All issues

Volume 57 (2014)
- Issue 6 Pages 237-
- Issue 5 Pages 197-
- Issue 4 Pages 155-
- Issue 3 Pages 95-
- Issue 2 Pages 65-
- Issue 1 Pages 1-
Volume 57, Issue 3
Displaying 1-6 of 6 articles from this issue
- |<
- <
- 1
- >
- >|
Review Paper
-
Ken MotokuraArticle type: Review Paper
2014 Volume 57 Issue 3 Pages 95-108
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSThis review paper treats with acid-base and Pd-complex-base bifunctional catalyst surfaces used to catalyze nucleophilic addition reactions. A silica–alumina-supported tertiary amine was found to be a highly active heterogeneous catalyst for Michael additions and cyano-ethoxycarbonylation. These reactions barely proceeded with either a homogeneous amine or silica–alumina support, indicating that synergistic catalysis occurred between the silica–alumina surface and basic amine. Silica–alumina, which contains both tertiary and primary amines, was an efficient catalyst for one-pot synthesis of 1,3-dinitroalkanes from aldehydes and nitromethane. The acid-base synergistic catalysis was applied to metal complex-base synergistic catalysis. Both the Pd-complex and tertiary amine-immobilized silica was an excellent heterogeneous catalyst for the Tsuji-Trost reaction. Spectroscopic analyses, such as solid-state MAS NMR, revealed the structures of prepared catalysts, immobilization mechanism of amine groups on the silica–alumina surface, and reaction intermediates and catalytic reaction mechanism. Substrate scope for the cyanation, Michael reaction, 1,3-dinitroalkane synthesis, and the Tsuji-Trost reaction are also described. View full abstractDownload PDF (4539K)
View full abstractDownload PDF (4539K)
Regular Paper
-
Atsuo Nishizawa, Tetsushi Kitano, Na-oki Ikenaga, Takanori Miyake, Tos ...Article type: Regular Paper
2014 Volume 57 Issue 3 Pages 109-117
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSFischer-Tropsch synthesis (FTS) using a Co–Mn/oxidized diamond (O-Dia) catalyst was compared among a fixed bed reactor (FBR), a trickle bed reactor (TBR), and a slurry phase reactor (SPR). For TBR and SPR, hexadecane was employed as a solvent. Regardless of the reactor, the addition of 10 mol% of Mn to Co/O-Dia catalyst greatly increased CO conversion without changing selectivity to C5+ liquid products. The highest initial CO conversion was obtained with FBR. However, with an increase in the time on stream, CO conversion decreased, due mainly to the accumulation of high molecular weight hydrocarbons (wax) on the catalyst bed. Although with TBR the initial CO conversion was 5 % lower than that with FBR, at a feed rate of the solvent from 3 to 12 mL/(g-catalyst h), CO conversion did not decrease during a prolonged run. SPR maintained the initial CO conversion for 24 h on stream, but the CO conversion was half of that with TBR. From these findings, we propose that TBR is the most appropriate reactor type for FTS. View full abstractDownload PDF (748K)
View full abstractDownload PDF (748K) -
Yuki Matsui, Ryo Katayama, Yuki Tamura, Shogo Teratani, Masaki Ota, Yo ...Article type: Regular Paper
2014 Volume 57 Issue 3 Pages 118-124
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSA flow-type apparatus was developed to measure vapor-liquid phase equilibrium of water–heavy oil mixtures at high temperature and pressure conditions. The validity of the apparatus and experimental procedures were confirmed by measuring the vapor-liquid equilibrium of water–tetralin mixtures. For the phase equilibrium measurements of water–heavy oil at isothermal and isobaric conditions (603-653 K and 1.0-17 MPa), water was distributed more in the liquid phase by increase in pressure and/or decrease in temperature. At the isothermal condition, the effect of water/oil (W/O) ratio was almost negligible for the range from 1 to 4 g/g at any pressure conditions chosen in our study. During the experiments with controlling residence time, it was recognized that thermal decomposition of heavy oil could be also negligible within 30 min under the studied temperature conditions. From the experiments using the developed apparatus, we succeeded for acquirements of the fundamental data towards the practical process constructions. View full abstractDownload PDF (815K)
View full abstractDownload PDF (815K) -
