
- |<
- <
- 1
- >
- >|
-
Takahiro OKUO, Tetsuhei KONDO, Minoru ASHIZAWA, Fumihiro SAGANE, Hidet ...2023 Volume 91 Issue 7 Pages 077001
Published: July 01, 2023
Released on J-STAGE: July 01, 2023
Advance online publication: June 08, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataMagnesium bis(trifluoromethanesulfonyl)amide (Mg(TFSA)2)/glyme solutions are promising electrolytes for rechargeable magnesium batteries, but the passivation of the Mg electrode because of the decomposition of [TFSA]− anion from the coordination state is a critical issue in practical use. In the present study, functionalized nanofiber gel electrolytes swollen in 0.5 M Mg(TFSA)2/triglyme solution were prepared using the silica nanofibers modified with oxymagunesium chloride (–OMgCl) groups (f-SiO2NFs) and used them as a gelator. The f-SiO2NF gel electrolyte improved the Mg plating/stripping reaction compared to the SiO2NF gel without –OMgCl groups.
View full abstractDownload PDF (5651K) Full view HTML -
Yuki FUJII, Ryoichi TATARA, Daisuke IGARASHI, Tomooki HOSAKA, Rena TAK ...2023 Volume 91 Issue 7 Pages 077002
Published: July 01, 2023
Released on J-STAGE: July 01, 2023
Advance online publication: June 03, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataHerein, the diluted-electrode method is applied to a hard carbon (HC) electrode to estimate sodium-ion (Na+) insertion kinetics. As metallic nickel (Ni) particles do not accommodate Na+ ions in the potential range of 0–2.0 V vs. Na+/Na, the HC powder electrode is diluted by adding inert Ni particles, enabling the adjustment of the HC concentration while maintaining the composite electrode structure. By examining the rate capabilities of the HC electrodes with different dilutions, we confirm that the Na+ insertion rate for the highly diluted electrode is 10 times higher than that for the undiluted electrode. These improved kinetics can be attributed to the alleviation of Na+ depletion, which results in insignificant concentration polarization under dilute conditions. For a highly diluted electrode, the Na+ insertion kinetics must be controlled by the Na+ mobility in the HC particles and across the HC/electrolyte interface. Therefore, our study reveals that the inherent kinetics of Na+ insertion into HC is very high and provides a basis for developing high-power Na-ion batteries.
 View full abstractDownload PDF (3699K) Full view HTML
View full abstractDownload PDF (3699K) Full view HTML -
Ryo SAKAMOTO, Nobuaki SHIRAI, Liwei ZHAO, Atsushi INOISHI, Hikari SAKA ...2023 Volume 91 Issue 7 Pages 077003
Published: July 04, 2023
Released on J-STAGE: July 04, 2023
Advance online publication: June 08, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataPerovskite-type CsSnCl3 is an attractive candidate for use as a solid electrolyte in all-solid-state chloride-ion batteries because it exhibits high ionic conductivity. However, perovskite-type CsSnCl3 is metastable at room temperature and easily undergoes a phase transition to a stable phase. Here, we prepared perovskite-type CsSn0.95Mn0.05Cl3, in which the Sn2+ in CsSnCl3 is partly substituted with Mn2+, via a mechanical milling method. Differential scanning calorimetry showed that the perovskite-type CsSn0.95Mn0.05Cl3 is stable to −15 °C. Moreover, it exhibits a high chloride ionic conductivity of 2.0 × 10−4 S cm−1 at 25 °C. We demonstrated the room-temperature operation of an all-solid-state chloride-ion battery with a BiCl3 cathode, an Sn anode, and CsSn0.95Mn0.05Cl3 as the electrolyte. The first discharge capacity of the all-solid-state cell at room temperature was 169 mAh g−1 based on the weight of BiCl3. X-ray diffraction and X-ray photoelectron spectroscopic analyses confirmed that the reaction mechanism of the cell is derived from the redox reaction of BiCl3 and Sn.
