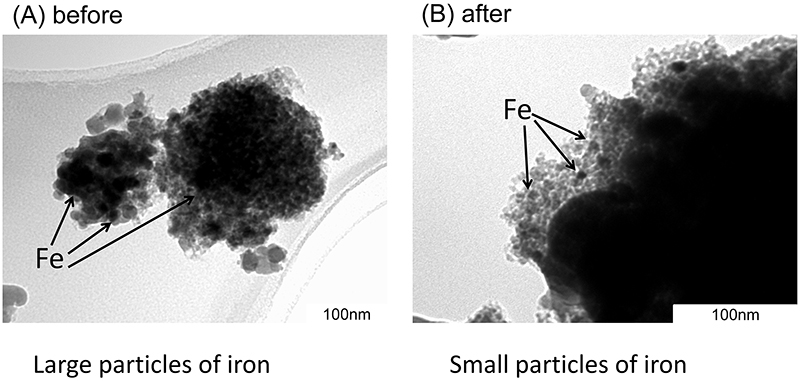
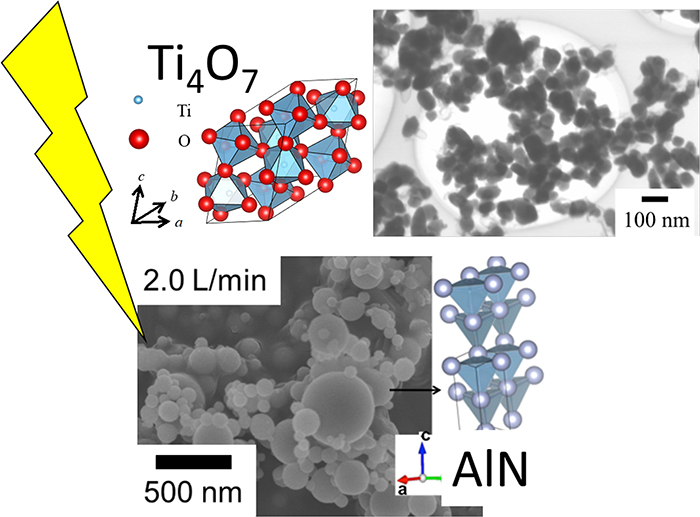
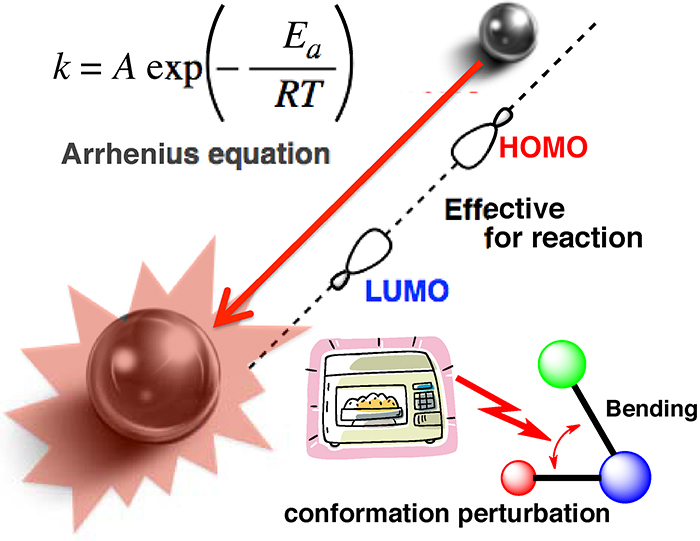
Volume 61, Issue 2
Displaying 1-15 of 15 articles from this issue
- |<
- <
- 1
- >
- >|
Regular Paper
-
Article type: Regular Paper
2018Volume 61Issue 2 Pages 51-58
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (3355K) -
Article type: Regular Paper
2018Volume 61Issue 2 Pages 59-71
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (7466K) -
Article type: Regular Paper
2018Volume 61Issue 2 Pages 72-79
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (635K) -
Article type: Regular Paper
2018Volume 61Issue 2 Pages 80-86
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1631K)
Preface — Feature Articles: Now and Future of Technology of Microwave Chemical Processes —
-
Article type: Preface
2018Volume 61Issue 2 Pages 87
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (151K)
Review Paper — Feature Articles: Now and Future of Technology of Microwave Chemical Processes —
-
Article type: Review Paper
2018Volume 61Issue 2 Pages 88-97
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (4222K) -
Article type: Review Paper
2018Volume 61Issue 2 Pages 98-105
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1141K) -
Article type: Review Paper
2018Volume 61Issue 2 Pages 106-112
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1647K) -
Article type: Review Paper
2018Volume 61Issue 2 Pages 113-120
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1808K) -
Article type: Review Paper
2018Volume 61Issue 2 Pages 121-128
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1420K) -
Article type: Review Paper
2018Volume 61Issue 2 Pages 129-139
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (3712K)
Regular Paper — Feature Articles: Now and Future of Technology of Microwave Chemical Processes —
-
Article type: Regular Paper
2018Volume 61Issue 2 Pages 140-149
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1899K) -
Article type: Regular Paper
2018Volume 61Issue 2 Pages 150-155
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (2136K)
Technical Report — Feature Articles: Now and Future of Technology of Microwave Chemical Processes —
-
Article type: Technical Report
2018Volume 61Issue 2 Pages 156-162
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1772K) -
Article type: Technical Report
2018Volume 61Issue 2 Pages 163-170
Published: March 01, 2018
Released on J-STAGE: March 01, 2018
Download PDF (1680K)
- |<
- <
- 1
- >
- >|