2023 Volume 40 Pages 124-148
2023 Volume 40 Pages 124-148
Photocatalytic H2O2 production based on graphitic carbon nitride (g-C3N4) materials has been attracting increasing attention. However, it is difficult to reveal the inner relationships among the structure, properties and performance of a g-C3N4-based photocatalyst by simply summarizing preparation methods, properties and performances in previous works. In this review, the three most important issues for improving H2O2 generation based on the band diagram and physicochemical properties of pristine g-C3N4 are proposed. Improvement of the charge separation, promotion of the light absorption and introduction of active sites for 2e− oxygen reduction reaction to suppress side reactions are the most three attractive strategies for enhancing the activities. Following discussion of these strategies, representative functionalization methods are summarized on the basis of the most desired properties for improving the photocatalytic activities for H2O2 production. Other influence factors for improving H2O2 production such as addition of electron donors and adjustment of pH value of the solution are also discussed. Future challenges for photocatalytic H2O2 based on g-C3N4 materials are also summarized to provide future directions in this field.
H2O2 has been recognized as one of the 100 most important chemicals worldwide (Jones C.W., 1999; Pesterfield L., 2009). The reactive oxygen content in H2O2 (47.1 % w/w) is the highest among all chemicals. No toxic by-products are produced from H2O2, making H2O2 a highly efficient and environmental-friendly oxidant. H2O2 has been widely applied for disinfection and sterilization (McDonnell G., 2014; Teng Z. et al., 2019), organic synthesis (Zhan W. et al., 2018) and wastewater treatment (Zhang M.-h. et al., 2019). A one-compartment fuel cell using H2O2 in which H2O2 is used as both an oxidant and a reductant has recently been developed (Mousavi Shaegh S.A. et al., 2012). Studies on this fuel cell showed that the theoretical output potential is 1.09 V, which is comparable to that of a conventional hydrogen (H2) fuel cell (1.23 V) (Mousavi Shaegh S.A. et al., 2012; Kim D. et al., 2015; Yamada Y. et al., 2013). Compared to H2, H2O2 showed a promising prospect as a water-soluble solar fuel (H2O2, 3.0 MJL−1, in an aqueous solution higher than the intensity of compressed H2 gas, 2.8 MJL−1, 35 MPa) (Kim D. et al., 2015). Unlike hydrogen, H2O2 is fully soluble in water and is easily transportable, making it an ideal energy carrier alternative to H2. The anthraquinone (AQ) method for H2O2 production is the main process used in industry (more than 95 % of total production, Fig. 1) (Campos-Martin J.M. et al., 2006). The AQ process includes the following four steps: (i) in an organic solvent, hydrogenation of anthraquinone (AQ) is firstly catalyzed on an Ni/Pd catalyst; (ii) hydrogenated anthraquinone (HAQ) is oxidized with the aid of a catalyst; (iii) H2O2 is extracted by pure water, and HAQ is used to reproduce AQ; and iv) the as-prepared H2O2 is purified and concentrated for following commercial use. These hydrogenation and oxidation reactions consume a lot of energy. Additionally, the AQ process is not environmental-friendly because large amounts of wastes are generated (Campos-Martin J.M. et al., 2006).

Schematic of the anthraquinone oxidation (AQ) process.
Efforts have been made to develop new methods for H2O2 production under more moderated reaction conditions with less waste production. (Yi Y. et al., 2016). Direct synthesis from H2 and O2 in the presence of a Pd catalyst or a bimetallic Au–Pd catalyst is a possible alternative route for H2O2 production based on the reaction of H2 + O2 = H2O2. Typical reaction conditions mainly include H2/O2 mixture gas catalyzed by noble metal catalysts (Xia C. et al., 2019; Freakley S.J. et al., 2016). The ratio of the H2/O2 mixture needs to be precisely controlled in the process to avoid the risk of explosions (Xia C. et al., 2019; Freakley S.J. et al., 2016). Recently, much attention has been given to the possibility of efficient H2O2 production by a photocatalytic reaction process, which would be a significant breakthrough in H2O2 chemistry. A photocatalytic approach would enable the use of explosive H2 gas to be avoided and it would only need light, O2 and water, with no pollutant generated in the overall process. Much interest has therefore been shown in photocatalytic strategies for H2O2 production.
In past one decade, many kinds of semiconductors including metal oxides, metal sulfides, metal organic compounds and organic semiconductors have been prepared for photocatalytic H2O2 production (Hou H. et al., 2020). Among these photocatalysts, graphite-like carbon nitride (g-C3N4), also named polymeric carbon nitride (PCN), as a metal-free polymer semiconductor (n-type), possesses a stacked two-dimensional (2D) structure of tri-s-triazine connected via tertiary amines (Wang X. et al., 2009). Because of its unique electrical, optical, structural and physicochemical properties, g-C3N4 has been recognized as a new class of cost-efficient multifunctional materials for electronic, catalytic and energy applications (Banerjee T. et al., 2021). Shiraishi and co-workers first found in 2014 that pristine g-C3N4 showed some photocatalytic activity for H2O2 production with the addition of ethanol as an electron donor (Shiraishi Y. et al., 2014b). Since then, more than 80 studies on photocatalytic H2O2 production based on g-C3N4 materials have been published. Several reviews summarizing some of the recent progress in photocatalytic H2O2 production have also been published (Hou H. et al., 2020; Zhang G. et al., 2020). Most of those reviews focused on how the various protocols for preparation of functionalized g-C3N4 influenced the photocatalytic activity (Hou H. et al., 2020; Zhang G. et al., 2020). However, it is difficult to reveal the inner relationships among the structure, properties and performance of a g-C3N4-based photocatalyst by simply summarizing preparation methods, properties and performances (Zhang G. et al., 2020; Torres-Pinto A. et al., 2019). Generally, the structure of a photocatalyst defines its properties, and the properties of the photocatalyst determine its performance. Thus, it is necessary to determine the desired properties of a g-C3N4-based photocatalyst for H2O2 production. Determination of the desired properties would lead to a comprehensive understanding of the photocatalyst from rational structure design to good performance.
In this review, we firstly show the principles of photocatalytic H2O2 production. Then the three most important issues for improving H2O2 generation based on the band diagram and physicochemical properties of pristine g-C3N4 are proposed. By combining the fundamental principles with preparation strategies and possible influencing factors in liquid-phase photocatalytic systems, future challenges and possible directions for H2O2 production using functionalized g-C3N4 catalysts are presented in this review with the hope that they will inspire design and preparation strategies for not only carbon nitrides but also a variety of photocatalysts with good performance.
Since solar energy is renewable and sustainable, photocatalysis using power systems has received much attention in the past few years and it has been applied to various fields including water splitting for H2 and O2 production (Wang X. et al., 2009; Wang Q. and Domen K., 2020), CO2 reduction (Liu R. et al., 2020), nitrogen fixation (Zhang G. et al., 2020), degradation of pollutants (Koe W.S. et al., 2020) and disinfections (Teng Z. et al., 2019), organic synthesis and H2O2 production (Hou H. et al., 2020). Photocatalytic power systems are cost-efficient and easy to operate compared with photo-electrochemical systems and photovoltaic-electrochemical systems (Hisatomi T. and Domen K., 2019). There are three typical steps in a photocatalytic process (Fig. 2) (Nosaka Y. and Nosaka A., 2016). Sufficient light absorption, efficient charge separation, and surface reactions are crucial properties for typical efficient photocatalysts.

Photoexcitation and charge decay pathway.
Photocatalytic production of H2O2 follows the photocatalytic reaction principles. H2O2 can be generated through either oxygen reduction reaction (ORR) or water oxidation reaction (WOR) (Fan W. et al., 2020) as shown by a schematic diagram of photocatalytic H2O2 production in Fig. 3. The light-driven 2e− WOR pathway (Eqn. (3), Table 1) is difficult to be achieved because of the uphill thermodynamics (1.76 V vs. a reversible hydrogen electrode, simplified as RHE, Table 1), i.e., the decomposition of as-produced H2O2 will occur at this potential (1.76 V) because H2O2 is an excellent hole scavenger (Shi X. et al., 2017). In the case of the ORR pathway, H2O2 can be produced by either a 2e− direct oxygen reduction (O2 → H2O2) route or a sequential 1e− indirect reduction (O2 →•O2− → H2O2). For h+ in consumption, the most effective reaction route is to oxidize H2O into O2 and H+ via a 4e− pathway (Eqn. (1)). Eqn. (7) to Eqn. (10) in Table 1 show production of H2O2 via the two-step 1e− reduction pathway. First, superoxide radicals (•O2−) are formed (Eqn. (7)) and further produce HO2• radicals with the reaction of H+ (Eqn. (8)). Then, the HO2• radicals could produce HO2− anions by undergoing another 1e− reduction (Eqn. (9)). Finally, HO2− reacts with H+ to generate H2O2. Eqn. (2) shows the one-step 2e− direct reduction for H2O2 production. In this process, O2 directly reacts with two protons to form an H2O2 product via two-electron photoreduction. It should be noted that the back reaction of WOR via a 4e− pathway is the ORR process via a 4e− pathway (Eqn. (1)), which could also be a competitive reaction for H2O2 production via 2e− ORR. The most ideal situation would be for photocatalytic production of H2O2 to be generated from H2O and O2 via 4e− WOR and 2e− ORR, which is an uphill reaction (ΔG0 = 117 kJ mol−1).

Schematic diagram for photocatalytic H2O2 production. (a) Photocatalytic H2O2 production via water oxidation reaction. (b) Photocatalytic H2O2 production via oxygen reduction. CB is the abbreviation for conduction band. VB is the abbreviation for valence band.
Standard electrode potentials for aqueous ORR half-reactions (Gao J. and Liu B., 2020). Reprinted with permission from Ref. (Gao J. and Liu B., 2020). Copyright: (2020) American Chemical Society.
| Eqn. No. | Half-reactions | E0 (V vs. SHE*) | E0 (V vs. RHE**) |
|---|---|---|---|
| (1) | O2(g) + 4H+ + 4e− ↔ 2H2O | 1.229 | 1.229 |
| (2) | O2(g) + 2H+ + 2e− ↔ H2O2 | 0.695 | 0.695 |
| (3) | H2O2 + 2H+ + 2e− ↔ 2H2O | 1.763 | 1.763 |
| (4) | O2(g) + 2H2O + 4e− ↔ 4OH− | 0.401 | 1.229 |
| (5) | O2 + H2O + 2e− ↔ HO2− + OH− | −0.065 | 0.764 |
| (6) | HO2− + H2O + 2e− ↔ 3OH− | 0.867 | 1.696 |
| (7) | O2(g) + e− ↔ O2− | −0.33 | −0.33 |
| (8) | O2(g) + H+ + e− ↔ HO2 • | −0.05 | −0.05 |
| (9) | HO2• + e− ↔ HO2− | 0.76 | 0.76 |
| (10) | HO2• + H+ + e− ↔ H2O2 | 1.44 | 1.44 |
| (11) | H2O2 + H+ + e− ↔ H2O + •OH | 0.793 | 0.793 |
| (12) | •OH + e− ↔ OH− | 1.89 | 2.72 |
Since Wang and his co-workers first discovered the photocatalytic activities of g-C3N4 (Wang X. et al., 2009), g-C3N4-based particulate photocatalysts have attracted much interest. The band gap of g-C3N4 (Carbon nitrides produced by thermal treatment at 500~550 °C with nitrogen-rich precursors have various names as polymeric carbon nitride (PCN), graphitic carbon nitride (GCN), and melon) is ~2.7 eV, corresponding to an optical wavelength of ~460 nm, which makes it a promising visible-light-response photocatalyst with an appropriate band structure (Fig. 4) (Ong W.-J. et al., 2016; Hou H. et al., 2020). Additionally, the conjugated heptazine ring in the PCN matrix can provide active sites for 2e− ORR (Shiraishi Y. et al., 2014a; Shiraishi Y. et al., 2014b), which is similar to the catalytic properties of graphene for 2e− ORR (Kim H.W. et al., 2018; Lu Z. et al., 2018). As such, g-C3N4 has emerged as a star material in the field of photocatalytic H2O2 production.

