
- |<
- <
- 1
- >
- >|
-
Koichi Yamashita2019Volume 18Issue 2 Pages A3
Published: 2019
Released on J-STAGE: July 30, 2019
JOURNAL FREE ACCESS FULL-TEXT HTMLDownload PDF (311K) Full view HTML
-
Jing ZHANG, Yang WANG, Qian CHEN, Yixin SU, Jingxiang XU, Yusuke OOTAN ...2019Volume 18Issue 2 Pages 103-104
Published: 2019
Released on J-STAGE: July 30, 2019
JOURNAL FREE ACCESS FULL-TEXT HTMLDiamond-like carbon (DLC) is a class of amorphous carbon materials with excellent friction properties. Graphitization of DLC caused by friction induces wear of DLC. Further, the existence of water molecules decrease the graphitization rate of DLC. In order to reduce the wear caused by graphitization, it is essential to reveal the mechanism by which water molecules decrease graphitization of DLC. Therefore, the friction simulations of DLC in both water and vacuum environment were carried out by reactive molecular dynamics method. We find that terminal-H atoms are removed by friction and dangling bonds are generated in both environments. In water environment, the generated dangling bonds connect with H and OH generated by the dissociation of water molecule, inhibiting the transformation from sp3 to sp2 carbon atoms and the formation of double bonds.We find that these are reasons for the suppression of DLC graphitization and the decrement of DLC wear.
 View full abstractDownload PDF (907K) Full view HTML
View full abstractDownload PDF (907K) Full view HTML -
Shuichi UEHARA, Zhongmin LIU, Narumasa MIYAZAKI, Yusuke OOTANI, Nobuki ...2019Volume 18Issue 2 Pages 105-107
Published: 2019
Released on J-STAGE: July 30, 2019
JOURNAL FREE ACCESS FULL-TEXT HTMLBottlebrush polymer (BBP) absorbed on a negatively charged ceramics surface creates a low friction surface. However, the effect of the contact pressure on friction force of the surface covered by BBP is still unknown due to the difficulty in in-situ observation of the friction interface. Moreover, conventional coarse-grained molecular dynamics simulation is difficult to be directly compared to experimental data because the conventional coarse-grained model cannot consider chemical specificity of monomers. Herein, we developed a coarse-grained molecular dynamics friction simulator which can consider chemical specificity. Then, we performed friction simulation between substrates covered by BBP in water. We showed that the friction coefficient rapidly increased at contact pressure of 16 MPa, when we increased the contact pressure. We found that a sufficient coordination of water to the side chain of BBP facilitated slip between substrates under low pressure (< 16 MPa), leading to a low friction coefficient. On the other hand, under high pressure (> 16 MPa), the number of side chain of BBP contacts with the BBP on the counter surface increased because water beads were squeezed out from the side chain of BBP. Therefore, the main chain of BBP was strongly stretched to the sliding direction, which induced shear between substrate and BBP. We found that the shear under high pressure caused a high friction coefficient.
View full abstractDownload PDF (1019K) Full view HTML
-
Kazuaki KUWAHATA, Yui SAKUMA, Yukio KAWASHIMA, Atsushi FUKUSHIMA, Umpe ...2019Volume 18Issue 2 Pages 108-114
Published: 2019
Released on J-STAGE: July 30, 2019
Advance online publication: July 14, 2019JOURNAL FREE ACCESS FULL-TEXT HTMLPlants can produce various types of compounds classified as primary and secondary metabolites to survive and adapt against their given environments. These plant secondary metabolites have large chemical diversities in terms of their physicochemical properties. However, in most of the cases, information on the physicochemical properties of these compounds can be obtained by referring to articles. As it takes a tremendous amount of time due to curating published papers manually, the development of novel computational methods for prediction of these properties to shorten time with high accuracy, is needed. One of the key scientific fields, quantum chemical calculation has a potential ability because compound structures can be directly used as parameters to represent compounds' physicochemical characteristics. Thus, we aimed to develop a novel method for improving the accuracy of spectroscopic data of secondary metabolites predicted by quantum chemical calculation when comparing publicly-available data. We chose the six representative metabolites that are accumulated by UV-B irradiation for this study. The UV-vis absorption spectrum of each compound was obtained by CNDO/S semi-empirical molecular orbital calculation at the optimized structure by PM3 one. Subsequently, obtained data for several excited states were corrected by Gaussian fitting and the regression analysis was employed for these data. The absorption maximum of the corrected UV-vis spectrum was corresponded reasonably well with the reported experimental data. Our method enables us not only to shorten the data acquisition time, but also to predict spectroscopic data of every compound from the corresponding chemical structures.
 View full abstractDownload PDF (1239K) Full view HTML
View full abstractDownload PDF (1239K) Full view HTML -
Shinnosuke TAKANO, Hiromasa KANEKO2019Volume 18Issue 2 Pages 115-121
Published: 2019
Released on J-STAGE: August 16, 2019
JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, we focused on refractive index (RI) and glass transition temperature (Tg) as polymer properties. Physical property estimation models were constructed using a polymer database between molecular descriptor X calculated from chemical structures of monomers and physical properties of the polymers obtained by polymerizing the monomers. The constructed models were used to design new chemical structures of monomers, with which polymers with high RI and high Tg could be polymerized. We discussed pretreatment methods of monomer structures, molecular descriptors and regression analysis methods, and then, the most predictive models for RI and Tg could be obtained when free bonds were replaced with carbon atom, molecular descriptors were calculated with RDKit, and regression models were constructed with support vector regression. After generating virtual chemical structures by breaking of retrosynthetically interesting chemical substructure (BRICS) and evaluating the reliability of estimated values with applicability domain of the models, RI and Tg were estimated using the models. As a result, it was confirmed that various chemical structures could be obtained, and there were chemical structures with high estimated RI and Tg values. We hope that the proposed method will accelerate the development of polymer materials with multiple target physical properties.
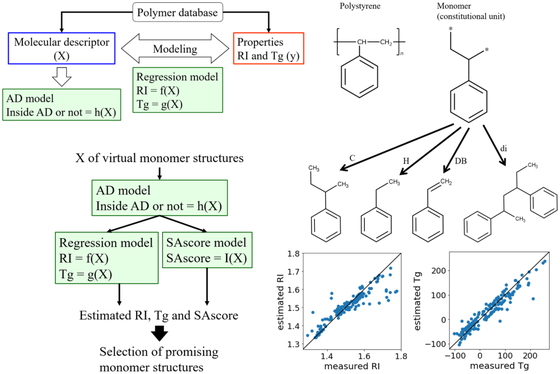 View full abstractDownload PDF (1768K) Full view HTML
View full abstractDownload PDF (1768K) Full view HTML
- |<
- <
- 1
- >
- >|