All issues

Volume 59, Issue 1
Displaying 1-5 of 5 articles from this issue
- |<
- <
- 1
- >
- >|
Regular Paper
-
Tadashi SAWAI, Takahiro YONEHARA, Makoto SANO, Toshimitsu SUZUKI, Taka ...Article type: Regular Paper
2016 Volume 59 Issue 1 Pages 1-8
Published: January 01, 2016
Released on J-STAGE: March 01, 2016
JOURNAL FREE ACCESSMetal-organic framework (MOF) compounds consisting of terephthalic acid and cations (Cr3+, In3+, Al3+, Cu2+ and Zn2+) were prepared by the hydro- and solvo-thermal methods and characterized by XRD, N2 adsorption-desorption isotherm, TG-DTA and FE-SEM. The prepared MOFs were crystalline and phase-pure. MOFs were used as column packings for chromatographic separation of alkylbenzenes. MOFs recognized differences in molecular sizes in addition to boiling points of alkylbenzenes. Activation energy for diffusion in the MOF micropores ranged from 25 to 60 kJ mol−1. Temperature-programmed XRD measurement in air revealed that no expansion or contraction of MOF frameworks occurred up to 230 °C.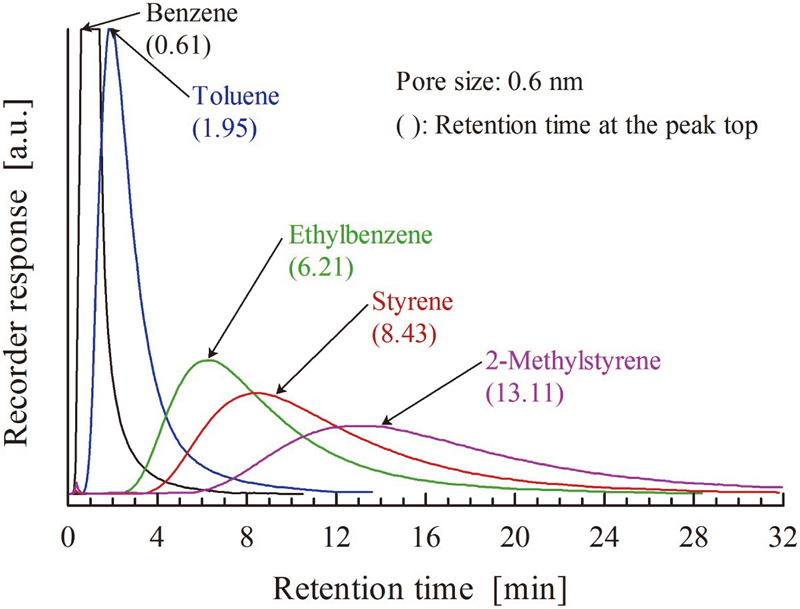 View full abstractDownload PDF (1994K)
View full abstractDownload PDF (1994K) -
Akihisa KITA, Kounosuke KOBAYASHI, Toyokazu MIURA, Yoshiko OKAMURA, Ts ...Article type: Regular Paper
2016 Volume 59 Issue 1 Pages 9-15
Published: January 01, 2016
Released on J-STAGE: March 01, 2016
JOURNAL FREE ACCESSHigh-rate production of methane from acetate using a fixed-bed reactor filled with marine sediment containing halotolerant aceticlastic methanogens and 3 % NaCl was investigated. In continuous culture, the methane production rate increased with increasing dilution rate. The maximum methane production rate of 750 mM d−1 was observed at a dilution rate of 16.2 d−1, which is impossible without immobilization of the cells in the sediment. Using scanning electron microscopy, we observed Methanosaeta-like filamentous microorganisms and Methanosarcina-like coccoid microorganisms in granule-like structures. The results demonstrated that marine sediment is not only a promising microbial resource of halophilic aceticlastic methanogens, but also a supporting material to fix aceticlastic methanogens in a fixed-bed reactor.