Band diagrams of g-C3N4 and summary of potentials for redox reactions during H2O2 production by using O2 and/or H2O. CBM is the abbreviation for conduction band minimum and VBM is the abbreviation for valence band maximum.
However, the activity of g-C3N4 for photocatalytic H2O2 production is still restricted by low efficiency because of some drawbacks of pristine g-C3N4. Firstly, a large concentration of defects is inevitably introduced into g-C3N4 during thermal polymerization (Kim D. et al., 2015; Ong W.-J. et al., 2016). This means that the inner-plane structure of g-C3N4 is not completely composed of covalent bonds, i.e., the interactions are hydrogen bonds between melons ([C6N9H3]n) (Fig. 5) (Kessler F.K. et al., 2017). As a result, the charge separation and charge migration are significantly hindered, resulting in a high charge recombination rate of PCN. Secondly, the large band gap (2.7 eV) and small absorption of visible light (Kubelka-Munk absorbance usually less than 2, λ = 420 nm) result in insufficient visible-light harvesting. Thirdly, although the side-on adsorption of molecular oxygen on the π-conjugated heptazine ring enables g-C3N4 (Shiraishi Y. et al., 2014b) to have some selectivity for 2e− ORR, it is difficult for the stepwise 1e− to 1e− ORR reaction to be prevented since signals of superoxide radicals can still be detected during the photocatalytic reactions with electron spin resonance during the photocatalytic reactions (Li S. et al., 2016). In this case, reaction sites with higher selectivity for 2e− ORR have to be constructed for improving the overall activity. To overcome these drawbacks, many protocols including tuning morphologies, defect engineering, loading cocatalysts, copolymerization of semiconductors, doping elements and hybridization have been developed. Each of these functionalization strategies overcome one or two drawbacks by changing the following properties of pristine PCN: (i) suppressing charge recombination, (ii) narrowing the bandgap or/and promoting light absorbance, and (iii) introducing active sites for selective 2e− ORR (Fig. 6). Based on the improved physicochemical properties, functionalization strategies for improvement in the activity for photocatalytic H2O2 production can also be divided into three parts, which provide a comprehensive understanding from material preparation to physicochemical properties, thus providing the insight into the relationships among structure, properties and performance.
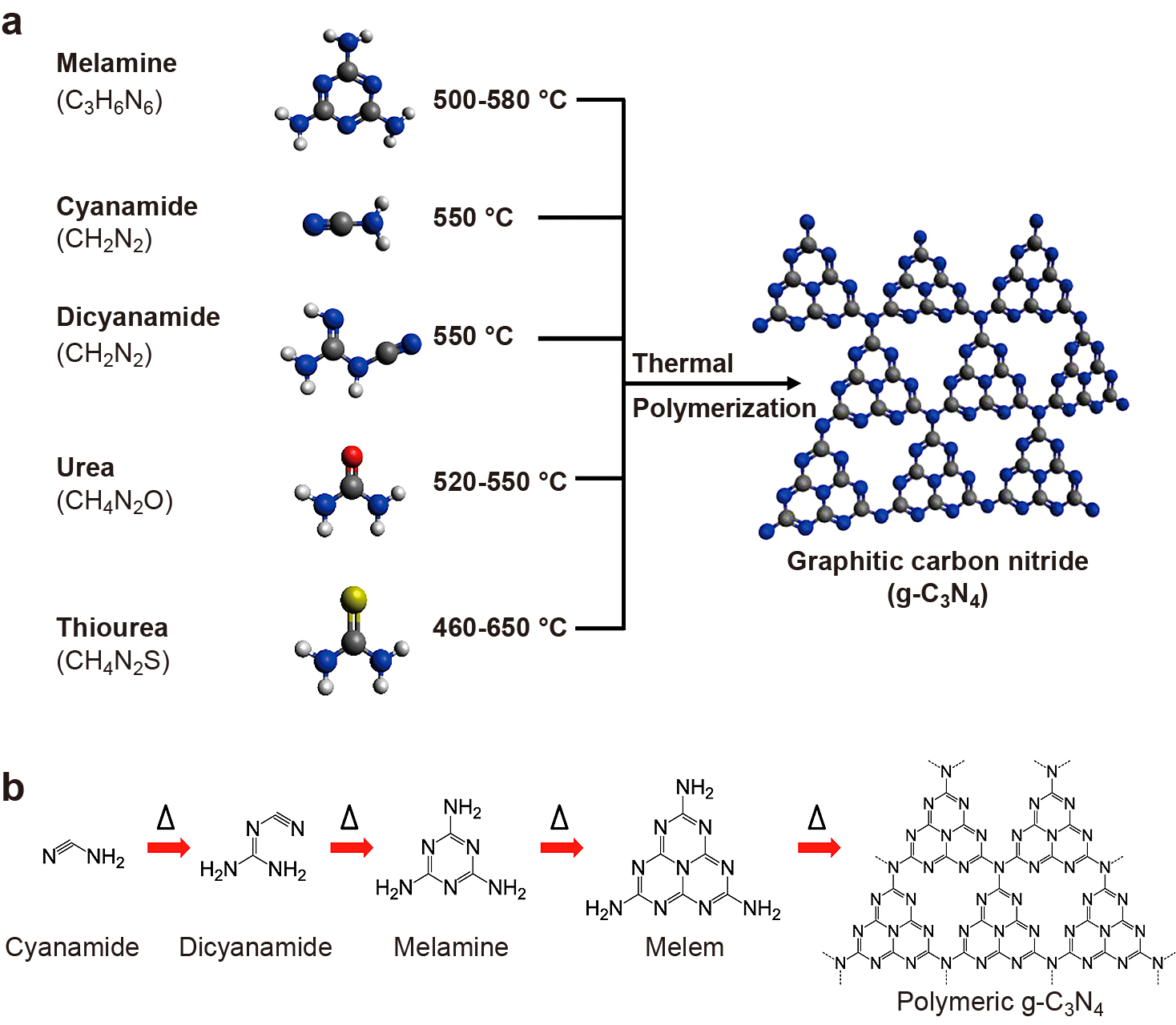
(a) Schematic illustration of the synthesis process of g-C3N4 by thermal polymerization of different precursors such as melamine, cyanamide, dicyanamide, urea, and thiourea. The black, blue, white, red, and yellow balls denote C, N, H, O, and S atoms, respectively. (b) Reaction pathway for the development of g-C3N4 using cyanamide as the precursor. Reprinted with permission from Ref. (Ong W.-J. et al., 2016). Copyright: (2016) American Chemical Society.

Solutions for overcoming the disadvantages of pristine g-C3N4 by manipulating physicochemical properties via 2e− ORR.
Carbonaceous nanomaterials are promising modifiers for photocatalysts because of their sp2-hybridized electronic structures (Shiraishi Y. et al., 2014a; Kim H.W. et al., 2018; Lu Z. et al., 2018; Yang S. et al., 2018). These materials usually work as electron transfer materials and photosensitizers to broaden the adsorption edge and improve the charge migration (Yang S. et al., 2018; Thakur S. et al., 2017; Zeng X. et al., 2017).
Carbon nanotubes (CNTs) with a π-conjugative structure are capable of accepting, transporting and storing electrons for g-C3N4 (Tasis D. et al., 2006). An amination reaction process was developed by Zhao and co-workers to introduce CNTs into nanosheets of g-C3N4, in which g-C3N4 was covalently combined with CNTs (Fig. 7a, b) (Zhao S. et al., 2018a). The CNTs covalently combined with g-C3N4 promoted charge migration by shifting the CB level to enhance the single-electron reduction of O2 to ·O2−, thus leading to enhanced ORR activity (Zhao S. et al., 2018a). As shown in Fig. 7c, the photocurrent of g-C3N4/CNTs was significantly increased compared with that pristine PCN. Shiraishi and co-workers also used reduced graphene oxide (rGO) for hybridization with a g-C3N4/perylene diimide (PDI) catalyst for further activity improvement (Fig. 7d, e). The improved activity may due to the ultra-thin two-dimensional nature of rGO (g-C3N4/PDI/rGO0.05) with high charge carrier mobility (Kofuji Y. et al., 2016a). As shown in Fig. 7f, the photocurrent of g-C3N4/PDI/rGO0.05 is significantly larger than that of g-C3N4/PDI. However, no current response is found by using a rGO electrode, indicating a synergic effect of g-C3N4/PDI and rGO. It should be noted that the formation of tight junctions between the g-C3N4-based photocatalyst and carbon-based material is extremely important for the formation of electron transfer pathways since the photocurrent intensity of the physical mixture of rGO and g-C3N4/PDI is 20 % smaller than the photocurrent density of g-C3N4/PDI (Kofuji Y. et al., 2016a).

Hybridization with a carbon-based material for improved charge separation. (a) TEM images of g-C3N4-CNTs. (b) Process for preparation of g-C3N4-CNTs. (c) Photocurrent response of g-C3N4-CNTs or g-C3N4-loaded electrodes under light at a bias of 0.25 V vs. SCE (λ ≥ 400 nm). (d) TEM image of g-C3N4/PDI/rGO0.05 and its SAED pattern (inverse contrast) with indexed graphite pattern. (e) Schematic illustration of the proposed mechanism for photocatalytic H2O2 production on g-C3N4/PDI/rGO. (f) Photocurrent responses of g-C3N4/PDI/rGO0.05 and other g-C3N4-based catalysts measured on FTO in 0.1 M Na2SO4 solution under visible light (λ > 420 nm) at a bias of 0.5 V (vs Ag/AgCl). SCE is an abbreviation for saturated calomel electrode, SAED is an abbreviation for selected area electron diffraction, and FTO is an abbreviation for fluorine-doped tin oxide. Reprinted with permission from Ref. (Zhao S. et al., 2018a; Kofuji Y. et al., 2016a). Copyright: (2018) Elsevier and (2016) American Chemical Society.
