
- Issue 6 Pages 195-
- Issue 5 Pages 133-
- Issue 4 Pages 95-
- Issue 3 Pages 69-
- Issue 2 Pages 31-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Katsuya SHIMURAArticle type: Review Paper
2023 Volume 66 Issue 2 Pages 31-39
Published: March 01, 2023
Released on J-STAGE: March 01, 2023
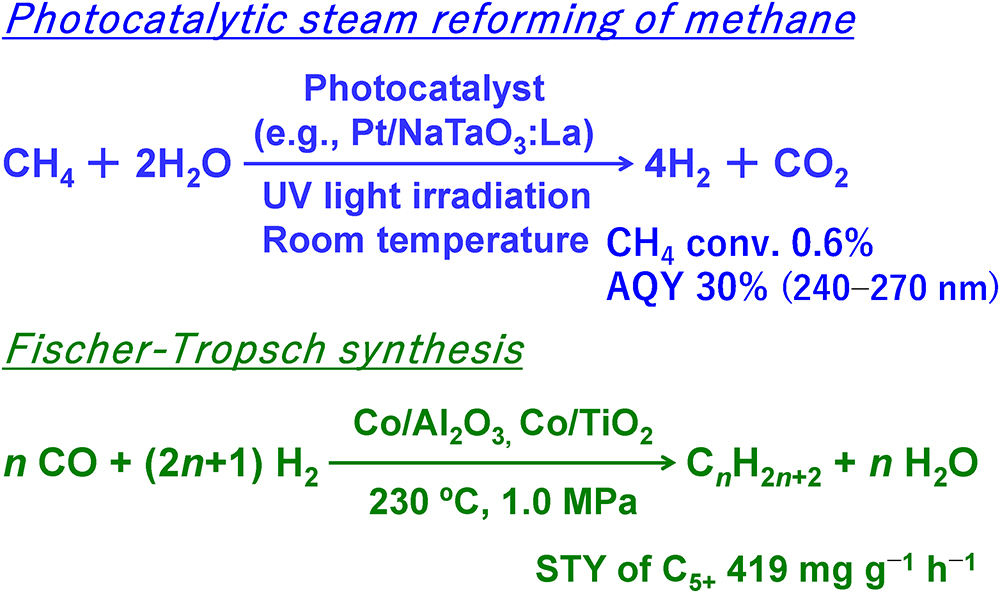
JOURNAL FREE ACCESSIn this review, I introduce our group’s development of improved catalysts for use in photocatalytic steam reforming of methane and in Fischer–Tropsch synthesis. Although methane is a promising alternative to petroleum, its use as a chemical feedstock is currently limited. This is because methane is a very stable hydrocarbon and its conversion usually requires high temperatures, which not only increases operating costs but also causes severe catalyst deactivation. In our first set of studies, we examined the use of photoenergy and photocatalysts at low temperature for methane conversion. We found that hydrogen could be efficiently produced from methane and water at room temperature by using semiconductor photocatalysts such as platinum-loaded, lanthanum-doped sodium tantalate (Pt/NaTaO3:La). In our second set of studies, we examined noble metal-free, cobalt-based catalysts as an inexpensive means of catalyzing Fischer–Tropsch synthesis. We found that the dispersion and electronic state of cobalt metal particles could be precisely controlled by optimizing the crystal phase and pore structure of the support, by carefully choosing the type and amount of promoter, and by selecting an appropriate catalyst preparation method, which together afforded catalysts with high catalytic activity for the formation of long-chain hydrocarbons with five or more carbon atoms.