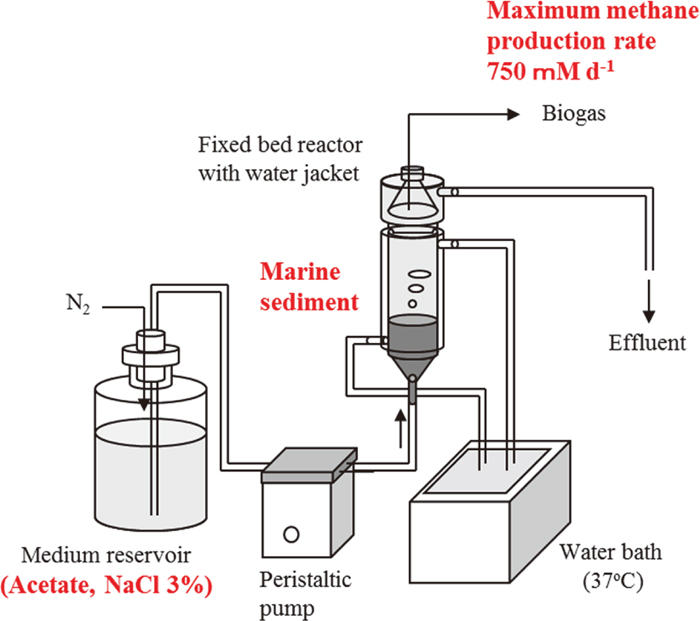 View full abstractDownload PDF (1064K)
View full abstractDownload PDF (1064K) -
Takanori SAEKI, Kensaku TSUDA, Hironobu OHKITA, Noriyoshi KAKUTA, Taka ...Article type: Regular Paper
2016 Volume 59 Issue 1 Pages 16-23
Published: January 01, 2016
Released on J-STAGE: March 01, 2016
JOURNAL FREE ACCESSWe investigated the effects of the Ni : Cu ratio and metal loading of a CeO2-supported bimetallic Ni–Cu catalyst on its reduction behavior and catalytic performance during steam reforming of ethanol for H2 production. Compared with monometallic Ni and Cu catalysts, both the NiO and CuO phases in the bimetallic catalysts were reduced at lower temperatures to form fine NiCu alloy crystallites. At a reaction temperature of 673 K, Ni/CeO2 exhibited a higher H2 yield than Cu/CeO2 but also produced a large quantity of CH4 and carbon deposits. The undesired byproducts were substantially inhibited by replacing a portion of the Ni with Cu. The highest H2 yield and an excellent carbon inhibition were achieved when the content of each metal was 5 wt%. Notably, a physical mixture of Ni/CeO2 and Cu/CeO2, each with the same metal contents, exhibited a lower H2 yield and heavy carbon deposition. This indicates that the higher reducibility and alloying in the bimetallic catalyst are key factors for synergistic improvements of the catalytic properties.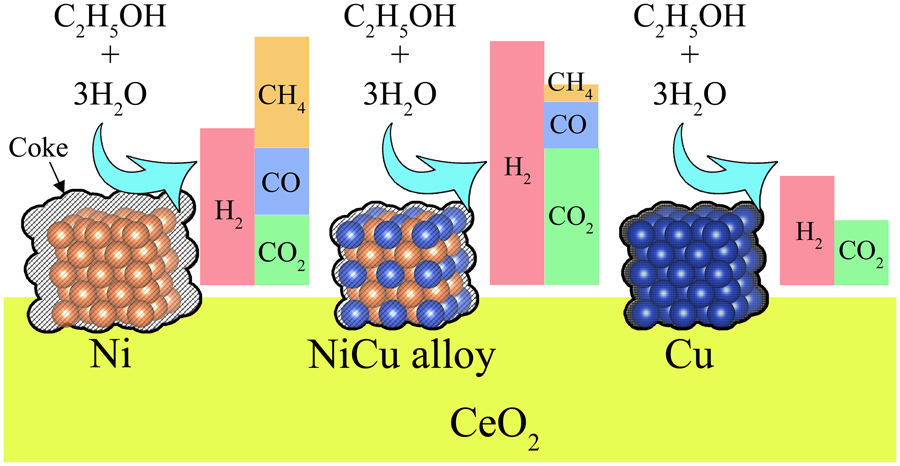 View full abstractDownload PDF (1029K)
View full abstractDownload PDF (1029K) -
Hiromi MATSUHASHI, Daichi FUNAKIArticle type: Regular Paper
2016 Volume 59 Issue 1 Pages 24-30