Fabrication of metal compounds to form a Schottky barrier between metal-based semiconductors and PCN material has been proved to be an efficient approach for enhancing the photocatalytic efficiency for several reactions (Ong W.-J. et al., 2016; Teixeira I.F. et al., 2018). The nature of the formation of Schottky barriers in PCN/metal compounds is due to the differences between the charge potentials of metal compounds and PCN under light irradiation (Zhang Z. and Yates J.T., 2012). The electrons tend to migrate to semiconductors that have more positive potentials, while holes tend to migrate to those with negative potentials (Nosaka Y. and Nosaka A., 2016). Although the overall redox potential of the photocatalyst is decreased, the charge separation of charge carriers is significantly improved, thus leading to a significantly improved charge separation (Fig. 8a). However, there are very few reports on the promotion of photocatalytic efficiency for H2O2 production by construction of heterojunctions based on PCN/metal compounds. There are two possible reasons. On the one hand, the potential of the conduction band minimum (0.9~1.3 V vs. SHE) of PCN (Fig. 4) is more negative than that of common metal compounds. In this case, the photoelectrons tend to accumulate on the metal oxides. Thus, the potential of the metal compounds determines the reduction potential of the heterojunction materials. As shown in Fig. 8b, the photocatalytic reduction potential of TiO2, Cu2O, CdS and ZnS can hardly facilitate 2e− ORR because of the kinetically favored 1e− ORR to form •O2−, thus resulting in a stepwise ORR to generate H2O2. For other metal compound semiconductors, 1e− ORR can be prevented because of the positively shifted CBM. However, the potential differences between the redox potentials of O2, 2H+/H2O2 and CBM of Fe2O3, CuWO4, SnO2, WO3, etc. are quite small, which may lead to a poor thermodynamic driving force for 2e− ORR. On the other hand, formed H2O2 can be decomposed by metal compounds by disproportionation or photoreaction (Li X. et al., 2001). Therefore, the formation of heterojunctions by contact of PCN with one metal compound may be an unacceptable strategy unless a unique electron transfer pathway between PCN and metal compounds is formed, such as in Z-scheme catalysts, so that reduction will occur on the surface of PCN (Wang S. et al., 2020a).

Schematic diagram of (a) semiconductor heterojunctions and (b) diagram of band positions based on experimental results for common metal-based photocatalysts. NHE is an abbreviation for normal hydrogen electrode.
Loading active noble metal nanoparticles (NPs) is one of the effective approaches for boosting the photocatalytic activity of a photocatalyst for H2O2 production (Hirakawa H. et al., 2016; Tsukamoto D. et al., 2012). Zuo and coworkers observed a similar improvement in the activity of an Au/C3N4 photocatalyst for H2O2 production (Zuo G. et al., 2019). In that study, the effects of different noble-metal cocatalysts on g-C3N4 for H2O2 production were also compared, and the results showed that g-C3N4 loaded with Au nanoparticles (NPs) had the highest activity for H2O2 production (Zuo G. et al., 2019). The enhancement of H2O2 production was attributed to the efficient separation of photogenerated electrons and holes between Au NPs and g-C3N4 (Zuo G. et al., 2019). The function of Au NPs on PCN for charge separation is similar to that of carbon materials. The Au NPs can improve the carrier mobility of photoelectrons so that the charge recombination can be suppressed.
3.1.1.3 Hybridization with metal-free semiconductors and moleculesThe advantages of cost efficiency, mechanical flexibility and easy operation makes organic semiconductors promising alternatives for inorganic materials to improve the charge separation (Chung D.D.L., 2019). Triazine (Jiang X. et al., 2015) and aromatic diimides have been used as modifiers for g-C3N4 based photocatalysts (Shiraishi Y. et al., 2014a; Kofuji Y. et al., 2016a; Kofuji Y. et al., 2018; Kofuji Y. et al., 2017; Kofuji Y. et al., 2016b). Shiraishi and co-workers incorporated several aromatic diimides (Shiraishi Y. et al., 2014a; Kofuji Y. et al., 2018; Kofuji Y. et al., 2016b; Kofuji Y. et al., 2017) into the g-C3N4 matrix by thermal condensation (Fig. 9a). All of the g-C3N4/PDI, g-C3N4/biphenyl diimide (BDI) and g-C3N4/mellitic triimide (MTI) catalysts successfully produced H2O2 (millimolar level) in pure water with O2 (Shiraishi Y. et al., 2014a; Kofuji Y. et al., 2016a; Kofuji Y. et al., 2018; Kofuji Y. et al., 2017; Kofuji Y. et al., 2016b). The photocurrent showed a significant improvement after the copolymerization of aromatic diimides. It should be noted that the introduction of more C=O groups significantly improved the photocurrent (Fig. 9b) (Kofuji Y. et al., 2017). The C=O groups in these aromatic diimides may accumulate negative charges and then increase the thickness of the space-charge region, which might improve the separation efficiency of charge carriers (Koe W.S. et al., 2020) and disinfections (Teng Z. et al., 2019). Based on this phenomenal result from the properties of C=O groups, Ohno and co-workers prepared C=O functionalized PCN by copolymerization of 2,5,8-triamino-tri-s-triazine (melem) and barbituric acid (BA) (PCNBA, Fig. 9c) (Teng Z. et al., 2020). In the photoluminescence (PL) spectra (Fig. 9d), there was a significant decrease in intensity together with a notable increase in photocurrent intensity (Teng Z. et al., 2020). The copolymerization of BA significantly suppressed recombination and accelerated the charge separation.

Hybridization with electron-deficient organic semiconductors for improved charge separation. (a) Scheme of the synthesis of g-C3N4 hybridized by aromatic diimide using g-C3N4/PDIx as an example. (b) Photocurrent responses of g-C3N4/MTI49 and g-C3N4/PDI51 in 0.1 M Na2SO4 (bias: 0.5 V vs Ag/AgCl). (c) Process for polymerization of PCNBA with barbituric acid and melem as precursors. (d) Photoluminescence spectra of PCN and PCNBA samples. Reprinted with permission from Ref. (Kofuji Y. et al., 2017; Teng Z. et al., 2020). Copyright: (2017) American Chemical Society and (2020) Elsevier.
In the case of incorporation with semiconductors, Shiraishi and co-workers combined PCN/PDI with boron nitride (BN) to separate holes since BN has a relatively negative VBM (Kofuji Y. et al., 2018). In the system of g-C3N4-PDI-BN0.2-rGO, the photocurrent increased about 20 % (Kofuji Y. et al., 2018). It is notable that the oxygen evolution of g-C3N4-PDI-BN0.2-rGO was increased by more than 15 % compared with that of g-C3N4-PDI-rGO (Kofuji Y. et al., 2016a), further confirming that the charge separation introduced by BN separated photogenerated holes, leading to an improved charge separation. Perylene imides (PIs) were modified on g-C3N4 nanosheets (Yang L. et al., 2017) to fabricate a Z-scheme configuration for promotion of charge separation. As a result, more electrons from the CB of the g-C3N4 part efficiently generated H2O2, while the holes of g-C3N4/PI oxidized OH− to OH (Yang L. et al., 2017), which also subsequently reacted to produce H2O2.
3.1.2 Doping 3.1.2.1 Metal and non-metal dopingOptical, electronic and other physical properties of g-C3N4 materials could be manipulated by doping strategies that introduce additional elements and impurities into the framework (Liu X. et al., 2020). In the case of charge separation, one of the most efficient strategies is to incorporate alkaline metal ions. Alkaline metal ions were used in several works to functionalize PCN, leading to a promoted charge separation for organic synthesis (Qiu C. et al., 2019) (Fig. 10a). Hydrogen evolution reduction (HER) (Sun S. et al., 2019), carbon dioxide reduction (COR) (Wang S. et al., 2020b), and nitrogen fixation, (Li X. et al., 2018a) can all be facilitated by incorporation of alkaline metal ions into PCN. Moon and co-workers incorporated potassium (K), phosphorus (P), and oxygen (O) elements into a g-C3N4 matrix by thermal calcination of melamine with potassium phosphate (KPD, K2HPO4) (Moon G.-h. et al., 2017). The charge separation of KPD was significantly improved because of the significantly decreased PL intensity (Fig. 10b). The hector-elements in the g-C3N4 matrix increased the carrier lifetime to a picosecond range. Consequently, the generation rate of H2O2 by K-P-O-doped g-C3N4 was notably promoted compared to that by pure g-C3N4 (Moon G.-h. et al., 2017) (Fig. 10c). Halogen (Br or Cl)-doped g-C3N4 nanorods could also accelerate H2O2 production (Zhang C. et al., 2018). The charge carrier transfer in the matrix of g-C3N4 was promoted by the introduction of heteroatoms (Zhang C. et al., 2018). Kim et al. studied the co-doping of metal (K) and nonmetal ions (P and F) into the g-C3N4 framework (Kim S. et al., 2018). The co-doped g-C3N4 also significantly promoted charge separation. Under visible light irradiation, the co-doped g-C3N4 exhibited higher activity for photocatalytic production of H2O2 than that of bare g-C3N4 (Fig. 10d).

Metal and/or non-metal doping for improving the charge separation. (a) XRD patterns of pristine PCN and K+-incorporated PCN. (b) PL emission spectra (λ = 400 nm) of bare carbon nitride (CN), KPD-CN-3, and KPD-CN-7.5. (c) Apparent quantum yield (AQY, left axis) and turnover frequency (right axis) of bare CN and those of KPD-CN-7.5 for H2O2 production with monochromatic light irradiation. (d) Time profiles of photocatalytic H2O2 generation using CN samples modified with various dopants and a control sample of a physical mixture of CN and KPF6 (CN + KPF). The experimental conditions were 0.5 g L−1 photocatalyst, 10 vol% ethanol, pH 3, continuously purged with O2 and polychromatic light through a long pass filter (k > 420 nm). Charge distributions of (e) K-GCN and (f) KCl-GCN obtained from density functional theory (DFT) calculations. Reprinted with permission from Ref. (Qiu C. et al., 2019; Moon G.-h. et al., 2017; Kim S. et al., 2018; Zhang P. et al., 2019). Copyright: (2018) The Authors (Qiu C. et al.), published by John Wiley & Sons, (2017) American Chemical Society, (2018) Elsevier and (2019) Springer Nature Ltd.
Zhang et al. used density functional theory (DFT) calculation to reveal how the incorporated alkaline ions change the charge distribution over different atoms (Zhang P. et al., 2019). The introduction of incorporated potassium species resulted in an accumulation on the first PCN layer. With the further assistance of Cl atoms, the electron distribution between the layers was more balanced (Fig. 10e and 10f), thus leading to a significantly improved interlayer charge transfer (Zhang P. et al., 2019).
Non-metal doping of PCN also enables manipulation of the electronic configuration of PCN with significantly improved charge separation (Zhang P. et al., 2020). Wei et al. developed an oxygen-enriched g-C3N4 (OCN) photocatalyst that achieved enhanced H2O2 production (Wei Z. et al., 2018). Compared to g-C3N4, the OCN samples achieved significantly enhanced no-sacrificial H2O2 production efficiency. Electrochemical impedance spectroscopy (EIS) and photoluminescence (PL) spectra all proved that charge separation efficiency was significantly promoted after the O element had been introduced into the PCN matrix.