View full abstractDownload PDF (3260K) Full view HTML -
Cheng QU, Minggang ZHENG2023 Volume 91 Issue 7 Pages 077004
Published: July 04, 2023
Released on J-STAGE: July 04, 2023
Advance online publication: June 08, 2023JOURNAL OPEN ACCESS FULL-TEXT HTMLThis article uses the topological optimization method to optimize the flow field of radial proton exchange membrane fuel cells. Due to the high computational cost of this method, the model was simplified to a two-dimensional model, and the cathode flow field was studied under isothermal and steady-state conditions. Using the gradient-based algorithm SNOPT, the flow field evolves freely in the sector design domain to maximize battery power and minimize reaction energy loss. Perform 3D modeling of the obtained optimized model and optimize the longitudinal depth by applying different tilt angles and obstacles. The results indicate that the application of obstacles has a significant optimization effect on speed. Setting the gradient has a better effect on the oxygen concentration distribution. With the increase of the gradient, the uniformity of the oxygen concentration distribution is also increased. As the inclination increases, the pressure difference of the reaction gas gradually increases, and the pressure uniformly decreases. After setting obstacles, the pressure of the reaction gas decreases step by step after passing through each obstacle. The effect of increasing the inclination to increase the average current density is better than setting obstacles, and the comprehensive effect of setting an inclination of 2° is the best at working voltages of 0.5 V and 0.6 V.
View full abstractDownload PDF (5173K) Full view HTML -
Lu YIN, Ryoichi TATARA, Shogo YAMAZAKI, Rena TAKAISHI, Eisuke SHIIYAMA ...2023 Volume 91 Issue 7 Pages 077005
Published: July 07, 2023
Released on J-STAGE: July 07, 2023
Advance online publication: June 06, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataA new combination of styrene acrylic rubber (SAR) and sodium carboxymethylcellulose was developed as a water-borne binder for LiCoO2 composite electrodes operating at high voltages. Four novel SAR-based latex binders were synthesized with butyl acrylate or 2-ethylhexyl acrylate monomer and styrene via copolymerization with low and high crosslinking degrees. Composite electrodes prepared using lower-crosslinking-degree SAR binders became more stretchable and flexible. Surface analysis using electron microscopy and X-ray