Published: January 01, 2016
Released on J-STAGE: March 01, 2016
JOURNAL FREE ACCESSSolid-base catalysts consisting of CaO covered with 5-20 mol% TiO2 (TiO2/CaO) were prepared by deposition of titanium tetraisopropoxide (Ti(OCH(CH3)2)4) on a Ca(OH)2 surface in an ethyl acetate solution, followed by thermal decomposition in air at 773-1073 K for 3 h. The catalyst heated at 773-973 K showed higher activity for the base catalyzed retro-aldol reaction to convert diacetone alcohol (4-hydroxy-4-methyl-2-pentanone) to acetone at 299 K. 10 mol% TiO2/CaO heat-treated at 773 K was the most active catalyst for the retro-aldol reaction among the metal oxide-covered CaO catalysts. The catalyst treated at 1073 K was inactive for the same reaction, as was uncovered CaO. The catalytic activity of TiO2/CaO poisoned by water was recovered by washing with acetone, confirming that TiO2-covered CaO had sufficient stability against H2O. No leaching of Ca2+ into the polar solvent diacetone alcohol was observed in any covered catalyst.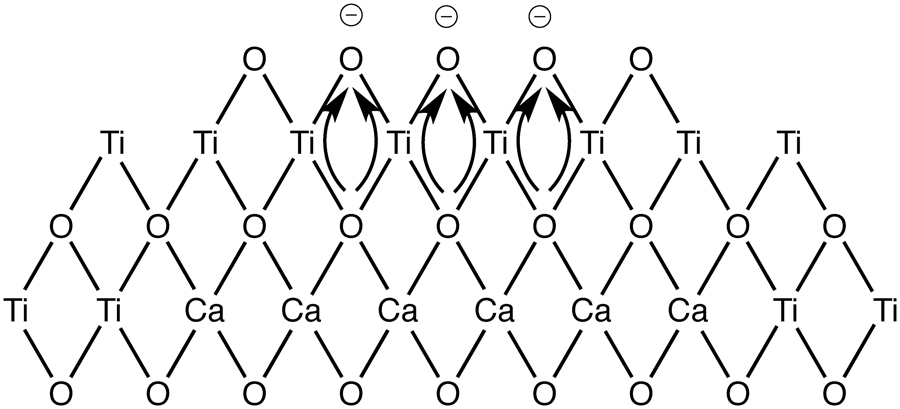 View full abstractDownload PDF (554K)
View full abstractDownload PDF (554K)
Letter
-
Lina MAHARDIANI, Yuichi KAMIYAArticle type: Letter
2016 Volume 59 Issue 1 Pages 31-34
Published: January 01, 2016
Released on J-STAGE: March 01, 2016
JOURNAL FREE ACCESSCatalytic activity of Co3O4 for oxidative decomposition of NH4+ with O3 in water was greatly enhanced with each reuse of the catalyst under the reaction conditions while maintaining its high selectivity towards gaseous compounds. On the other hand, other metal oxide catalysts showed no or only a little enhancement of the activity. Formation of −NHx groups on the surface of Co3O4 during the reaction caused the enhancement of the catalytic activity.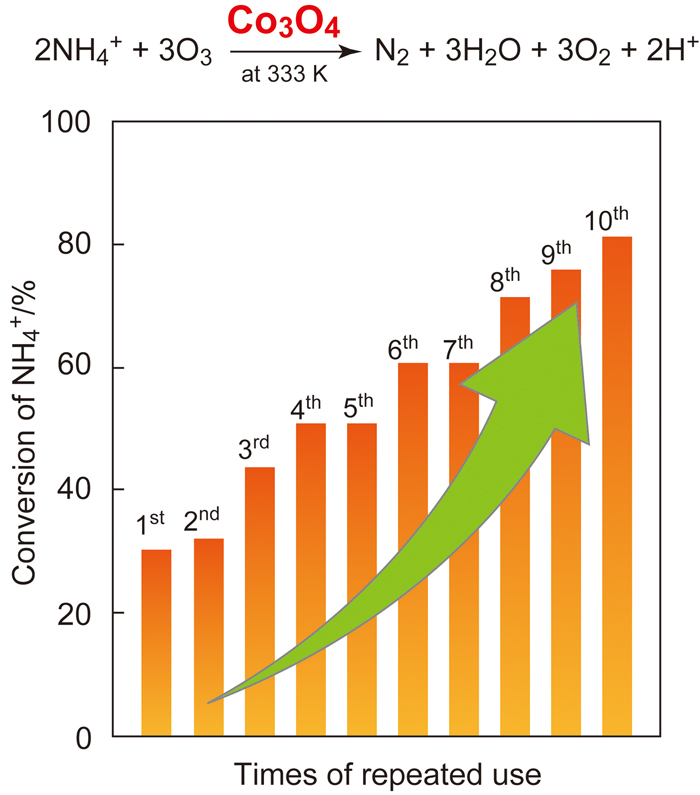 View full abstractDownload PDF (1031K)
View full abstractDownload PDF (1031K)
- |<
- <
- 1
- >
- >|