3.1.2.2 Doping with polyoxometalates and their derivativesPolyoxometalates (POMs) are composed of cations and polyanion clusters with high structural diversity (Han X.-B. et al., 2015). Under light irradiation, a charge transfer from O2− to Mn+ (n = 5, 6) usually occurred in these catalysts and lead to the formation of a hole center (O−) and trapped electron center (M(n−1)+) pair. POMs in the PCN matrix can serve as electron donor-acceptor pairs, which could significantly promote the charge separation or/and serve as co-catalysts (Han X.-B. et al., 2015). Zhao and coworkers synthesized a series of POMs clusters-modified g-C3N4 samples for efficient photocatalytic H2O2 evolution by the thermal condemnation method (Zhao S. et al., 2017; Zhao S. and Zhao X., 2019; Zhao S. et al., 2018b). The amount of H2O2 formed by g-C3N4/PW11 reached 3.5 μmol within 60 min, while the catalytic performance of pure g-C3N4 was only 1.3 μmol (Zhao S. et al., 2017). That research group also used other POM clusters, including [SiW11O39]8− (SiW11), (NH4)3PW12O40 (NH4-PW12) and (NH4)8Co2W12O42 (NH4-Co2W12), for covalent combination with g-C3N4 (Zhao S. and Zhao X., 2019). All of the clusters significantly improved the charge separation of pristine PCN. Action spectra measurements revealed that the band excitation results in the generation of H2O2 in both g-C3N4 and g-C3N4-CoWO systems (Han X.-B. et al., 2015; Zhao S. et al., 2017; Zhao S. and Zhao X., 2019), indicating that the function of POM incorporation may be as the same as that of ion doping since POM clusters are all well dispersed in the PCN matrix. The inner-panel charge transfer and the charge transfer between the layers may be significantly promoted by the introduction of highly dispersed POM clusters.
3.1.3 Defect engineeringRecently, it was found that defects in semiconductors enhance photocatalytic activity if they are precisely controlled (Pei Z. et al., 2013; Xiong J. et al., 2018; Meng A. et al., 2020). Introduction of carbon vacancies (Li S. et al., 2016; Goclon J. and Winkler K., 2018) and introduction of nitrogen vacancies are usually defect engineering used in a g-C3N4 photocatalyst (Li X. et al., 2018b; Qu X. et al., 2018; Shi L. et al., 2018; Zhu Z. et al., 2018). These strategies can change the electronic configuration and reactant molecules to promote H2O2 production. For example, Li et al. synthesized g-C3N4 functionalized carbon vacancies (Cv-g-C3N4) through thermal annealing in an oxygen-deficient environment (Ar flow) (Li S. et al., 2016). This Cv-g-C3N4 was also functionalized with a large number of amino groups with the formation of carbon vacancies (Li S. et al., 2016). Under visible light irradiation (Kessler F.K. et al., 2017), the H2O2 yield of Cv-g-C3N4 was much higher than that of pure g-C3N4 (Li S. et al., 2016). The improved photocatalytic activity of Cv-g-C3N4 for H2O2 production can be ascribed to the promoted charge separation and alteration of the H2O2 production pathway from a stepwise 1e− to 1e− pathway to a 2e− pathway.
Nitrogen vacancies in g-C3N4 can also boost the photocatalytic activity for H2O2 production (Zhu Z. et al., 2018). Li et al. (Li X. et al., 2018b) and Qu et al. (Qu X. et al., 2018) showed that nitrogen vacancies can be introduced in the matrix of g-C3N4 by dielectric barrier discharge (DBD) plasma treatment. Their results showed that H2O2 evolution can be improved by up to 10 times compared with that produced by pristine PCN (Li X. et al., 2018b). In another related study, Shi et al. created a holey defective C3N4 photocatalyst with nitrogen vacancies via a hydrazine photoreduction method. The transfer of charge carriers from the bulk to the surface was significantly improved, as confirmed by PL measurements (Shi L. et al., 2018). The introduced nitrogen vacancies narrowed the bandgap and the formation of defect states within the bandgap together with a notably suppressed electron-hole recombination. Therefore, the holey defective g-C3N4 photocatalyst showed much higher photocatalytic activity for visible light-driven H2O2 production than that of pristine g-C3N4.
In summary, the functionalization strategies of doping and defect engineering for improving the charge separation for PCN-based materials used for the 2e− ORR process are similar to the functionalization strategies for other reactions such as HER, COR, and ORR. However, the hybridization of PCN-based materials, especially hybridization with electron-deficient semiconductors, provides a PCN-based photocatalyst with unique electronic properties for improving the charge separation. We believe that copolymerization strategies to introduce other electron-deficient groups with higher charge separation efficiency might enable the generation of π-conjugated and π-stacked donor–acceptor couples (Shiraishi Y. et al., 2019) in the PCN matrix, leading to a higher efficiency for photocatalytic H2O2 synthesis.
3.2 Improving light harvestingAlthough pristine PCN can use visible light for a photocatalytic reaction, the large band gap (2.7 eV) results in insufficient visible light harvesting for further efficiency improvement (Ong W.-J. et al., 2016). Thus, improving the light absorbance of a g-C3N4-based photocatalyst and narrowing the band gap are two practical strategies for H2O2 production.
Copolymerization of PCN with other organic molecules/semiconductors, such as triazine, barbituric acid and aromatic diimides, not only improves the light absorbance of the photocatalyst but also narrows the bandgap. As mentioned in Section 3.1.1.3, Shiraishi and co-workers prepared a series of PCN samples hybridized with atomic diimides with a significantly narrowed band gap from 2.7 eV of pristine PCN to 2.4 eV of PCN/PDI (Shiraishi Y. et al., 2014a; Kofuji Y. et al., 2017; Kofuji Y. et al., 2016a). The light absorbance of PCN samples hybridized with aromatic diimides improved to about ~20 % at λ = 400 nm, indicating improved light absorption. Additionally, Ohno and co-workers co-polymerized melem with barbituric acid to expand the light absorption edge from 445 nm (2.76 eV) of pristine PCN to ~550 nm (2.18 eV) of PCNBA0.5 (Fig. 11a) (PCNBA is an abbreviation for polymetric carbon nitride doped by barbituric acid, 0.5 means calcination of 3 g melamine and 0.5 g barbituric acid) (Teng Z. et al., 2020). The absorbance of PCNBA0.2 at 420 nm was also increased by 200 % compared with that of pristine PCN (Fig. 11b) (Teng Z. et al., 2020).

Typical strategies for improving light harvesting. (a) Photographs show the corresponding powders of PCNBA samples. (b) UV-vis spectra of PCN and PCNBA samples: absorbance spectra. (c) Diffuse reflectance UV-vis spectra (DR-UVS) of modified CN samples prepared with various KPF6 contents. (d) UV-vis spectra and apparent quantum yield (AQY) of H2O2 production as a function of irradiation wavelength with 10 % EtOH (v/v) under visible light irradiation (λ ≥ 420 nm) and T = 25 °C. Reprinted with permission from Ref. (Teng Z. et al., 2020; Zhang P. 2019). Copyright: (2019) Springer Nature Ltd. and (2020) Elsevier.
Doping of alkaline metal ions can also expand the light absorption to a small extent (0.1 eV~0.3 eV) combined with significantly light improved absorbance (50 %). Alkaline metal doping and non-metal doping were also proved to be efficient strategies for improving light absorbance (Moon G.-h. et al., 2017; Kim S. et al., 2018; Zhang P. et al., 2020). Choi and co-workers found that the light absorption ability of CN samples was gradually promoted by increasing the amount of KPF6, even though KPF6 alone did not exhibit any visible-light absorption (Moon G.-h. et al., 2017) (Fig. 11c). A very recent work using K and S-co-doped PCN with functionalization of the –OH group (AKMT) also showed significant improvement in both light absorption and narrowing of the bandgap (Zhang P. et al., 2020). The light absorption edge expanded from ~450 nm of pristine PCN to ~520 nm of AKMT (Zhang P. et al., 2020) (Fig. 11d). With the addition of a sacrificial reagent, AKMT showed apparent quantum yields (AQYs) of 76 % and 40 % at 480 nm and 500 nm for H2O2 production, respectively. By comparison, pristine PCN exhibited negligible activities at the same wavelengths.
Defect engineering can also slightly improve the light absorption of photocatalysts, especially for semiconductor-based materials (Li S. et al., 2016) and Zhu et al. (Zhu Z. et al., 2018) reported that the UV-vis absorption spectra of g-C3N4 and carbon vacancy-functionalized g-C3N4 showed a red shift of absorption edges compared with that of pristine ones. Nitrogen vacancies also significantly improve light absorption (Li X. et al., 2018b; Qu X. et al., 2018; Shi L. et al., 2018). Ye and co-workers compared the optical properties of bulk PCN (BCN) and those of N-defected CN samples (DCN) (Shi L. et al., 2018). The absorption edges of the samples showed red shifts of ~40 nm compared with that of pristine g-C3N4 samples without thermal treatment (Shi L. et al., 2018). They also revealed that the electrons could be accommodated by forming defect states in BCN, which greatly promoted light harvesting (Shi L. et al., 2018). Compared with the copolymerization of an electron-deficient semiconductor and metal/non-metal doping, the influence of defect engineering is relatively small because defect states are usually located just below the CBM. In this case, the extent of narrowing of the bandgap by introducing defects is limited. Copolymerization of electron-deficient semiconductors seems to be the most promising functionalization strategy for both improving light absorbance and expanding the absorption edge.
3.3 Introduction of active sites for selective 2e− ORRIntroduction of active sites with specific physicochemical properties can significantly improve both selectivity and activities (Bo Y. et al., 2020). To give a systematic review for this topic, three dominant strategies for introducing active sites for photocatalytic H2O2 production are presents as follows.