photoelectron spectroscopy revealed that the cycled LiCoO2 electrode was covered with approximately 10-nanometer-thick decomposition products when a conventional poly(vinylidene fluoride) binder was used. The electrodes with SAR-based binders with low cross-linkage formed a stable passivation surface during the initial cycle, and further continuous electrolyte decomposition was successfully suppressed. This passivation improved the cycle stability of LiCoO2 electrode up to 4.5 V, i.e., 87.1 % capacity retention, even after 100 cycles and suppressed self-discharge performance at 45 °C.
 View full abstractDownload PDF (6959K) Full view HTML
View full abstractDownload PDF (6959K) Full view HTML -
Daisuke TAKIMOTO, Keisuke SUZUKI, Sho HIDESHIMA, Wataru SUGIMOTO2023 Volume 91 Issue 7 Pages 077006
Published: July 08, 2023
Released on J-STAGE: July 08, 2023
Advance online publication: June 09, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
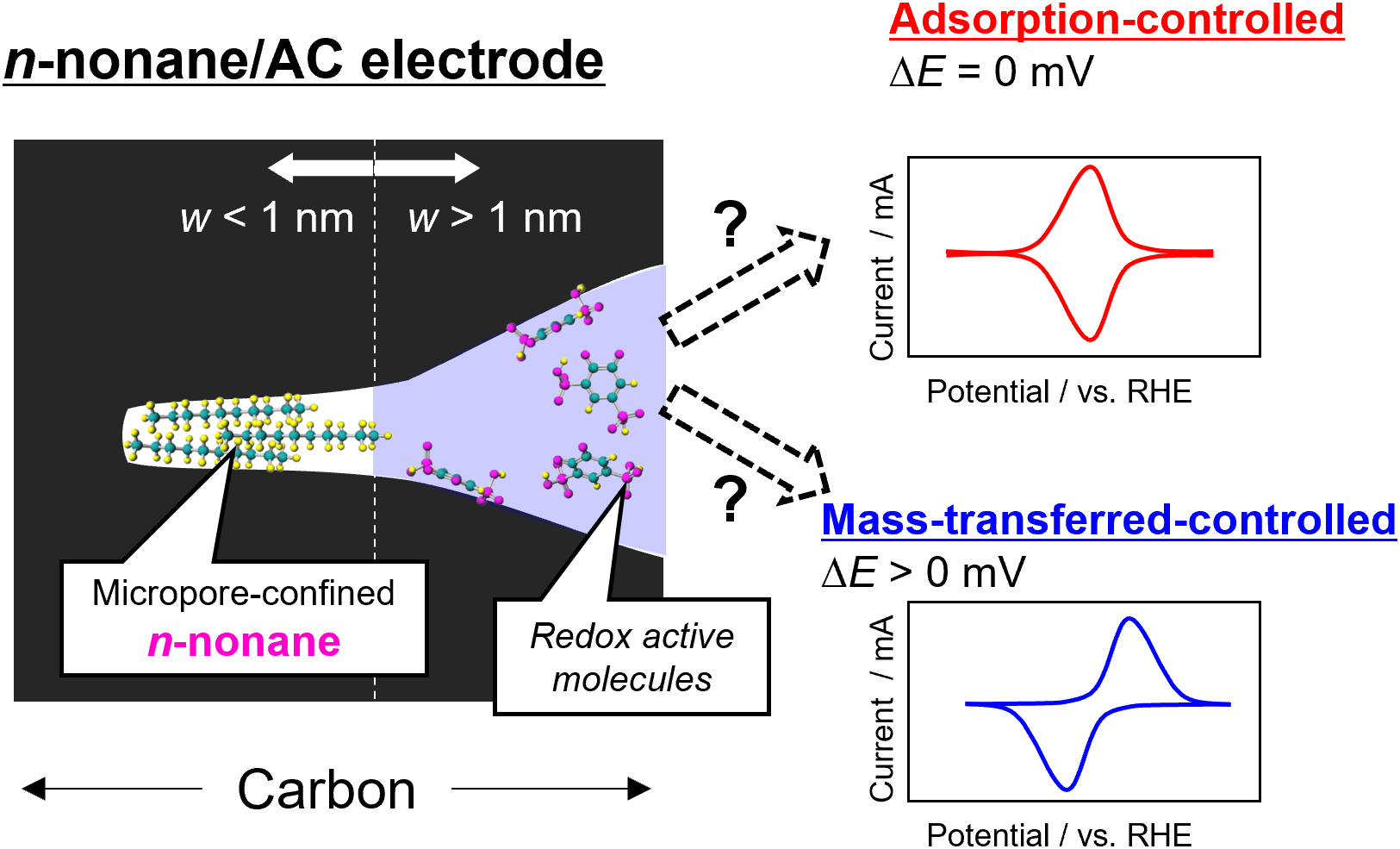
J-STAGE DataRedox-active organic materials have emerged as promising alternatives to inorganic electrode materials in electrochemical devices owing to advantages such as low cost and flexible design. However, the kinetics of their electrochemical reactions are typically slow due to the slow diffusion of organic materials dissolved in the electrolyte. Generally, peak separation of the redox reaction is observed (mass-transfer-controlled system), while no peak separation is obtained when the active molecules, such as high surface carbon material, are adsorbed onto the electrode material (adsorption-controlled system). Aromatic compounds confined in activated carbon (AC) micropores exhibit an adsorption-controlled reaction, improving the reaction kinetics. To elucidate this behavior, a well-defined and accurate understanding of the pore geometry is required. Although various synthetic techniques have been used to tune the micropore size, these afford different surface properties. This study reports an approach to achieve an adsorption-controlled redox reaction of quinone-based molecules and a tool to analyze their reaction environment. AC micropores sized <1 nm were filled with n-nonane without any change occurring in the AC surface properties. It was thus concluded that AC micropores in the sub-nanometer scale are necessary for an adsorption-controlled redox reaction to occur. This study reveals new insights on the micropore confinement effect in electrochemistry.