Yoshihisa HayashiArticle type: Regular Paper
2014 Volume 57 Issue 3 Pages 125-132
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSA method for determination of the solubilities of methane and carbon dioxide in high-pressure water was developed by direct injection of the sample into a gas chromatograph column. This method used a DVB-EVB-EGDMA packings (80-100 mesh) packed column (3 mm i.d. × 2 m). The column temperature and carrier gas flowrate were optimized for the separation of methane, carbon dioxide and water. The measurement accuracy was greatly improved by installing a check valve in the gas supply system. Solubilities of methane and carbon dioxide were determined from the values obtained from the absolute calibration curve. View full abstractDownload PDF (739K)
View full abstractDownload PDF (739K) -
Kazuhisa Murata, Wasana Khongwong, Siriporn Larpkattaworn, Yanyong Liu ...Article type: Regular Paper
2014 Volume 57 Issue 3 Pages 133-145
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSFast pyrolysis with three typical modified zeolite catalysts was evaluated to produce bio-oil from Jatropha waste. Jatropha waste was pyrolyzed in a stainless-steel reactor at 600 °C under N2 gas flow. The aromatic hydrocarbon selectivity in the bio-oil was in the order: PtPd/ZSM(30) (73.7 %)>PtPd/Beta(22.5) (68.6 %)>PtPd/USY(20) (48.7 %), where the weight ratio of Jatropha/catalyst was 1. In addition, catalyst regeneration was carried out to study the catalyst efficiency. The analysis of fresh and regenerated catalysts by XRD, NH3-TPD, and TG/DTG, as well as product selectivity and surface properties showed that coke deposition and removal were associated with the zeolite structure and surface acid property. Due to pore size regulation, H-ZSM-5 with 10-membered ring (10 MR) could promote the pyrolysis reaction on the outside surface rather than in the inside channels. In USY zeolite with 12 MR, the reaction could occur inside the channels, but the moderate acid nature resulted in only slightly developed coke formation, which could be removed, at least partly, after regeneration. In beta zeolite with 12 MR, the total amount of surface acidity was more than twice that of USY, which undergoes more condensed coke formation, and was more difficult to remove by regeneration than the coke over USY. Thus, under these pyrolysis conditions, PtPd/ZSM(30) seems to be a better candidate for pyrolysis of Jatropha waste compared to PtPd/Beta(22.5) and PtPd/USY(20). View full abstractDownload PDF (1271K)
View full abstractDownload PDF (1271K) -
Hideki Kurokawa, Ryota Ogawa, Kazuhiro Yamamoto, Tsutomu Sakuragi, Mas ...Article type: Regular Paper
2014 Volume 57 Issue 3 Pages 146-154
Published: 2014
Released on J-STAGE: July 01, 2014
JOURNAL FREE ACCESSHeterogeneous α-diiminenickel(II) catalysts were prepared by the direct reaction between nickel(II) ion-exchanged fluorotetrasilicic mica and the ligand [Ph’–N = C(Me)–C(Me) = N–Ph’; Ph’ = C6H5–, 2-FC6H4–, 4-FC6H4–, 2,4-F2C6H3–, and 2,4,6-F3C6H2–]. When the prepared procatalysts were activated by a conventional alkyl aluminum compound, such as triethylaluminum or triisobutylaluminum, the high activity for ethylene oligomerization was readily obtained with the formation of linear α-olefins. The activity of the catalysts increased with the addition of fluorine atoms on the phenyl groups in the ligand, and the maximum activity (420 g-C2 g-cat−1 h−1 at 0.7 MPa and 50 °C) was obtained by the catalysts containing 2,4-difluorophenyl groups, because fluorine atoms decreased the electron density of the nickel center. The oligomers were formed in good accordance with the Schulz-Flory distribution, and the values of the distribution constant were within 0.53-0.65. The selectivities of the linear α-olefins were not less than 90 mol% in all the runs. During all the oligomerization runs, long induction periods were clearly observed, but the period was shortened when the reaction temperature was raised. It seemed that the characteristic catalytic behavior was due to a complicated activation mechanism derived from the specific layered structure of the procatalysts.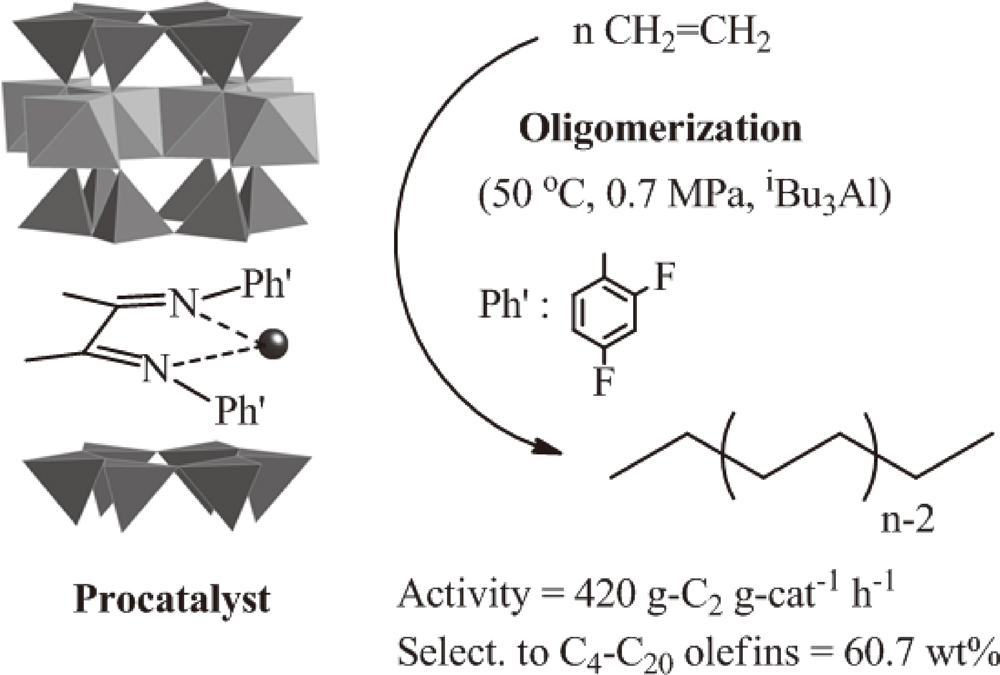 View full abstractDownload PDF (702K)
View full abstractDownload PDF (702K)
- |<
- <
- 1
- >
- >|