3.3.1 Increasing the surface areaPCN prepared by the thermal polymerization method is usually quite small (<15 m2 g−1) (Teng Z. et al., 2017). As previously mentioned, the conjugated heptazine ring in the PCN matrix can provide active sites for 2e− ORR (Shiraishi Y. et al., 2014a). A plausible strategy for increasing the number of active sites for 2e− ORR is to increase the surface area (Mohamed N.A. et al., 2019; Ou H. et al., 2017; Liao G. et al., 2019). However, there is still some controversy about whether the overall 2e− ORR rate can be improved by increasing the specific surface area of PCN. Shiraishi and co-workers investigated the photocatalytic production of H2O2 with the existence of O2 and Et-OH. A series of mesoporous g-C3N4 catalysts having different specific surface areas (SSA) were prepared by a hard template (silica nanoparticles with different diameters) method (Fig. 12a) (Shiraishi Y. et al., 2015). In these systems, photogenerated holes oxidize Et-OH and the conduction band electrons localized at the melem unit reduce O2 to form H2O2. This g-C3N4 catalysts exhibit significant higher SSA of 56 and 160 m2 g−1 compared with that of pristine g-C3N4 (10 m2 g−1) and higher activity for H2O2 production (Shiraishi Y. et al., 2015). However, g-C3N4 with the largest SSA (228 m2 g−1) showed significantly decreased activity and selectivity for H2O2 formation (Fig. 12b) (Shiraishi Y. et al., 2015). Mesoporous g-C3N4 with the largest surface area inherently contains the largest number of primary amine moieties (Shiraishi Y. et al., 2015). The H2O2 selectivity was significantly suppressed since these defects can also serve as active sites for 4e− reduction of O2 (Fig. 12c) (Shiraishi Y. et al., 2015). These defects also further decomposed the formed H2O2, leading to significantly suppressed activity. Results of density function theory (DFT) calculations confirmed (i) a large distribution of lowest unoccupied molecular orbital (LUMO) electrons onto the primary amine moieties and (ii) a decrease in H2O2 selectivity with an increase in the amount of primary amine moieties, strongly suggesting that the primary amine moieties on the surfaces of mesopores behave as the active sites for four-electron reduction (Fig. 12d–f) (Shiraishi Y. et al., 2015). However, the specific types of those defects were not described in their reports. Specification of the types and fine structures of defects is important to further improvement by defect engineering strategies.

Influence of surface defects on H2O2 production by using a PCN-based material. (a) Different N atoms of PCN. (b) Time-dependent change in the amounts of H2O2 during photoreaction on the respective GCN(x) catalysts. (c) Electron transfer numbers during the photocatalytic H2O2 production. Interfacial plots of the main orbitals for (d) single, (e) double, and (f) triple melem-conjugated models, calculated by the function of B3LYP at the 6–31G (d) level. Reprinted with permission from Ref. (Shiraishi Y. et al., 2015). Copyright: (2015) American Chemical Society.
Copolymerization with electron-deficient semiconductors such as PDI, BDI and MTI has also been proved to be one of the most efficient strategies for promoting the selectivity of a photocatalyst (Shiraishi Y. et al., 2014a; Kofuji Y. et al., 2017; Kofuji Y. et al., 2016a). Calculations based on the density functional theory (DFT) were investigated to clarify the influence of the PDI unit on the electronic structure of the g-C3N4 framework (Shiraishi Y. et al., 2014a). Time-dependent results showed that the main transitions are S0→S1 (highest occupied molecular orbitals, HOMO→LUMO and HOMO→LUMO+2). The iso-surfaces showed that electron distributions of the melem-PDI model (main transitions HOMO→LUMO+2) are located mainly at the melem units with partial distribution to the PDI units (Shiraishi Y. et al., 2014a). The high electron affinity of the PDI units in g-C3N4/PDI may significantly improve the polarization of the whole frameworks.
Being specific, the electrons on HOMO are concentrated at the N2 and N6 atom positions of the melem unit, and those on LUMO+2 are located at the C1 and N4 atoms (Shiraishi Y. et al., 2014a). The Raman spectra of g-C3N4/PDI and g-C3N4 confirmed that the side-on adsorption of O2 was significantly promoted on g-C3N4/PDI, indicating a crucial role of copolymerization of electron-deficient semiconductors.
3.3.3 Doping/Heteroatom incorporationIncorporation of metal species or no-metal species in the PCN matrix can both improve the charge separation and introduce active sites for specific reactions (Hu S. et al., 2018; Zhang Z. et al., 2022). By the template-assisted method, Hu and co-workers fabricated hollow copper-doped g-C3N4 microspheres. The g-C3N4 with copper incorporation showed a much higher H2O2 production ability (~4.8 mmol·L−1) than that of pristine g-C3N4 (0.45 mmol·L−1). DFT simulations showed that the Cu(I)-N sites work as reactive sites to activate molecular O2 (Hu S. et al., 2018). The OCN proposed by Zhu and co-workers was proven to show a high selectivity for 2e− ORR for photocatalytic H2O2 production, which is attributed to the functionalized C-O-C groups on OCNs (Wei Z. et al., 2018). Choi and co-workers revealed by DFT calculations that the K incorporation could result in improved adsorption for O2 molecules (Zhang P. et al., 2019) (Fig. 13a, b). They also investigated whether electrons on S-doped g-C3N4 can be donated to the antibonding π* orbital of adsorbed *O2 (with –0.40 |e|) for forming efficient ORR sites, as well as AQ doping as cocatalysts (Fig. 13c). The strong electron pushing effect between carbon and oxygen facilitates subsequent protonation in ORR kinetics (Chu C. et al., 2020). Both the activity and selectivity of ORR can be changed by manipulating the properties of metallic sites (Kulkarni A. et al., 2018). As elaborated in several electrochemical catalysts, the end-on O2 adsorption usually lead to highly selective 2e− ORR. It is difficult to prevent the splitting of O-O bond breaking on surface of metal particles since end-on O2 molecular adsorption and side-on O2 molecular adsorption occur on metal particles (Choi C.H. et al., 2014; Chu C. et al., 2019). Benefiting from the desirable features of a single atom catalyst (SAC) (Sun H. et al., 2021), the adsorption of O2 molecules on atomically isolated sites is often end-on type, which could reduce the possibility of O-O bond splitting (Kulkarni A. et al., 2018; Wang A. et al., 2018). For example, SACs with Pt2+ (Shen R. et al., 2019) and Co-N4 (Gao J. et al., 2020; Jung E. et al., 2020) centers could electrochemically reduce O2 to H2O2 via a 2e− ORR pathway with ultrahigh selectivity (>96 %). However, it is difficult for Pt2+ and Co-N4 sites to be coupled in the photocatalytic system due to their high charge recombination characteristics, which originate from the intermediate band formed by the half-filled d electrons (Teng Z. et al., 2021a). Very recently, Ohno and co-workers developed an Sb single atom photocatalyst (Sb-SAPC) for non-sacrificial photocatalytic H2O2 synthesis in a water and oxygen mixture under visible light irradiation (Teng Z. et al., 2021a). The Sb-SAPC was prepared by a wet chemical method using NaSbF6 and melamine as precursors. As shown in the image acquired from high-angle annular dark field scanning TEM (HAADF-STEM) measurement, the bright spots with high density are uniformly dispersed in the entire carbon nitride matrix (Fig. 14a). Electron energy loss spectroscopy (EELS) (Fig. 14b) measurement revealed bright spots corresponding to Sb atoms, in which the oxidation state of Sb is regulated to +3 with a 4d105s2 electron configuration (Fig. 14c, d). The results of experimental and theoretical investigations indicated that the adsorption of O2 on isolated Sb atomic sites is end-on type, which promotes the formation of Sb-μ-peroxide (Sb-OOH), leading to an efficient 2e− ORR pathway for H2O2 production (Fig. 14e). The 2e− ORR efficiency of Sb species improved by almost one magnitude in the presence of 2-propanol (Fig. 14f). The apparent charge recombination of Sb-SAPC was significantly suppressed by introducing Sb species into the PCN matrix (Fig. 14g, h). With highly concentrated holes, this catalyst also achieved non-sacrificial H2O2 production using water as an electronic donor.
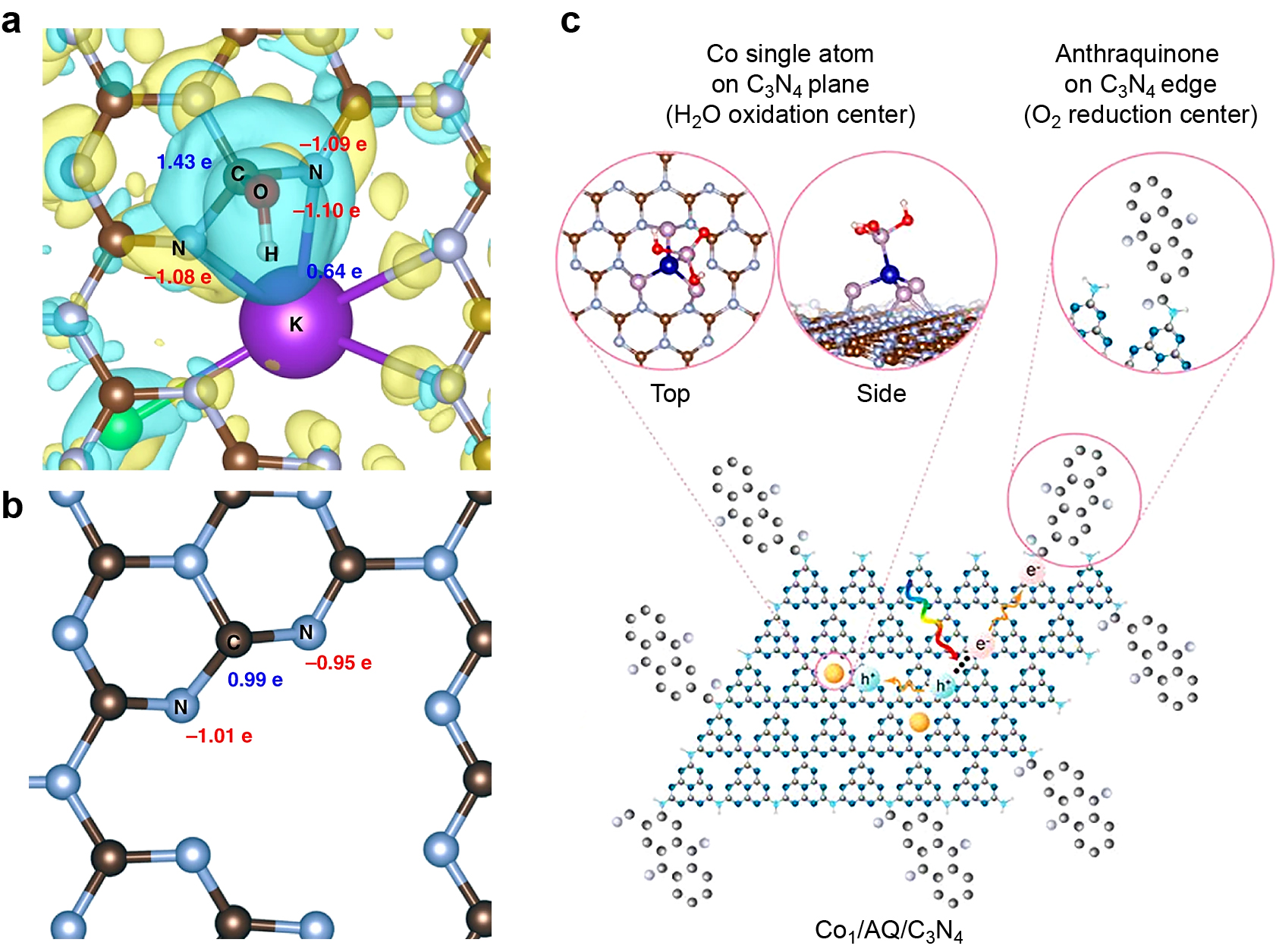
Typical strategies for introducing active sites for efficient 2e− ORR. (a) An enlarged top view of KCl-OH-GCN, and (b) an enlarged top view of pristine GCN. The yellow and blue colors represent electron accumulation and depletion at an isosurface value of 0.002 Å e−3. The brown, gray, and violet colors represent carbon, nitrogen, and potassuim, respectively. (c) Spatial separation of Co single atom (as an oxidation center) and AQ (as a reduction center) cocatalysts by anchoring them in the center (i.e., pyridinic N) and on the edge (i.e., primary/secondary amine N) of 2D ultrathin C3N4. Reprinted with permission from Ref. (Zhang P. et al., 2019; Chu C. et al., 2020). Copyright: (2019) Springer Nature Ltd. and (2020) The Authors (Chu C. et al.), published by National Academy of Science.