 View full abstractDownload PDF (4288K) Full view HTML
View full abstractDownload PDF (4288K) Full view HTML -
Mayu SHIOZAKI, Hiroki YAMASHITA, Yuko HIRAYAMA, Takaaki OGAMI, Kiyoshi ...2023 Volume 91 Issue 7 Pages 077007
Published: July 08, 2023
Released on J-STAGE: July 08, 2023
Advance online publication: June 13, 2023JOURNAL OPEN ACCESS FULL-TEXT HTMLThe effects of LiMn0.7Fe0.3PO4/C (LMFP) species on the electrochemical performance of the blended cathodes of LiNi0.5Mn0.3Co0.2O2 (NMC) and LMFP are examined. Two types of LMFPs are synthesized by the hydrothermal method (LMFP-1) and the solid-state reaction (LMFP-2) leading to different physical characteristics and uniformity of primary particles. The blended cathodes of NMC and LMFP-1 show higher discharge capacity, gravimetric energy density, and rate capability than those of NMC and LMFP-2 for the same blending ratio (10, 20, 30, 40, and 50 wt% LMFP to NMC) because of the differences in the electronic conductivity, specific surface area, mean particle size, and uniformity of primary particles between LMFP-1 and LMFP-2. The discharge capacity and gravimetric energy density of NMC : LMFP-1 = 9 : 1 and 8 : 2 at 0.2 C-rate are comparable to those of NMC. Further, the rate capability is the highest at the blending ratio of NMC : LMFP-1 = 7 : 3. An optimal range for the blending ratio of LMFP to NMC is revealed based on the discharge capacity, energy density, and rate capability of the blended cathode. Moreover, LMFP has a considerable impact on the electrochemical characteristics of the blended cathodes of NMC and LMFP.
View full abstractDownload PDF (4201K) Full view HTML -
Yuki FUJII, Keisuke SUGATA, Yukikazu OMURA, Narumi KUBOTA, Kento KISA, ...2023 Volume 91 Issue 7 Pages 077008
Published: July 15, 2023
Released on J-STAGE: July 15, 2023
Advance online publication: June 13, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataCurrent efforts to improve sodium-ion batteries are heavily focused on developing high performance carbon materials for the negative electrode. With significant research, hard carbons have come to show massive storage capacities and fast discharge rates. On the other hand, soft carbons have received very little attention, though they likewise encompass a wide variety of materials with structures highly dependent on the starting material and preparation temperature. In our contribution, we systematically evaluate the electrochemical performance of soft carbon electrodes made from mesophase-pitch carbon fibers (MCF). By using felt electrodes, we evaluate the cyclic voltammetry of MCFs prepared at 600–1300 °C and show the best performance with MCF prepared between 700–950 °C. In addition, using a surface modification step with silver showed significantly improved voltammetry for all the materials. Electrochemical impedance measurements further indicated that the surface modification step could decrease both of charge transfer resistances and film resistances attributed to the solid electrolyte interphase. Upon comparing lithium- and sodium-cell, it was revealed that sodium-cell demonstrated more significant increase in current density and decrease in resistance through surface treatment. We further verified our results with measurements on single-fiber electrodes; an increase in currents and a decrease in impedance were also observed by the surface modification, as with the felt electrodes. Overall, we speculate our surface modification removes inhibitors, such as functional groups or impurities, on the MCF surface to prevent sluggish ion transfer or trapping during sodium insertion/extraction.
View full abstractDownload PDF (2082K) Full view HTML -
Yuya OKADA, Takuya KIMURA, Kota MOTOHASHI, Atsushi SAKUDA, Akitoshi HA ...2023 Volume 91 Issue 7 Pages 077009
Published: July 29, 2023
Released on J-STAGE: July 29, 2023
Advance online publication: July 05, 2023JOURNAL OPEN ACCESS FULL-TEXT HTML
J-STAGE DataAll-solid-state batteries (ASSBs) have attracted significant attention as alternatives to Li-ion batteries. In ASSBs, solid electrolytes (SEs) play a key role. While many halide Li-ion conductors have been reported, only a few Na-ion conductors have been reported. In this study, a new phase of Na3InCl6 with a cryolite-type monoclinic structure was prepared using a mechanochemical method. The new phase showed higher conductivity than the previously reported trigonal Na3InCl6 and underwent a phase transition to trigonal phase when heat-treated at 90 °C. A Zr-substituted system of Na3−xIn1−xZrxCl6 was mechanochemically prepared. The obtained solid solutions with monoclinic structures based on Na3InCl6 were formed in the compositions of x = 0.1–0.9. The Rietveld refinement results showed a decrease in Na occupancy at the octahedral sites and slight change at the prismatic sites. Bond valence sum mapping results showed that Na ions diffused alternately through two types of sites, suggesting that the introduction of Na vacancies at either site had a positive effect on Na-ion conduction. The ionic conductivity increased to approximately 10−5 S cm−1 with an increase in the number of Na vacancies when x was greater than 0.6. This report describes one of the few Na-ion conducting chlorides with high conductivity.
View full abstractDownload PDF (2921K) Full view HTML
- |<
- <
- 1
- >
- >|