 View full abstractDownload PDF (540K)
View full abstractDownload PDF (540K) -
Shohei TADAArticle type: Review Paper
2023 Volume 66 Issue 2 Pages 40-47
Published: March 01, 2023
Released on J-STAGE: March 01, 2023
JOURNAL FREE ACCESSCu-based catalyst is one of the major catalysts for CO2-to-methanol hydrogenation, but the catalytic performance must be further improved. This review introduces a new development strategy for Cu-based catalysts: Cu coordination structure in a catalyst precursor will control the morphology of the active sites. The strategy requires preparation of Cu-doped metal oxides containing unique Cu clusters. Cu doping allows (i) control of the crystal phase of the metal oxide support, (ii) formation of Cu nanoparticles with quite small size via H2 reduction, and (iii) regeneration of the spent catalysts. Based on the strategy, we developed Cu/a-ZrO2 (a- = amorphous) and Cu/MgAl2O4 for CO2-to-methanol hydrogenation. Crystal phase control and Cu nanoparticle formation were related to the catalyst activity, whereas the regeneration ability was related to the catalyst durability. Control of the coordination structure of the active metal species in the catalyst precursor will lead to the development of improved catalysts and to the elucidation of the unique structure and behavior of the active metal species.
 View full abstractDownload PDF (4655K)
View full abstractDownload PDF (4655K) -
Atsushi FUKUOKA, Hirokazu KOBAYASHIArticle type: Review Paper
2023 Volume 66 Issue 2 Pages 48-56
Published: March 01, 2023
Released on J-STAGE: March 01, 2023
JOURNAL FREE ACCESSThis review article describes our research on heterogeneous catalysis for valorization of cellulose and chitin, which are abundant plant and marine biomass. First the background and history of this topic are presented, and importance of synthesis of valuable chemicals is emphasized in countries like Japan where the amount of biomass resource is limited. In cellulose degradation, we discovered that Pt supported on alumina catalyzes hydrolytic hydrogenation of cellulose to sorbitol. Then we found that Pt supported on carbon (Pt/C) is more active and durable than Pt/Al2O3, and Pt/C is a bifunctional catalyst in which carbon works as solid acid. This finding led us to develop weakly acidic carbon catalysts for cellulose hydrolysis, and ball-milling of mixed cellulose and the carbon catalyst is imperative to increase physical contact between the solid substrate and the solid catalyst. This method enabled fast and highly selective synthesis of glucose from cellulose even in real biomass. In our mechanistic study, it is found that aromatic surface of carbon adsorbs cellulose through CH-π bonding and that weakly acidic sites on carbon attack glycosidic bonds in cellulose. In comparing with benchmarks, the heterogeneous carbon catalyst is superior to both cellulase enzyme and homogeneous sulfonic acid. The weakly acidic carbon is also effective for the synthesis of valuable cello-oligosaccharides, which work as biostimulants. We have also been studying the depolymerization of chitin to oligomers and N-acetylglucosamine (NAG). Hydrolysis of chitin was promoted by mechanocatalysis combining acid catalysis with mechanical force of ball-milling. NAG can be further converted to various organonitrogen compounds.
 View full abstractDownload PDF (2687K)
View full abstractDownload PDF (2687K)
-
Satoshi INAGAKI, Yuki MAEKAWA, Yuki ORUI, Rikuto WAKATSUKI, Yuko NISHI ...Article type: Regular Paper
2023 Volume 66 Issue 2 Pages 57-67
Published: March 01, 2023
Released on J-STAGE: March 01, 2023
JOURNAL FREE ACCESSMethylcyclohexane (MCH) is a promising candidate for use as a liquid organic hydrogen carrier, but is isomerized into undesirable five-membered-ring compounds with similar boiling points to MCH, so purification requires selective conversion of the five-membered-ring compounds without reaction of the MCH. We found that an Ir/SiO2 catalyst efficiently promoted the selective hydrogenolysis of methylcyclopentane (MCP), a model five-membered-ring compound, in the presence of excess MCH in the reaction system. Ir/SiO2 and Rh/SiO2 catalysts provided high conversions in the hydrogenolysis of MCP at 200 °C, whereas almost no reaction of MCH occurred. In contrast, Pt/SiO2 catalyst was almost inactive in the hydrogenolysis of both MCP and MCH at 200 °C. The difference in reactivities for the Ir/SiO2 and Rh/SiO2 catalysts can be explained by the relatively high strain energy and formation enthalpy of MCP compared to MCH. The Ir/SiO2 catalyst also preferentially promoted the hydrogenolysis of ethylcyclopentane, which is a more realistic impurity, in the presence of excess MCH.
 View full abstractDownload PDF (9861K)
View full abstractDownload PDF (9861K)
- |<
- <
- 1
- >
- >|