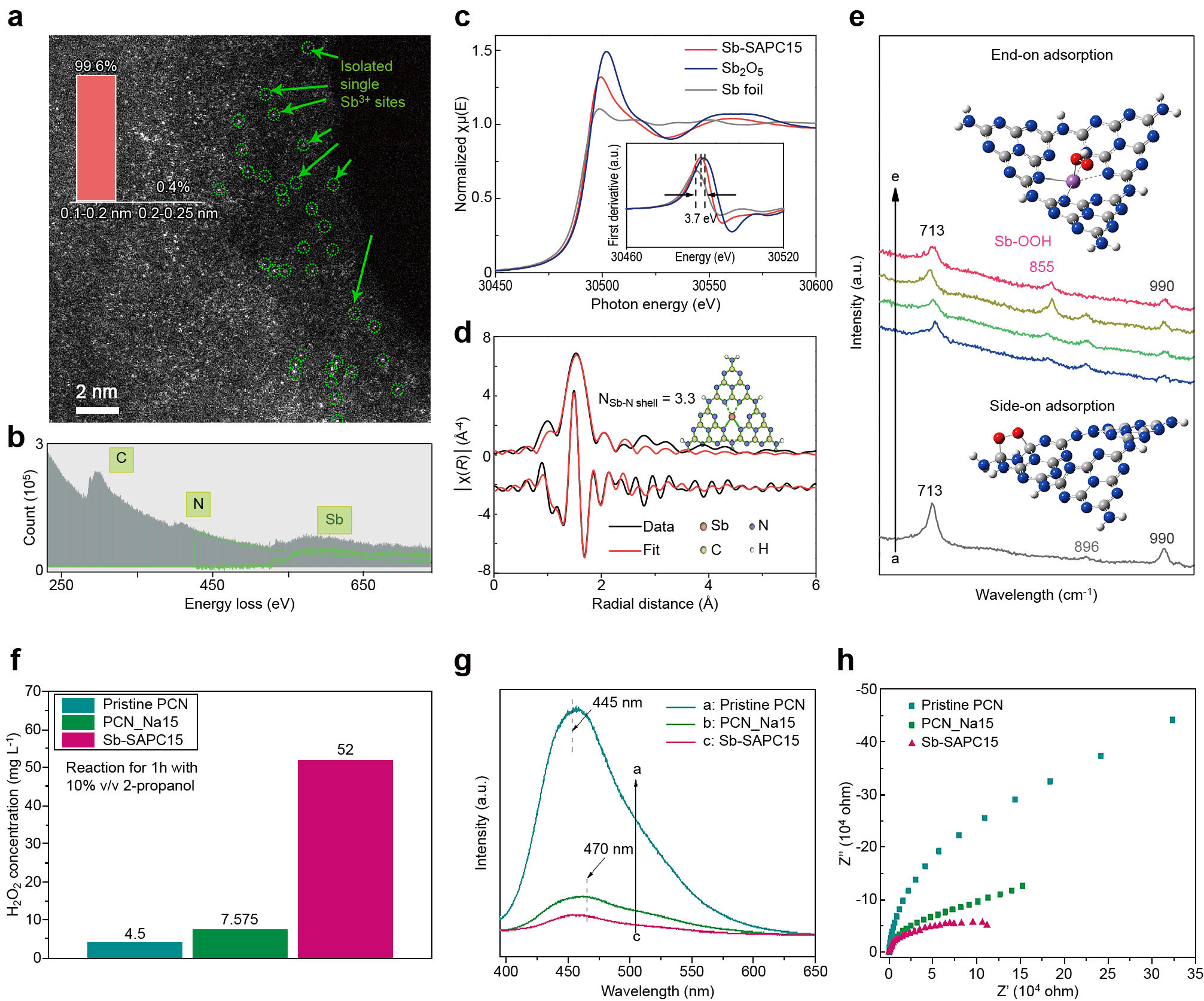
Single atomic sites for efficient ORR. (a) High-magnification HAADF-STEM image of Sb-SAPC15. The insert shows size distribution of the bright spots. (b) Electron energy-loss spectroscopy (EELS) spectrum of Sb-SAPC15. (c) Sb-K edge X-ray absorption near edge structure (XANES) of Sb foil, Sb2O5 and Sb-SAPC15. (d) Fitting of the extended X-ray absorption fine structure (EXAFS) data for Sb-SAPC15 based on the model obtained from DFT optimization. Inserted figures: optimized molecular models based on DFT for EXAFS fitting. (e) Experimental Raman spectra recorded during the photoreaction in a 2-propanol aqueous solution with saturated oxygen. Spectra a, b, c and d: PCN, Sb-SAPC1, Sb-SAPC5 and Sb-SAPC15 in 10 % (v/v) 2-propanol aqueous solutions. Spectrum e: Sb-SAPC15 in pure water. (f) Comparison of H2O2 production in 10 % (v/v) 2-propanol aqueous solutions catalyzed by pristine PCN, PCN_Na15 and Sb-SAPC15. (g) Photoluminescence spectra of PCN, PCN_Na15 and Sb-SAPC15 at an excitation wavelength of 380 nm (h) Electrochemical impedance spectroscopy (EIS) spectra (Nyquist plots) of pristine PCN and Sb-SAPC15 in the frequency range from 100 kHz to 0.01 Hz at 0.6 V (vs. Ag/AgCl) under visible light irradiation. Reprinted with permission from Ref. (Teng Z. et al., 2021b). Copyright: (2021) Springer Nature Ltd.
Cocatalyst loading is a traditional strategy for introducing active sites for specific reactions (Nosaka Y. and Nosaka A, 2016). For instance, pristine PCN shows quite low efficiency in photocatalytic hydrogen production, and loading of platinum species on PCN usually significantly improves the HER performance (Wang Q. and Domen K., 2020). The reason for loading a cocatalyst to boost the photocatalytic reactions is usually the decreased overpotential of the reactions on the co-catalyst surface compared with that on pristine photocatalysts (Hirakawa H. et al., 2016; Tsukamoto D. et al., 2012; Peng Y. et al., 2017). AQ species can enhance the selectivity of O2 reduction to H2O2 based on the mechanism of the current industrial H2O2 production process (Kim H.-i. et al., 2018; Chu C. et al., 2020). Kim and co-workers prepared several AQ-modified PCN samples with significantly improved selectivity for 2e− ORR (Chu C. et al., 2020). Loading an AQ cocatalyst onto ultrathin g-C3N4 significantly improved the selectivity of H2O2 production, combined with a 1.9-fold enhancement of the amount of H2O2 production (Chu C. et al., 2020). Several works in which Au nanoparticles were loaded onto the C3N4 matrix all showed significantly improved 2e− ORR efficiency (Chang X. et al., 2018; Zuo G. et al., 2019). This phenomenon may be attributed to the cocatalytic effects of the Au surface on the photocatalytic 2e− ORR, which has already been proved by investigating other photocatalysts and photocathodes (Hirakawa H. et al., 2016).
In summary, there are two fundamental strategies for introducing active sites for the improvement of 2e− ORR of a PCN-based photocatalyst. One is to manipulate the electronic configurations of the functionalized PCN catalyst so that the selectivity and activity of the π-conjugated heptazine ring for boosting H2O2 production can be significantly enhanced. Similar strategies have also been used in some other carbon-based materials for promoting selective H2O2 production. On the contrary, the nature of melem sites is side-on adsorption of oxygen (Shiraishi Y. et al., 2015), which might result in unexpected splitting of O-O bonds, thus leading to the possible 4e− ORR process and a limited selectivity for 2e− ORR (Siahrostami S. et al., 2013; Verdaguer-Casadevall A. et al., 2014). The other strategy is to introduce active sites beyond the π-conjugated heptazine ring for selective 2e− ORR. Up to now, only a few cocatalysts have been developed for selective ORR (Section 3.3.4). Further development of 2e− ORR sites beyond the π-conjugated heptazine rings may overcome the disadvantage of side-on O2 adsorption and shed light on further improvement for H2O2 production.
Sacrificial reagents are commonly used to consume the photogenerated electrons or holes so that half of the reaction can be significantly boosted (Schneider J. and Bahnemann D.W., 2013). In the case of g-C3N4-based photocatalysts, most researchers used electron donors so that reduction reactions could be maximized in a certain photocatalytic system since an electron donor prevents the photogenerated electrons from recombining with holes (Wang Y. et al., 2019; Wang M. et al., 2017). g-C3N4 has shown promising prospects for catalyzing several photochemical reduction reactions with the existence of electron donors. The reason may be as follows: the CBM of pristine PCN (–1.3 V) is quite negative, thus resulting in significantly larger redox potentials of hydrogen evolution reaction (HER), nitrogen reduction reaction (NRR), carbon dioxide reduction reaction (CRR) and 2e− ORR (0.695 V vs. SHE) (Wang Y. et al., 2019). Combined with the merits of the side-on O2 adsorption on melem units (forming 1–4 endoperoxide species) as acceptable 2e− reduction sites, PCN satisfied the thermodynamic potential for 2e− ORR with the existence of alcohol. However, a green and sustainable process for photocatalytic H2O2 synthesis requires the use of earth-abundant water as an electron donor, i.e., water oxidation reaction instead of alcohol oxidation should occur for hole consumption.
There have been several studies in which mechanisms for photoreduction reactions were systematically investigated by using g-C3N4 (Wang Y. et al., 2019). Since the functions of electron donors were clarified in those works, we will focus on the crucial properties and functionalization strategies for achieving H2O2 production with water and oxygen (Fig. 15a). Typically, water oxidation via a 4e− pathway is usually recognized as an oxygen evolution reaction (OER) that is usually needed to overcome the large overpotential. VBMs of photocatalysts for OER usually have quite positive potentials (VBMTiO2-rutile ~2.9 V vs. SHE, VBMBiVO4 ~2.53 V) (Fan W. et al., 2020; Hirakawa H. et al., 2016). As shown in Fig. 15b, pristine PCN is unable to generate H2O2 in the presence of water and O2 because the valence band maximum (VBM) lies at approximately 1.4 V, which shows an insufficient thermodynamic driving force for OER (ca. 0.8 V versus RHE, pH 7) (Shiraishi Y. et al., 2014a). To overcome this obstacle, band engineering and the use of a cocatalyst are two effective strategies.

