliated since 2013 in I.T.U. She has been assigned as researcher/scholar in 10 scientific projects. Now, she is working as a postdoctoral scholar in Materials Science and Engineering Department in Stanford University. Her main research activities are powder metallurgy, high-tech boron products and composite materials. She is author/co-author of 21 papers, 1 Patent cited in Web of Science and 75 international conference proceedings.
Özge Balcı
Özge Balcı received her M.Sc. degree in Metallurgical and Materials Engineering from Istanbul Technical University in 2010 and continues her Ph.D. in the same department. She has been assigned as researcher in 4 scientific projects and gained professional fellowships in 3 research projects. She has been as a visiting researcher at IFW Dresden for 6 months during her Ph.D studies. Her main research activities are powder metallurgy, high-tech boron products and composite materials. She is author/co-author of 11 papers and 1 Patent cited in Web of Science and 42 proceedings presented in international conferences.
M. Lütfi Öveçoğlu
Prof. Dr. M. Lütfi Öveçoğlu received his M.Sc. and Ph.D. degrees in Materials Science and Engineering from Stanford University in 1984 and 1987. He has been affiliated with the Department of Metallurgical and Materials Engineering at Istanbul Technical University since 1990. He is the founder and technical director of Particulate Materials Laboratories (PML), a cluster of 9 laboratories. His main research activities are mechanical alloying and mechanochemical synthesis of W-based, Al-based and boride-based materials. He is author/co-author of 145 papers and 1 patent cited in Web of Science, 2 book chapters and 3 edited conference proceedings having about 905 citations.
İsmail Duman
Prof. Dr. İsmail Duman received his M.Sc. degree from Istanbul Technical University in 1978 and Ph.D. degree from Berlin Technical University in 1985 in Metallurgical Engineering. He has been affiliated with Department of Metallurgical and Materials Engineering at Istanbul Technical University since 1980. His main research activities are extractive metallurgy, powder metallurgy, development of high-tech boron products and CVD technologies. He has been the supervisor of 17 M.Sc. and 8 Ph.D. dissertations and he has been assigned as project director or researcher in 23 industrial/scientific research projects. He is author/co-author of 49 papers and 7 Patents cited in Web of Science.