Typical strategies for preparation of a g-C3N4-based catalyst for photocatalytic H2O2 production with water and O2. (a) Real hydrogen electrode potentials of 2e− ORR and 4e− WOR compared with the band diagram of PCN. (b) Electronic band structures of pristine PCN and g-C3N4/BDIx. (c) Changes in the amounts of H2O2 formed during photoreaction with respective catalysts. For the reaction in (c), 100 mg of catalyst was used. The irradiance at 420–500 nm is 27.3 W m−2. Blue points show the results of photoreaction with water containing ca. 100 μmol of H2O2. (d) Calculated HOMO and LUMO of Melem_4 model (representing PCN), Melem_4BA1 (representing PCNBA with a low motif concentration) and Melem_4BA2 (representing PCNBA with a high motif concentration). (e) Schematic diagram of PCNBAxCox for accelerating water oxidation. (f) Absorption spectrum of PCNBA0.2 and action spectra for H2O2 formation on the respective catalysts. (g) Time course of O2 evolution measured under Ar pressure of 0.6-kPa and 300-W xenon lamp irradiation with 0.5 g L−1 of the catalyst, 1 g L−1 La2O3, and 20 mM AgNO3 in 100 mL of water. Reprinted with permission from Ref. (Kofuji Y., et al., 2016b; Teng Z. et al., 2020). Copyright: (2016) American Chemical Society and (2020) Elsevier.
The bandgap position of PCN can be easily manipulated by functionalization. Shiraishi and co-workers successfully manipulate the band positions of a PCN-based photocatalyst by copolymerized melamine with electron-deficient aromatic diimide (Shiraishi Y. et al., 2014a; Shiraishi Y. et al., 2014b; Kofuji Y. et al., 2016a; Kofuji Y. et al., 2017; Kofuji Y. et al., 2016b). The valence band of the as-prepared co-polymerized PCN is about 1.8~2.4 V (vs. NHE). Taking g-C3N4/PDI as an example, the activity results revealed a significant role of band engineering. Pristine g-C3N4 (VBM = 1.40 V vs. NHE) barely produces H2O2, and g-C3N4/BDI51 (VBM = 1.86 V vs. NHE) produces 31 μmol of H2O2 after photocatalytic reaction for 48 h (Kofuji Y. et al., 2016b) (Fig. 15c). g-C3N4/BDI50 (VBM = 1.83 V vs. NHE) shows a higher performance (41 μmol) (Kofuji Y. et al., 2016b). With the addition of electron acceptors, O2 can be detected by GC, which further confirmed that water oxidation to generate oxygen consumed the generated holes during the photocatalytic reactions (Kofuji Y. et al., 2016b). Introduction of boron nitride (BN) can further improve the activity of the g-C3N4/PDI-rGO photocatalyst because of the enhanced charge separation efficiency (Kofuji Y. et al., 2018). Following those works, Ohno and co-workers further revealed by analysis of the density of states that the copolymerization of electron-deficient units results in the introduction of O 2p states (Teng Z. et al., 2020). The introduced O 2p states compose the valence band with the states of N 2p and C 2p, resulting in a more positive potential than that of pristine PCN for which the VB is only composed of the states of N 2p and C 2p (Fig. 15d) (Teng Z. et al., 2020). Thus, the OER activity is significantly promoted (Fig. 15e). Additionally, preparation of POM-incorporated PCN samples with ideal electronic configurations and band positions also played similar roles (shifting the VBM to a more oxidative potential) for boosting the OER, which successfully achieved the H2O2 production via ORR and WOR (Zhao S. et al., 2018b; Zhao S. et al., 2017; Zhao S. and Zhao X., 2019). Another strategy for improving the OER activity of PCN is loading a cocatalyst for OERs. Ohno and co-workers loaded Na2CoP2O7 on the band-engineered PCNBA0.2 by a simple ball milling method (Fig. 15e) (Teng Z. et al., 2020). The time-dependent H2O2 production and action spectra confirmed the cocatalytic effects of Na2CoP2O7 (Teng Z. et al., 2020). The action spectra showed that its bandgap excitation produces H2O2 (Teng Z. et al., 2020). The apparent quantum yield (AQY) of PCNBA with loading of 5 % Na2CoP2O7 (PCNBA0.2Co5 %) at 420 nm (8.0 %) was two-times higher than that of PCNBA0.2 (4.0 %) (Fig. 15f) (Teng Z. et al., 2020). Additionally, no H2O2 was produced at the light-irradiation wavelengths between 550 nm and 650 nm, indicating that Na2CoP2O7 works as a cocatalyst and does not work as a photocatalyst for production of H2O2 (Teng Z. et al., 2020). More recently, Chu and co-workers prepared single-atom dispersed Co as a co-catalyst for OER (Chu C. et al., 2020). X-ray absorption fine-structure spectroscopy (FT-EXAFS) showed that the oxidation state of the Co single atoms coordinated by P atoms is close to +2 (Chu C. et al., 2020). The OER activity of g-C3N4 was significantly increased (8.4-fold) by the introduction of Co single atomic sites (Fig. 15g). The chemical states of P-coordinated Co atoms are close to +2, indicating that the unique electronic configuration of Co(II)-P may provide active sites for OER during solar H2O2 synthesis (Chu C. et al., 2020). It is notable that the VBM of this single-Co dispersed PCN (~1.4 V vs. SHE) (Chu C. et al., 2020), quite close to that of PCN, is significantly more positive than that of metal oxides (>2.3 V vs. SHE). Thus, the Co(II)-P single atomic sites may significantly boost the kinetic process of water oxidation. Very recently, the Sb-SAPC proposed by Ohno and co-workers also showed good activity based on non-sacrificial photocatalytic H2O2 activities. Based on the results detailed characterizations and analyses, the following reaction mechanism (Fig. 16) of Sb-SAPC for photocatalytic H2O2 production is proposed. Firstly, efficient charge separation occurs on Sb-SAPC under visible light irradiation, resulting in the generation of photoexcited electrons and holes for ORR and WOR, respectively. Then water molecules are oxidized to evolve O2 by photogenerated holes localized at the N atoms near the single Sb atoms. Simultaneously, O2 dissolved in water and that generated from the WOR both participate in the ORR process to produce H2O2. It is notable that the efficient charge separation, ideal single atomic sites for end-on type O2 adsorption and close spatial distribution of active sites boost both the 2e− ORR and 4e− WOR for efficient H2O2 production.

Mechanism of photocatalytic H2O2 production. (White, gray, blue, red and magenta spheres indicate hydrogen, carbon, nitrogen, oxygen and Sb atoms, respectively.) After shining visible light, the photogenerated electrons are localized at the Sb sites (with a blue glow), and the photogenerated holes are localized at the N atoms at the melem units (with a red glow). Subsequently, the dissolved O2 molecules are adsorbed (orange arrows) onto the Sb sites and then become reduced (blue arrows) via a 2e− transfer pathway through the formation of an electron μ-peroxide as the intermediate. Simultaneously, water molecules are oxidized (pink arrows) to generate O2 by the highly concentrated holes on the melem units. Reprinted with permission from Ref. (Teng Z. et al., 2021b). Copyright: (2021) Springer Nature Ltd.
In summary, accelerating the photocatalytic oxygen evolution reaction by using a PCN-based photocatalyst is the most crucial challenge for achieving photocatalytic H2O2 production with water and O2. Band engineering and loading cocatalyst for OER may respectively improve the oxidation potential and promote the kinetics for 4e− OER.
4.2 pH valueIt is difficult for the light-driven 2e− WOR pathway to be achieved because of the uphill thermodynamics (1.76 V vs. NHE), i.e., the as-synthesized H2O2 will decompose at this highly oxidative potential because H2O2 is an excellent hole scavenger (Fuku K. and Sayama K., 2016). Therefore, we will focus on the 2e− ORR pathway. Substantial efforts have been devoted to the 2e− ORR concept with the aim of achieving high-yield production of H2O2 in basic, neutral, and acidic media (Gao J. and Liu B., 2020; Kim H.W. et al., 2018; Lu Z. et al., 2018; Iglesias D. et al., 2018). The acid dissociation constant of H2O2 (H2O2 ↔ H+ + HO2−,
Kim and co-workers found that photocatalytic activity for H2O2 production was higher at lower pH values (Fig. 17a) with the existence of an electron donor. This is probably due to proton conduction within the CN framework and is much more facilitated in KPF_CN. The proton conduction of KPF_CN was greatly enhanced when PF6 − anions was introduced into the structure of the g-C3N4 framework (Kim S. et al., 2018). The higher charge carrier density in KPF_CN might be favorable for facilitating proton conduction. The higher proton conductivity within the KPF_CN framework promotes the production of H2O2, particularly when the reaction between O2 and protons occurs within the interlayer space (Kim S. et al., 2018). Ohno and co-workers also investigated the optimal pH conditions by measuring the amount of H2O2 produced by the photocatalytic reaction using PCNBA0.2 (Fig. 17b) (Teng Z. et al., 2020). When ethanol was introduced into the system, the amount of produced H2O2 remained almost constant when the pH value was increased from 1 to 9 and then decreased drastically (Teng Z. et al., 2020). When the pH value was lower than 7, the concentration of protons had almost no effect on the pH value (Teng Z. et al., 2020). This phenomenon revealed that protonation plays a less essential role in the PCNBA system, indicating that incorporation of BA units further improved the proton conductivity compared with that of KPF_CN. They also found that the lowest [H+] is 10−9 mol L−1 for O2 reduction to H2O2 production. When no sacrificial agent was added to the system, the amount of H2O2 rapidly increased before the pH value reached 7 and then gradually decreased, indicating that a low concentration of OH− also restricts the generation of O2 from water (Wang Q. and Domen K., 2020). Therefore, the optimal pH condition for H2O2 production from O2 and water using PCNBA is 7–9. The production of H2O2 significantly decreased in both of the works when pH was larger than 9, being consistent with the fact that H2O2 self-decomposes in alkaline conditions. Although there have been only few works in which the influence of pH on the amount of H2O2 generated was investigated, it was shown that the pH value did play an essential role during the photosynthesis process. Additionally, the pH value of the interfaces between materials and reaction solution might be even more important compared with the pH value of the whole solution. The rational design of Helmholtz layer by the manipulation of the surface properties are extremely important for improving the H2O2 production (Zeng X. et al., 2020). We believe that systematic investigations of the effects of pH on the amount of H2O2, surface properties and electronic configurations should be carried out to reveal the mechanisms of ORR and WOR during the photosynthesis of H2O2.
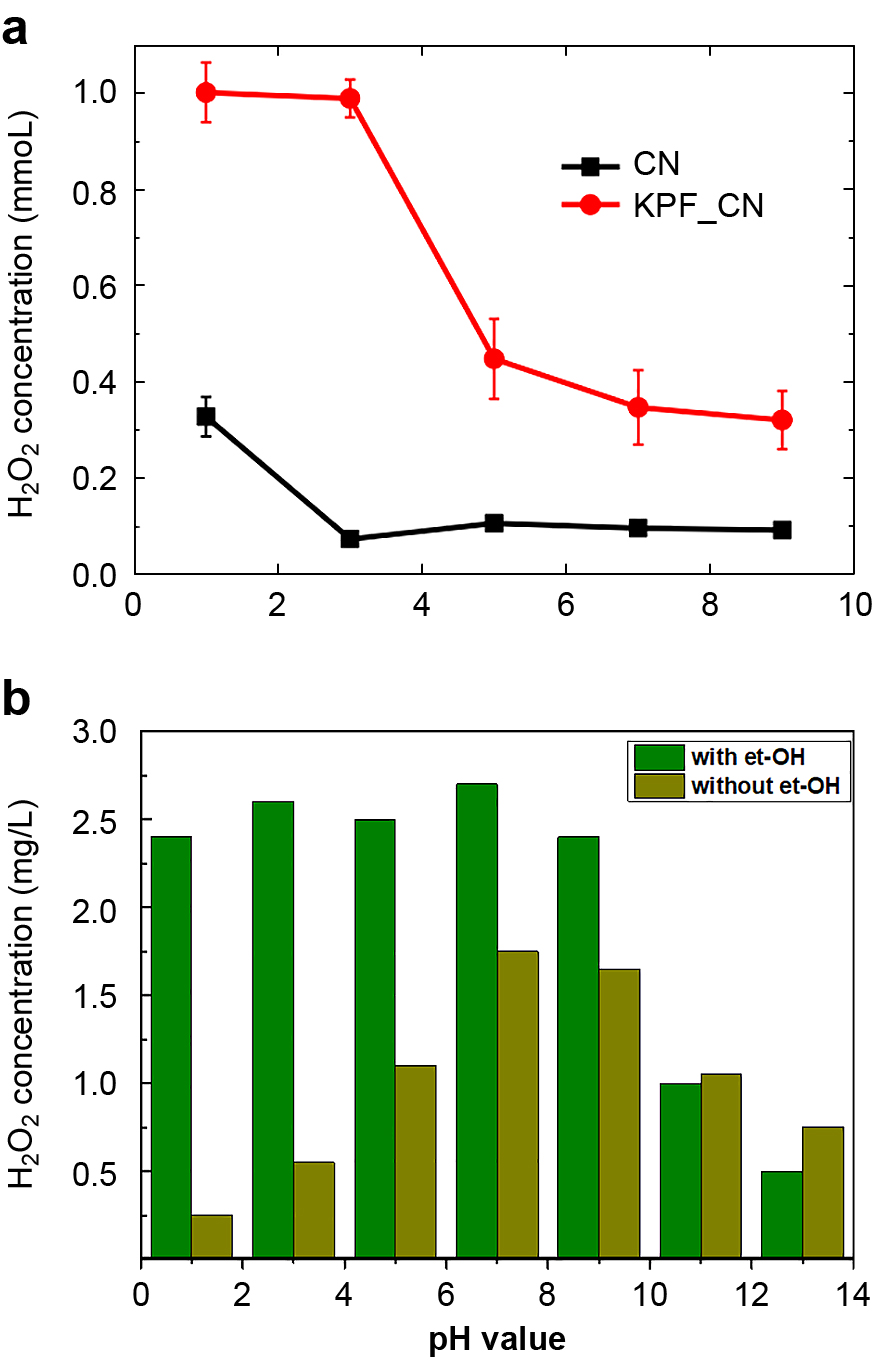
Influence of pH values during H2O2 production by using a PCN-based photocatalyst. (a) H2O2 production in an irradiated suspension of bare CN and KPF_CN_15 at different pH values. (b) Optimization of the pH condition for H2O2 production when PCNBA0.2 was used as a photocatalyst. Reprinted with permission from (Kim S. et al., 2018) and (Teng Z. et al., 2020). Copyright: (2018, 2020) Elsevier.
Photocatalytic H2O2 production by using a PCN-based material has shown a promising prospective in environmental and energy-related realms. We have summarized the principles for photocatalytic H2O2 production, especially for the photocatalytic H2O2 production using g-C3N4. Advantages and disadvantages for H2O2 production and the most favorable properties of PCN for photocatalytic H2O2 production were briefly summarized. Based on the intrinsic properties of pristine g-C3N4, the most urgent issue for overcoming the drawbacks was focused on. Notably, current PCN-based photocatalysts still suffer from relatively low H2O2 yield. Due to the low activity, g-C3N4-based photocatalysts are far from the requirements for industrial applications. Improving the charge separation, promoting the light absorption and introducing active sites for 2e− ORR to suppress the side reaction are three attractive strategies for boosting the activities. Following discussion of these strategies, the most representative functionalization method was summarized on the basis of the most desired properties for improving the photocatalytic activities for H2O2 production. Other factors for improving H2O2 production such as addition of electron donors and pH value of the solution were also discussed.
There are several outlooks and suggestions for further improvement of photocatalysts. First of all, further improvement in the charge separation of the PCN is necessary. New species of elements or organic semiconductors for incorporation are highly recommended. Secondly, the absorption edge of the functionalized PCN can hardly be extended to >550 nm with a reasonable AQY for H2O2 production (Teng Z. et al., 2020). Further narrowing of band gap is therefore still of great importance. It is notable that the narrowing of the bandgap could reduce the thermodynamic driving force, leading to weakened capability for reduction reactions and oxidation reactions. Thus, reducing the overpotential for 2e− ORR and oxidation reactions on the PCN surface is of great importance. Exploring new reaction sites with higher performance beyond the heptazine rings in the PCN matrix for 2e− ORR efficiency is also of great importance for further improvement of photocatalytic activity and selectivity for H2O2 production. Recently, single-atom catalysts (SACs) have shown excellent selectivity for H2O2 production with Pt2+ (Shen R. et al., 2019; Gao J. et al., 2020; Jung E. et al., 2020) via a 2e− ORR pathway. In the case of PCN, anchoring single-atom catalysts that have large amounts of nitrogen atoms with lone electron pairs is easy to perform. However, Pt2+ and Co-N4 sites can hardly be utilized for photocatalytic systems due to poor charge separation, which origin form the half-filled d electrons of Pt2+ and Co-N4 may result in a high bulk-recombination rate of PCN. The preparation of photocatalysts having atomically dispersed atoms with a d0 or d10 electronic configuration usually introduce intermediate bands with energetic levels that are slightly more negative than that of CBM, which can promote electron mobility, thus leading to a boosted performance (Inoue Y., 2009). It should be mentioned that measurement of H2O2 production was carried out in most studies with the addition of an organic sacrificial electron donor to consume the photogenerated holes and inhibit the recombination of photogenerated carries, which is energy-consuming and cost-inefficient. Despite of band engineering and cocatalyst loading, the construction of an all-solid-state Z-scheme photocatalytic system is another recommended strategy for achieving non-sacrificial H2O2 production with water and oxygen (Xu Q. et al., 2018). Additionally, it is necessary to develop approaches other than optimization of pH conditions to prevent decomposition of the generated H2O2. The stability of organic semiconductors during photocatalytic H2O2 production should also be investigated (Liu L. et al., 2021). Last not but least, attention should be given to precise determination of the amount of H2O2 generated because the species in the solution can influence the accuracy for determination (Wei Y. et al., 2021).
To optimize the design of functionalized PCN with maximized photocatalytic H2O2 production, a comprehensive understanding of the photocatalytic mechanism for H2O2 production is necessary. Both experimental and theoretical investigations should be simultaneously carried out. In situ measurements by Kelvin probe microscopy (Zhu J. et al., 2015) and X-ray absorption fine structure analysis (Yang H. et al., 2018) could be used to study the dynamic behavior of photogenerated carriers on the surface and the changes of oxidized states in a photocatalyst (Hou H. et al., 2020). In situ Raman spectroscopy may reveal the process from the adsorption of O2 to dissociation of H2O2, thus leading to a more precise understanding of the mechanism. In the case of fundamental calculations and simulations for investigation of the mechanism, ground state properties such as density of states, adsorption energy, and the structural and electronic properties have been widely used for explanation of physicochemical properties for the design of an ideal photocatalyst for H2O2 production (Dong J.-C. et al., 2020). However, investigation of the excitation properties of PCN materials has not been sufficient to obtain a fundamental understanding of the excitation behavior of a photocatalyst. We suggest that excitation properties of the functionalized PCN, such as transition densities based on time-dependent DFT calculations (Laurent A.D. et al., 2013; Teng Z. et al., 2021b; Che H. et al., 2021), should be investigated in future studies in order to establish a satisfactory blueprint for designing a PCN catalyst for H2O2 production. Last but not least, since H2O2 production is a green oxidant for many chemical reactions, the development of highly value-added chemicals based on the photocatalytic systems is needed. Using the in-situ generated H2O2 for synthesis of chemicals could also be achieved on the basis of previous work using D2O to produce deuterated chemicals (Zhang B. et al., 2021). We believe that better design strategies and guidelines for not only PCN-based materials but also other efficient photocatalysts can be established for H2O2 formation if the above-mentioned properties can be achieved.
The authors acknowledge the financial support of Mitsubishi Chemical Corporation, JSPS Grant-in-Aid for Scientific Research (B, No. 20H02847), and Grant-in-Aid for JSPS Fellows (DC2, 20J13064).
Zhenyuan Teng
Dr. Zhenyuan Teng is currently a JSPS Research Fellow (PD) in the Research Initiative for Supra-Materials (RISM), Interdisciplinary Cluster for Cutting Edge Research, Shinshu University. He obtained his B.S. degree in 2016 and then acquired the Master degree (2018) from Yangzhou University. He obtained his Doctoral degree from Kyushu Institute of Technology in 2021 under the supervision of Prof. Teruhisa Ohno. His research interest focuses on the design and synthesis of single-atom organic-inorganic photocatalytic systems for energetic and environmental applications.
Wenan Cai
Wenan Cai received his B.S. in Beijing Institute of Petrochemical Technology in 2016. Currently, he is a Ph.D. student under the supervision of Prof. Ohno Teruhisa in Kyushu Institute of Technology. His research interest is the development and design of long-wavelength response photocatalysts for H2O2 production.
Teruhisa Ohno
Prof. Teruhisa Ohno is currently the dean of faculty of engineering in Kyushu Institute of Technology. He obtained his Doctoral degree in Kyushu University in 1988. He worked as an Associate Professor in Kyushu University from 1992 to 1994 and in Osaka University from 1994–2003. Then he moved to Kyushu Institute of Technology as a Full Professor. From 2016 to 2021, He published 57 SCI papers. Field-Weighted Citation Impact: 2.04, Citation Count: 1,108, h-index: 48, h5-index: 15. A number of papers have published on influential periodical, such as Applied Catalysis B: Environmental, ACS catalysis and Nature Catalysis. From 1983 to now, published about 202 SCI papers, these papers were totally quoted more than 11,763 times and h-index is 49 (Scopus Data). His research interests are: 1. Development of visible light sensitive photocatalysts; 2. Nanoscale surface structure control of TiO2 photocatalysts; 3. Development of photocatalyst and photoelectrode system for CO2 reduction; 4. Visible light responsive photocatalyst and photoelectrode for H2O2 production.